Experimental Cannabinoid 2 Receptor Activation by Phyto-Derived and Synthetic Cannabinoid Ligands in LPS-Induced Interstitial Cystitis in Mice
Abstract
1. Introduction
2. Results
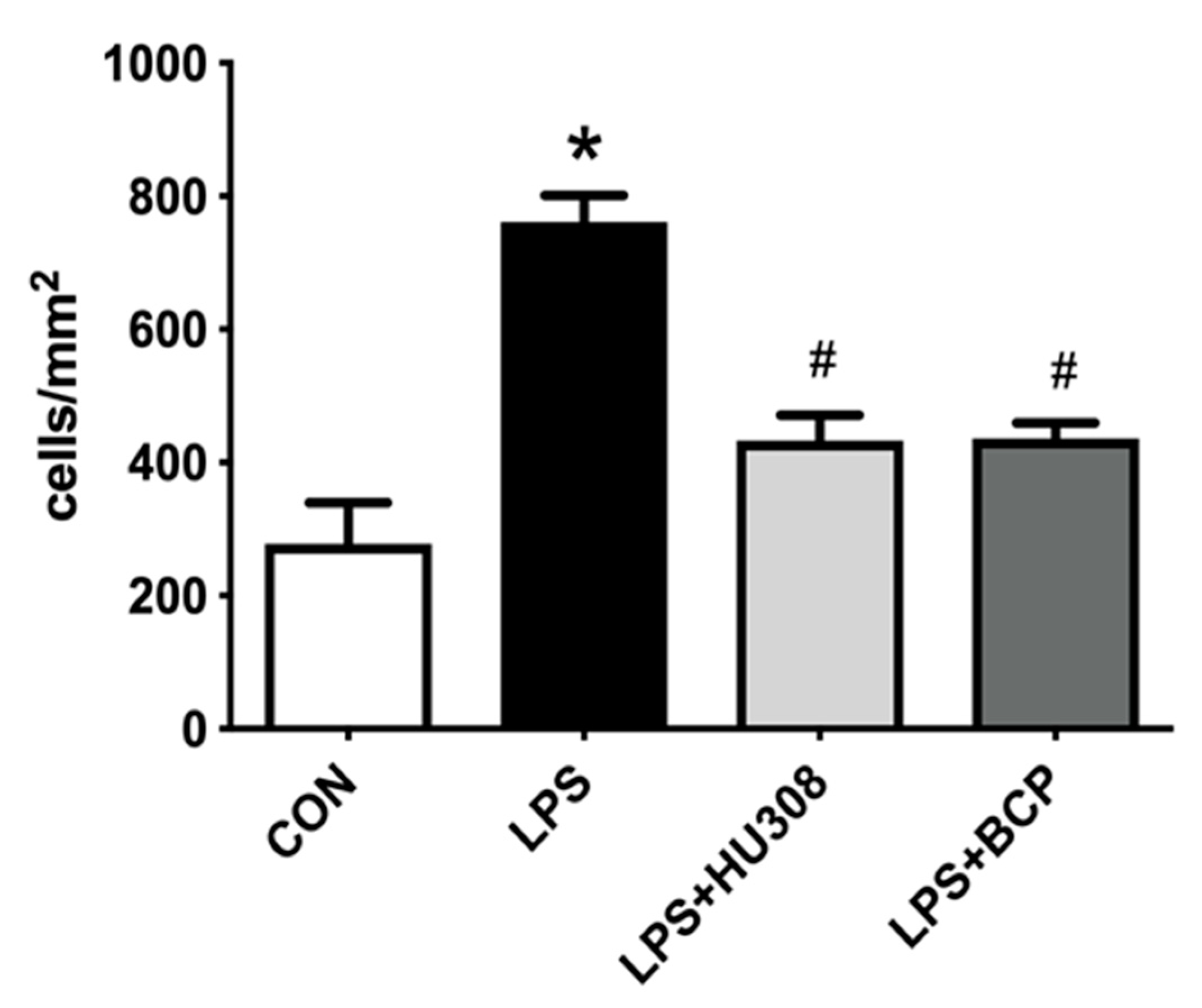
2.1. IC Induced by i.p. LPS Administration
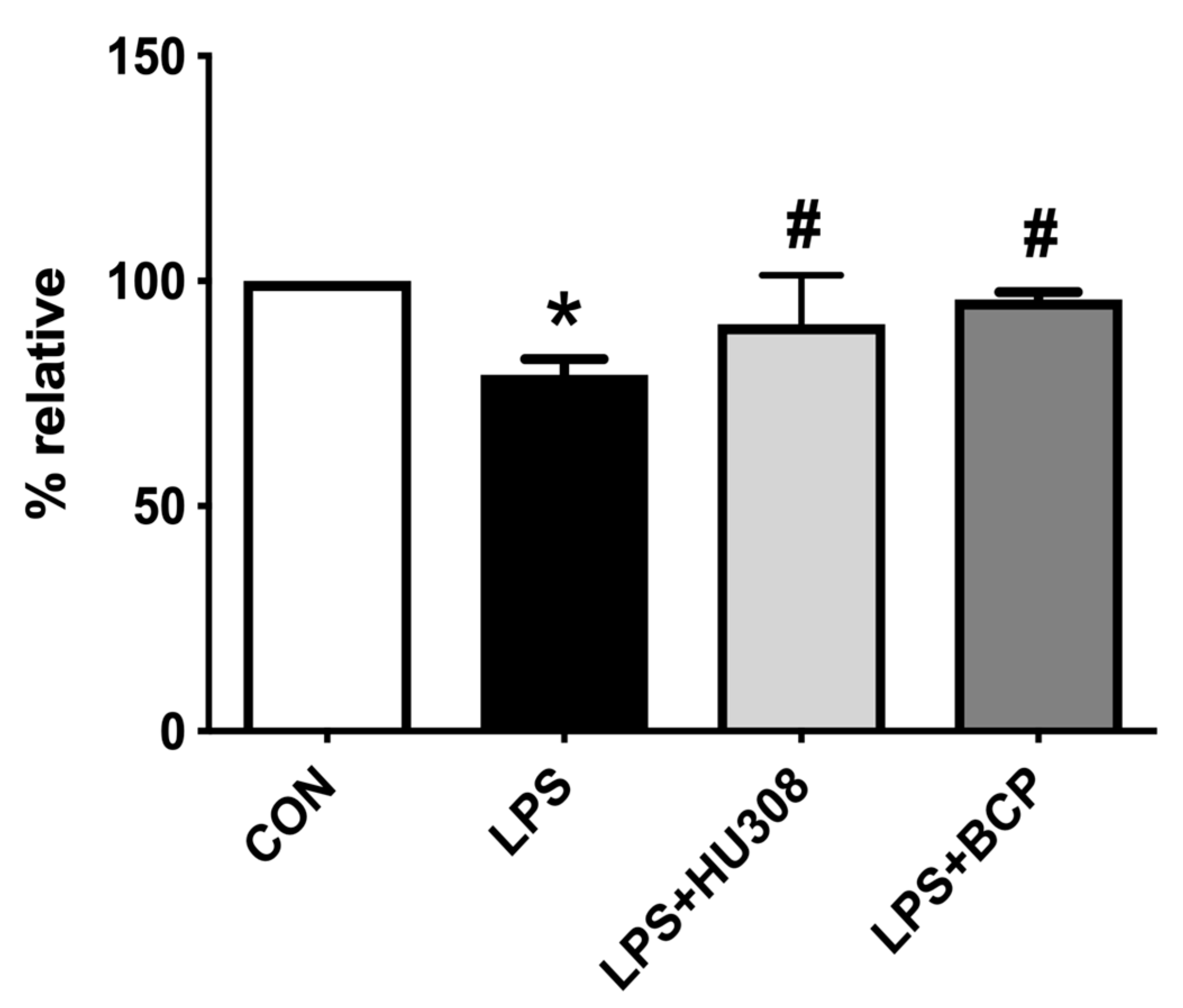
2.2. IC Induced by Intravesical LPS Administration
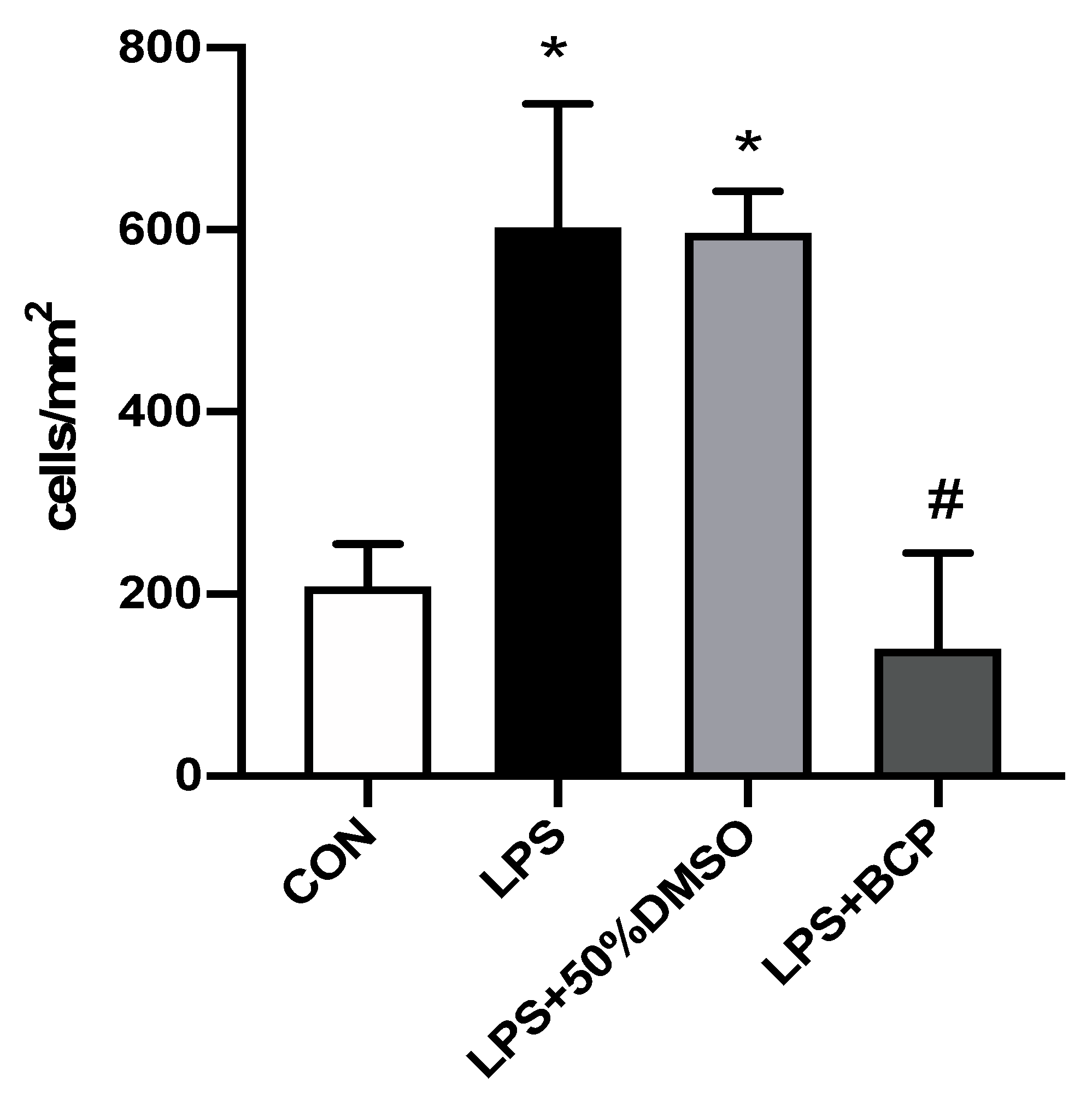
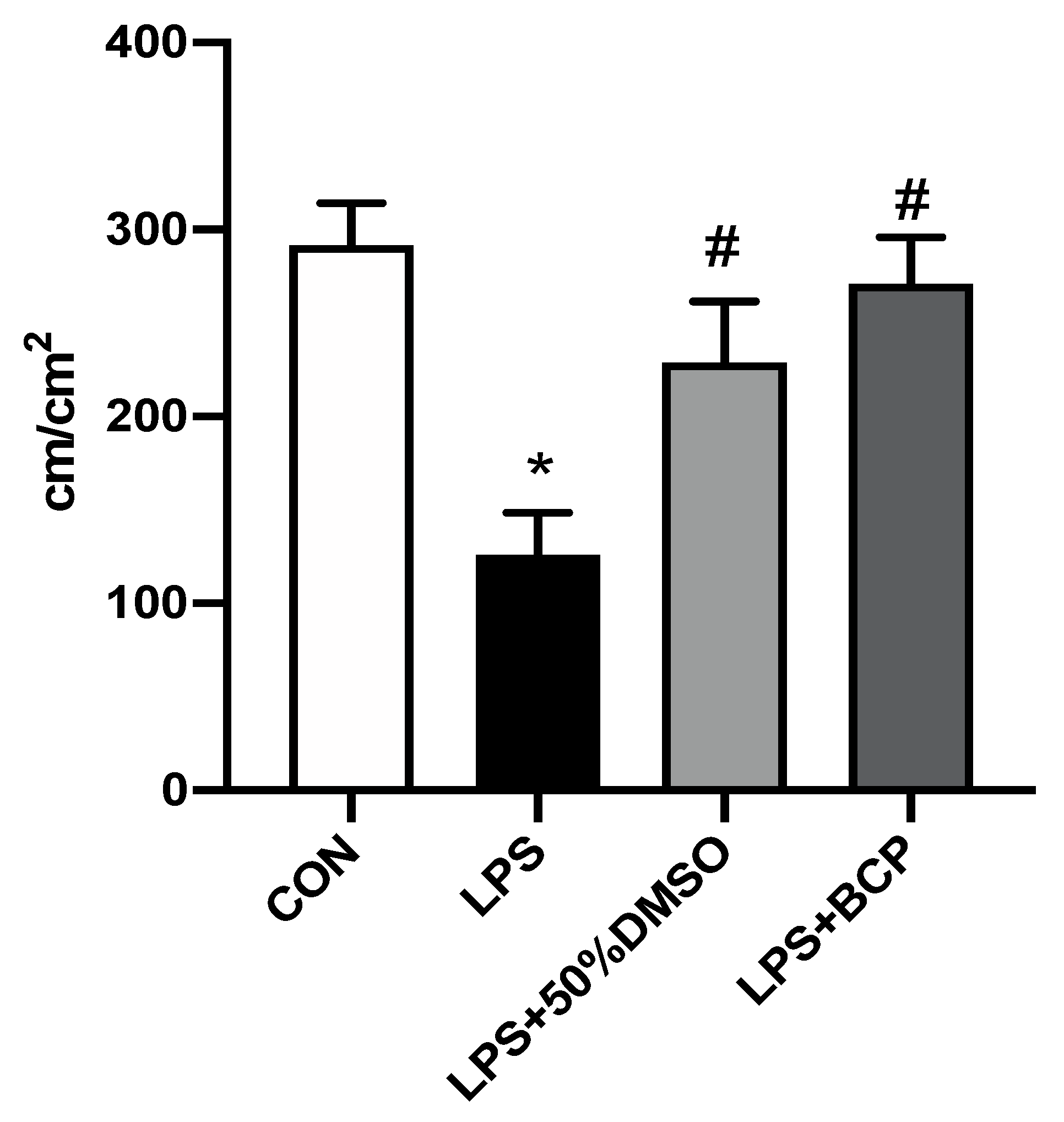
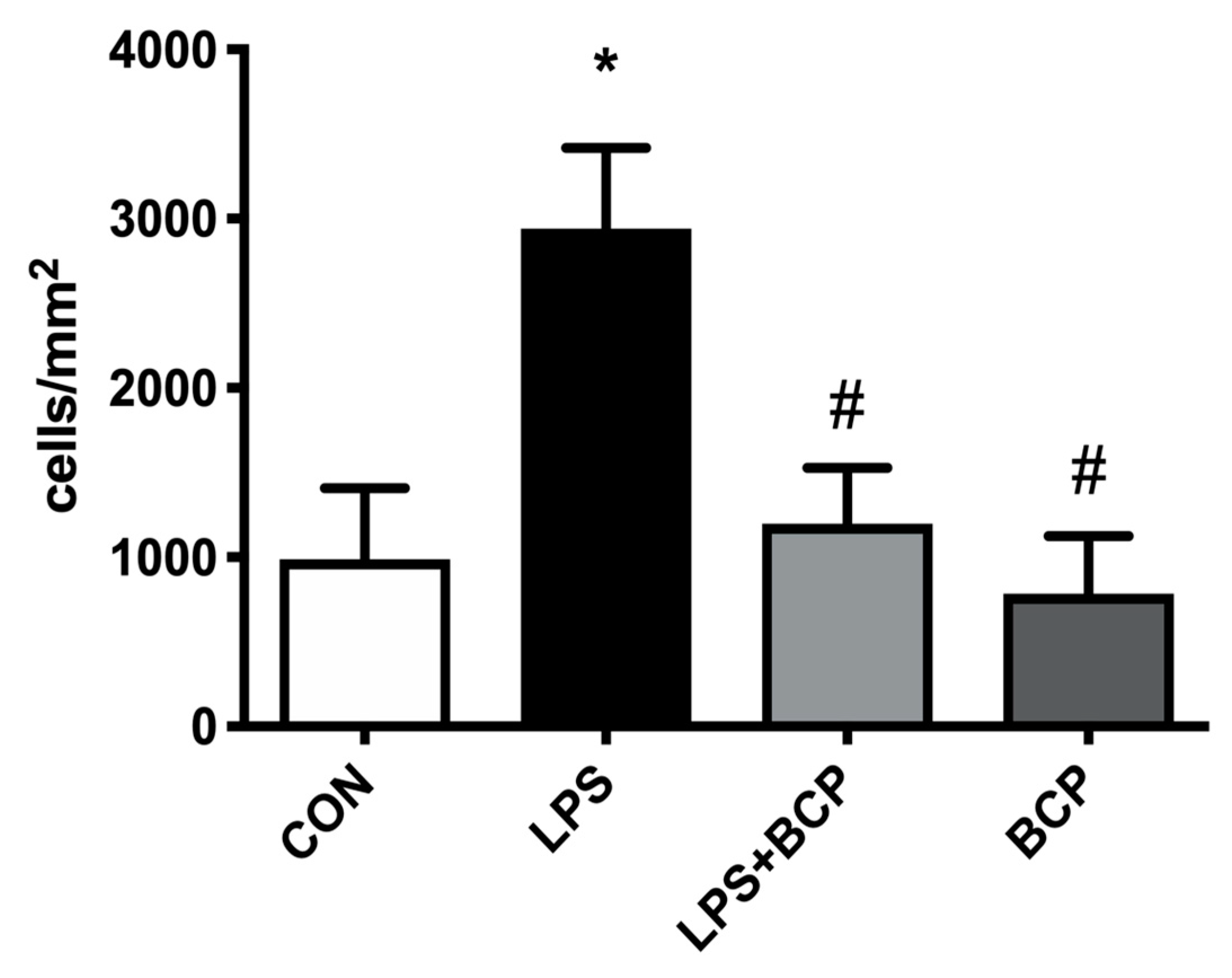
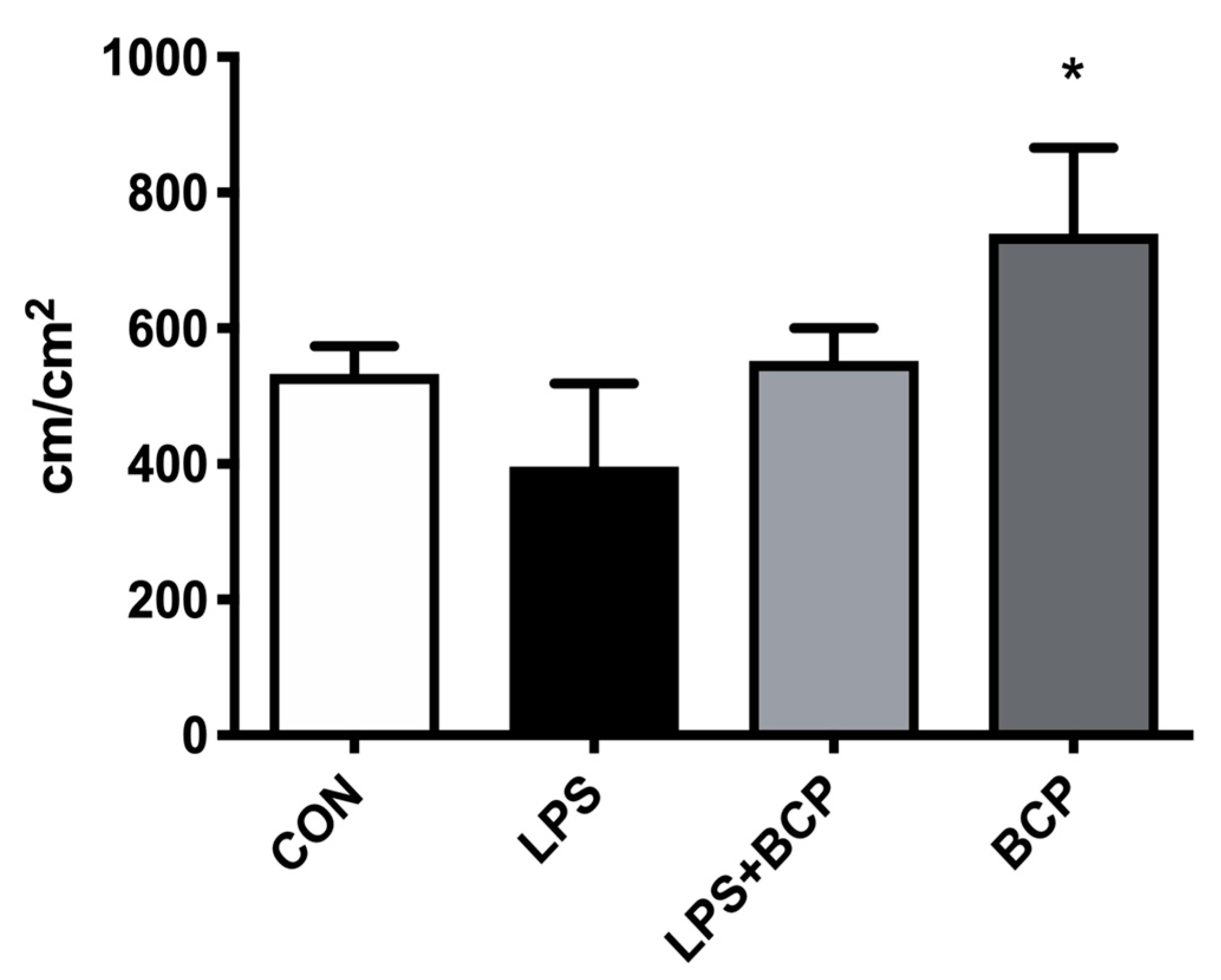
2.3. IC Induced by Intracesical LPS Administration Treated with Oral BCP
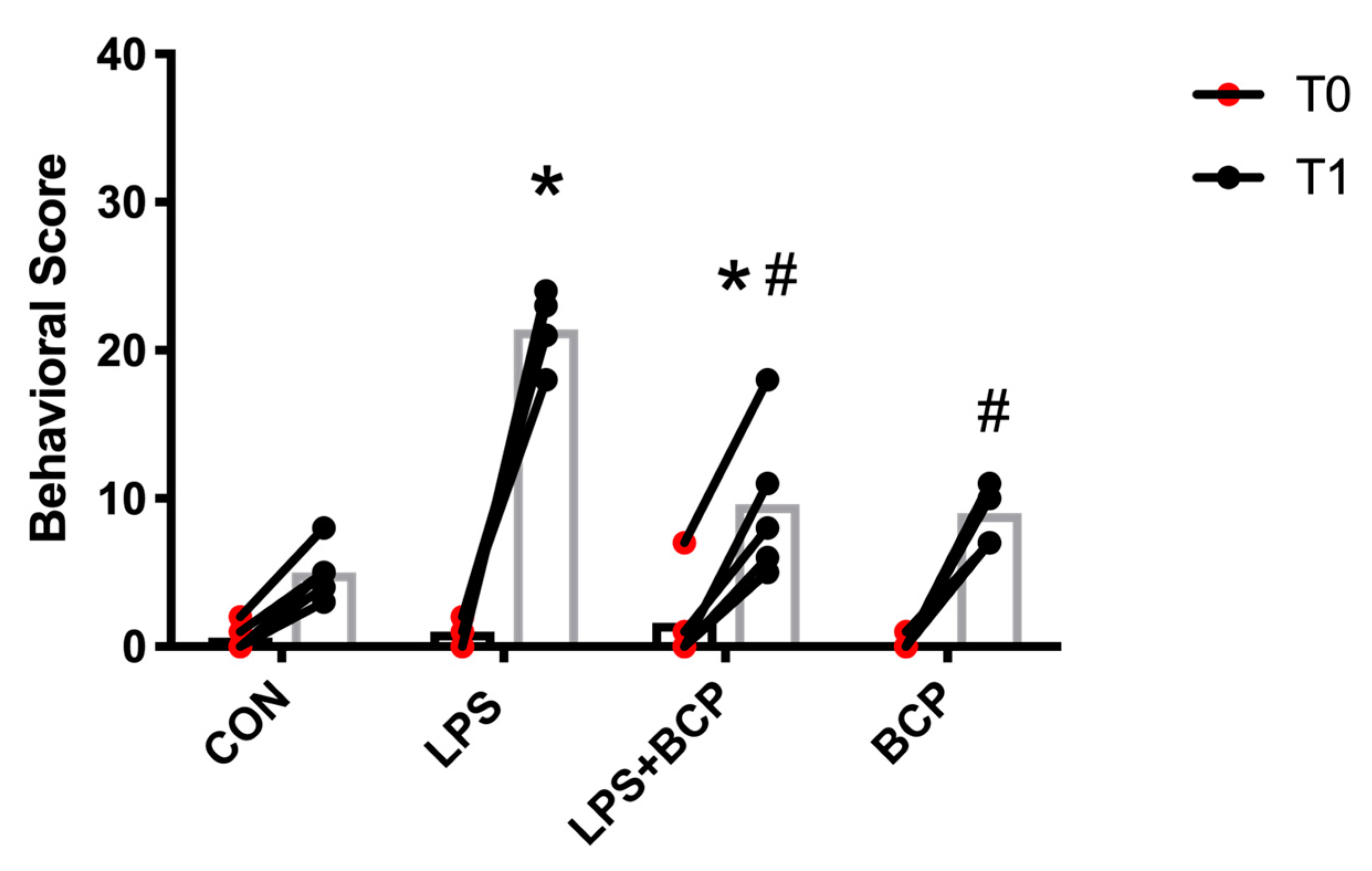
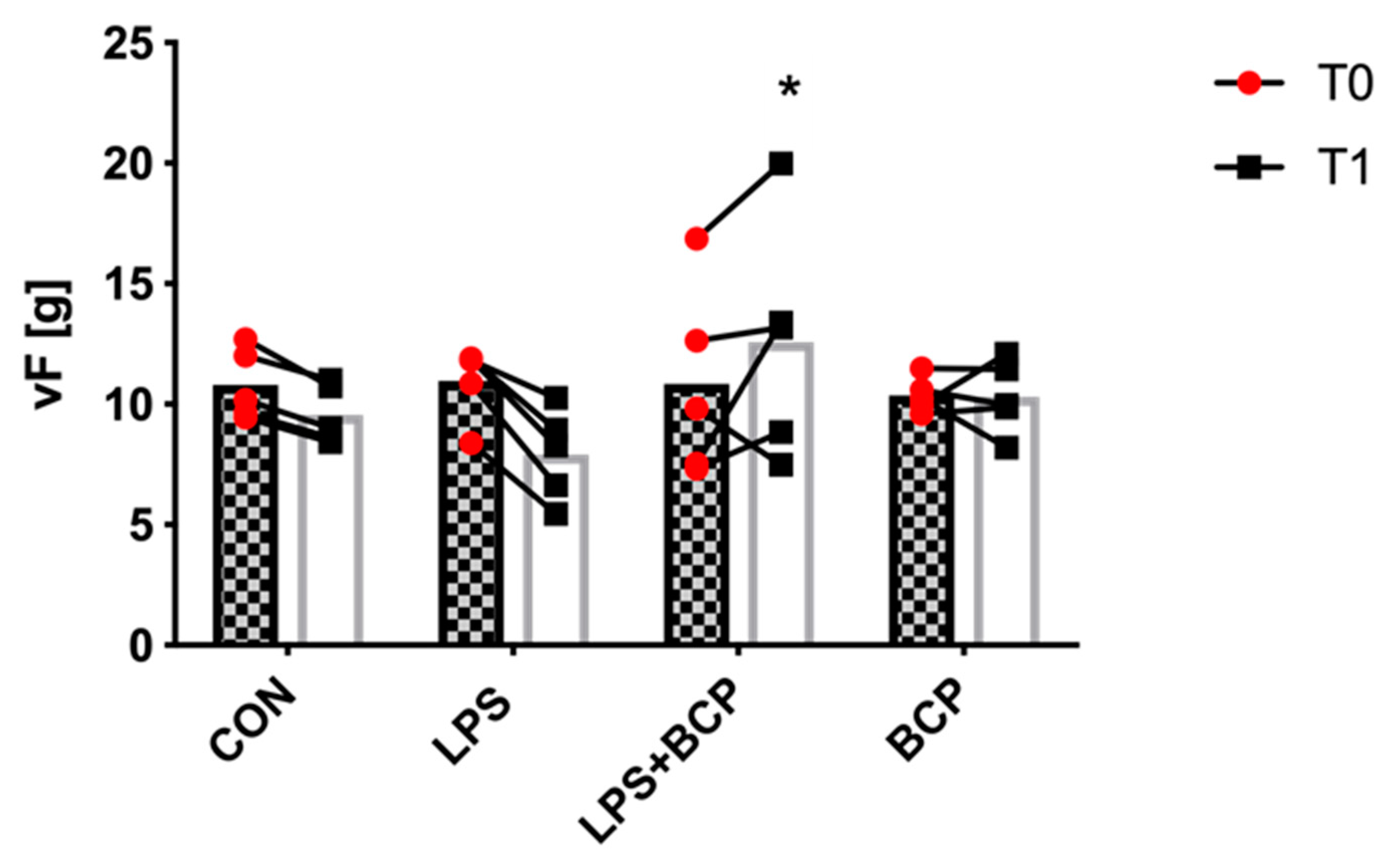
2.4. Behavioral Analysis and Von Frey Aesthesiometry
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.1.1. IC Induced by i.p. LPS Administration
4.1.2. IC Induced by Intravesical LPS Administration with Intravesical Treatment
4.1.3. IC Induced by Intravesical LPS Administration with Oral Treatment
4.2. Anesthesia and Surgery
4.3. Intravital Microscopy
4.4. Video Analysis
4.5. Behavior Assessment Scoring
4.6. Von Frey Aesthesiometry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hanno, P.; Dmochowski, R. Status of international consensus on interstitial cystitis/bladder pain syndrome/painful bladder syndrome: 2008 snapshot. Neurourol. Urodyn. 2009, 28, 274–286. [Google Scholar] [CrossRef]
- Van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; et al. Diagnostic Criteria, Classification, and Nomenclature for Painful Bladder Syndrome/Interstitial Cystitis: An ESSIC Proposal. Eur. Urol. 2008, 53, 60–67. [Google Scholar] [CrossRef]
