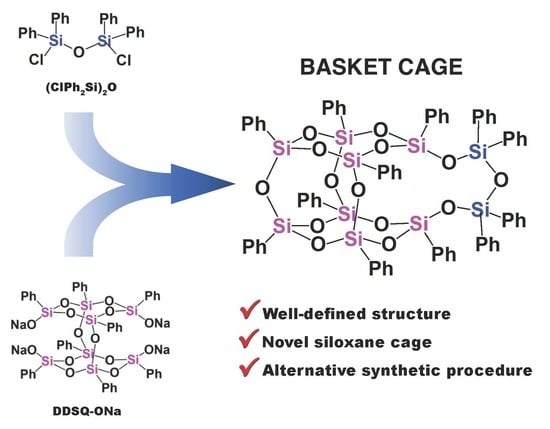
Synthesis and Characterization of Unsymmetrical Double-Decker Siloxane (Basket Cage)
Abstract
1. Introduction