- Clemens, J.Q.; Meenan, R.T.; Rosetti, M.C.O.; Gao, S.Y.; Calhoun, E.A. Prevalence and incidence of interstitial cystitis in a managed care population. J. Urol. 2005, 173, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Belknap, S.; Blalock, E.; Erickson, D. The Challenges of Interstitial Cystitis: Current Status and Future Prospects. Drugs 2015, 75, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, N.; Oguchi, T.; Yokoyama, H.; Funahashi, Y.; Yoshikawa, S.; Sugino, Y.; Kawamorita, N.; Kashyap, M.; Chancellor, M.; Tyagi, P.; et al. Bladder afferent hyperexcitability in bladder pain syndrome/interstitial cystitis. Int. J. Urol. 2014, 21, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Srivastava, A.; Lee, R.; Tewari, A.K.; Te, A.E. Role of inflammation in bladder function and interstitial cystitis. Ther. Adv. Urol. 2011, 3, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.; Chen, Y.; Shen, P.; Hsu, S.; Chen, G.; Ho, E. Risk factors that affect the treatment of interstitial cystitis using intravesical therapy with a dimethyl sulfoxide cocktail. Int. Urogynecol. J. 2012, 23, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.N.; Dwyer, P.; Murray, C.; Karmakar, D.; Rosamilia, A.; Thomas, E. Long-term outcomes of intravesical dimethyl sulfoxide/heparin/hydrocortisone therapy for interstitial cystitis/bladder pain syndrome. Int. Urogynecol. J. 2017, 28, 1085–1089. [Google Scholar] [CrossRef]
- Fowler, J.E. Prospective study of intravesical dimethyl sulfoxide in treatment of suspected early interstitial cystitis. Urology 1981, 18, 21–26. [Google Scholar] [CrossRef]
- Perez-Marrero, R.; Emerson, L.; Feltis, J. A controlled study of dimethyl sulfoxide in interstitial cystitis. J. Urol. 1988, 140, 36–39. [Google Scholar] [CrossRef]
- Parsons, C.; Benson, G.; Childs, S.; Hanno, P.; Sant, G.; Webster, G. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J. Urol. 1993, 150, 845–848. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Di Marzo, V. An introduction to the endocannabinoid system: From the early to the latest concepts. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol Res 2009, 60, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.P.; Apostu, V.I. Role of cannabinoids in multiple sclerosis. CNS Drugs 2011, 25, 187–201. [Google Scholar] [CrossRef]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30, S13–S18. [Google Scholar] [CrossRef]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 619–631. [Google Scholar] [CrossRef]
- Reggio, P.H. Endocannabinoid structure-activity relationships for interaction at the cannabinoid receptors. Prostaglandins. Leukot. Essent. Fatty Acids 2002, 66, 143–160. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Ross, R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fat. Acids 2002, 66, 101–121. [Google Scholar] [CrossRef]
- Hayn, M.H.; Ballesteros, I.; de Miguel, F.; Coyle, C.H.; Tyagi, S.; Yoshimura, N.; Chancellor, M.B.; Tyagi, P. Functional and Immunohistochemical Characterization of CB1 and CB2 Receptors in Rat Bladder. Urology 2008, 72, 1174–1178. [Google Scholar] [CrossRef]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Moro, M.A.; Martínez-Orgado, J. Cannabinoids in Neurodegenerative Disorders and Stroke/Brain Trauma: From Preclinical Models to Clinical Applications. Neurotherapeutics 2015, 12, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Rogers, T.J.; Lichtman, A.H. Turning Over a New Leaf: Cannabinoid and Endocannabinoid Modulation of Immune Function. J. Neuroimmune Pharmacol. 2015, 10, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Gratzke, C.; Streng, T.; Park, A.; Christ, G.; Stief, C.G.; Hedlund, P.; Andersson, K.-E. Distribution and function of cannabinoid receptors 1 and 2 in the rat, monkey and human bladder. J. Urol. 2009, 181, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J. Anti-inflammatory cannabinoids in diet: Towards a better understanding of CB(2) receptor action? Commun. Integr. Biol. 2008, 1, 26–28. [Google Scholar] [CrossRef]
- Hanus, L.; Breuer, A.; Tchilibon, S.; Shiloah, S.; Goldenberg, D.; Horowitz, M.; Pertwee, R.G.; Ross, R.A.; Mechoulam, R.; Fride, E. HU-308: A specific agonist for CB2, a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 14228–14233. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Toguri, J.T.; Lehmann, C.; Laprairie, R.B.; Szczesniak, A.M.; Zhou, J.; Denovan-Wright, E.M.; Kelly, M.E.M. Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis. Br. J. Pharmacol. 2014, 171, 1448–1461. [Google Scholar] [CrossRef]
- Ossola, C.A.; Surkin, P.N.; Mohn, C.E.; Elverdin, J.C.; Fernández-Solari, J. Anti-Inflammatory and Osteoprotective Effects of Cannabinoid-2 Receptor Agonist HU-308 in a Rat Model of Lipopolysaccharide-Induced Periodontitis. J. Periodontol. 2016, 87, 725–734. [Google Scholar] [CrossRef]
- Gómez-Gálvez, Y.; Palomo-Garo, C.; Fernández-Ruiz, J.; García, C. Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2016, 64, 200–208. [Google Scholar] [CrossRef]
- Lehmann, C.; Kianian, M.; Zhou, J.; Küster, I.; Kuschnereit, R.; Whynot, S.; Hung, O.; Shukla, R.; Johnston, B.; Cerny, V.; et al. Cannabinoid receptor 2 activation reduces intestinal leukocyte recruitment and systemic inflammatory mediator release in acute experimental sepsis. Crit. Care 2012, 16, R47. [Google Scholar] [CrossRef]
- Sardinha, J.; Kelly, M.E.M.; Zhou, J.; Lehmann, C. Experimental Cannabinoid 2 Receptor-Mediated Immune Modulation in Sepsis. Mediators Inflamm. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, C.L.; Chen, M.; Manivannan, A.; Cabay, L.; Pertwee, R.G.; Coutts, A.; Forrester, J. V Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 2007, 82, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Tambaro, S.; Casu, M.A.; Mastinu, A.; Lazzari, P. Evaluation of selective cannabinoid CB1 and CB2 receptor agonists in a mouse model of lipopolysaccharide-induced interstitial cystitis. Eur. J. Pharmacol. 2014, 729, 67–74. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Wang, P.; Bjorling, D.E. Activation of cannabinoid receptor 2 inhibits experimental cystitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R846–R853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Wang, P.; Bjorling, D.E. Treatment with a cannabinoid receptor 2 agonist decreases severity of established cystitis. J. Urol. 2014, 191, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Merriam, F.V.; Wang, Z.; Guerios, S.D.; Bjorling, D.E. Cannabinoid receptor 2 is increased in acutely and chronically inflamed bladder of rats. Neurosci. Lett. 2008, 445, 130–134. [Google Scholar] [CrossRef]
- Szczesniak, A.-M.; Porter, R.F.; Toguri, J.T.; Borowska-Fielding, J.; Gebremeskel, S.; Siwakoti, A.; Johnston, B.; Lehmann, C.; Kelly, M.E.M. Cannabinoid 2 receptor is a novel anti-inflammatory target in experimental proliferative vitreoretinopathy. Neuropharmacology 2017, 113, 627–638. [Google Scholar] [CrossRef]
- Spronk, P.E.; Zandstra, D.F.; Ince, C. Bench-to-bedside review: Sepsis is a disease of the microcirculation. Crit. care 2004, 8, 462–468. [Google Scholar] [CrossRef][Green Version]
- Astiz, M.; DeGent, G.; Lin, R.; Rackow, E. Microvascular function and rheologic changes in hyperdynamic sepsis. Crit. Care Med. 1995, 23, 265–271. [Google Scholar] [CrossRef]
- Cho, J.; Kim, S. A Novel Mitogen-Activated Protein Kinase Mechanism of Gene Transrepression by GR. Mol. Endocrinol. 2009, 23, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Mukhopadhyay, P.; Kechrid, M.; Patel, V.; Tanchian, G.; Wink, D.A.; Gertsch, J.; Pacher, P. β-Caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic. Biol. Med. 2012, 52, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Rawls, W.F.; Cox, L.; Rovner, E.S. Dimethyl sulfoxide (DMSO) as intravesical therapy for interstitial cystitis/bladder pain syndrome: A review. Neurourol. Urodyn. 2017, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Lusty, A.; Kavaler, E.; Zakariasen, K.; Tolls, V.; Nickel, J.C. Treatment effectiveness in interstitial cystitis/bladder pain syndrome: Do patient perceptions align with efficacy-based guidelines? Can. Urol. Assoc. J. 2018, 12, E1–E5. [Google Scholar] [CrossRef]
- Tomoe, H. In what type of interstitial cystitis/bladder pain syndrome is DMSO intravesical instillation therapy effective? Transl. Androl. Urol. 2015, 4, 600–604. [Google Scholar]
- Han, E.; Nguyen, L.; Sirls, L.; Peters, K. Current best practice management of interstitial cystitis/bladder pain syndrome. Ther. Adv. Urol. 2018, 10, 197–211. [Google Scholar] [CrossRef]
- Zacchè, M.M.; Srikrishna, S.; Cardozo, L. Novel targeted bladder drug-delivery systems: A review. Res. Reports Urol. 2015, 7, 169–178. [Google Scholar] [CrossRef]
- Andrade-Silva, M.; Correa, L.B.; Candéa, A.L.P.; Cavalher-Machado, S.C.; Barbosa, H.S.; Rosas, E.C.; Henriques, M.G. The cannabinoid 2 receptor agonist β-caryophyllene modulates the inflammatory reaction induced by Mycobacterium bovis BCG by inhibiting neutrophil migration. Inflamm. Res. 2016, 65, 869–879. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Dai, X.; Ao, Y.; Li, Y. Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicol. Appl. Pharmacol. 2017, 329, 326–333. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Lu, G.; Xu, T.; Qin, L.; Wang, X.; Jin, X.; Liu, B.; Zhao, Z.; Shen, Z.; et al. Specific inhibition of ICAM-1 effectively reduces bladder inflammation in a rat model of severe non-bacterial cystitis. Sci. Rep. 2016, 6, 35672. [Google Scholar] [CrossRef] [PubMed]
- Malan, T.P.; Ibrahim, M.M.; Deng, H.; Liu, Q.; Mata, H.P.; Vanderah, T.; Porreca, F.; Makriyannis, A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 2001, 93, 239–245. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Deng, H.; Zvonok, A.; Cockayne, D.A.; Kwan, J.; Mata, H.P.; Vanderah, T.W.; Lai, J.; Porreca, F.; Makriyannis, A.; et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. USA 2003, 100, 10529–10533. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, M.; Bernardini, N.; Bertorelli, R.; Campanella, M.; Nicolussi, E.; Fredduzzi, S.; Reggiani, A. CB2 receptor-mediated antihyperalgesia: Possible direct involvement of neural mechanisms. Eur. J. Neurosci. 2006, 23, 1530–1538. [Google Scholar] [CrossRef]
- Clayton, N.; Marshall, F.H.; Bountra, C.; O’Shaughnessy, C.T. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain 2002, 96, 253–260. [Google Scholar] [CrossRef]
- Klauke, A.-L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef]
- Bjorling, D.; Wang, Z.-Y.; Bushman, W. Models of Inflammation of the Lower Urinary Tract. Neurourol. Urodyn. 2011, 30, 673–682. [Google Scholar] [CrossRef]
- Birder, L.; Andersson, K.-E. Animal Modelling of Interstitial Cystitis/Bladder Pain Syndrome. Int. Neurourol. J. 2018, 22, S3–S9. [Google Scholar] [CrossRef]
- Boucher, M.; Meen, M.; Codrom, J.-P.; Coudore, F.; Kemeny, J.-L.; Eschalier, A. Cyclophosphamide-incuced cystitis in freely-moving conscious rats: Behavioral approach to a new model of visceral pain. J. Urol. 2000, 164, 203–208. [Google Scholar] [CrossRef]
Sample Availability: All of the compounds used in this study are commercially available (Beta-Caryophyllene (BCP), HU308, DMSO, Saline). Samples are not available from authors. |



| Parameter | Score Description |
|---|---|
| Breathing Rate | Each decrease/increase of 10 breaths per minute from baseline warrants an increase/decrease in score. |
| Eye opening/closing | 0: eyes completely open 5: eyes half closed 10: eyes fully closed |
| Posture | 0: normal posture, 5: moderately hunched back but able to stretch for food/water, 10: fully rounded back or limp posture, no attempt to reach for water or food. Intermediate scores were assigned based on the discretion of observer. |
| Motor activity | Activity (exploring, grooming, feeding) within 20 s (Ex. No movement for 10 s (=50%) = 5 points, no movement for 10 s (=10%) = 1 point |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berger, G.; Arora, N.; Burkovskiy, I.; Xia, Y.; Chinnadurai, A.; Westhofen, R.; Hagn, G.; Cox, A.; Kelly, M.; Zhou, J.; et al. Experimental Cannabinoid 2 Receptor Activation by Phyto-Derived and Synthetic Cannabinoid Ligands in LPS-Induced Interstitial Cystitis in Mice. Molecules 2019, 24, 4239. https://doi.org/10.3390/molecules24234239
Berger G, Arora N, Burkovskiy I, Xia Y, Chinnadurai A, Westhofen R, Hagn G, Cox A, Kelly M, Zhou J, et al. Experimental Cannabinoid 2 Receptor Activation by Phyto-Derived and Synthetic Cannabinoid Ligands in LPS-Induced Interstitial Cystitis in Mice. Molecules. 2019; 24(23):4239. https://doi.org/10.3390/molecules24234239
Chicago/Turabian StyleBerger, Geraint, Nipun Arora, Ian Burkovskiy, Yanfang Xia, Anu Chinnadurai, Robert Westhofen, Georg Hagn, Ashley Cox, Melanie Kelly, Juan Zhou, and et al. 2019. "Experimental Cannabinoid 2 Receptor Activation by Phyto-Derived and Synthetic Cannabinoid Ligands in LPS-Induced Interstitial Cystitis in Mice" Molecules 24, no. 23: 4239. https://doi.org/10.3390/molecules24234239
APA StyleBerger, G., Arora, N., Burkovskiy, I., Xia, Y., Chinnadurai, A., Westhofen, R., Hagn, G., Cox, A., Kelly, M., Zhou, J., & Lehmann, C. (2019). Experimental Cannabinoid 2 Receptor Activation by Phyto-Derived and Synthetic Cannabinoid Ligands in LPS-Induced Interstitial Cystitis in Mice. Molecules, 24(23), 4239. https://doi.org/10.3390/molecules24234239








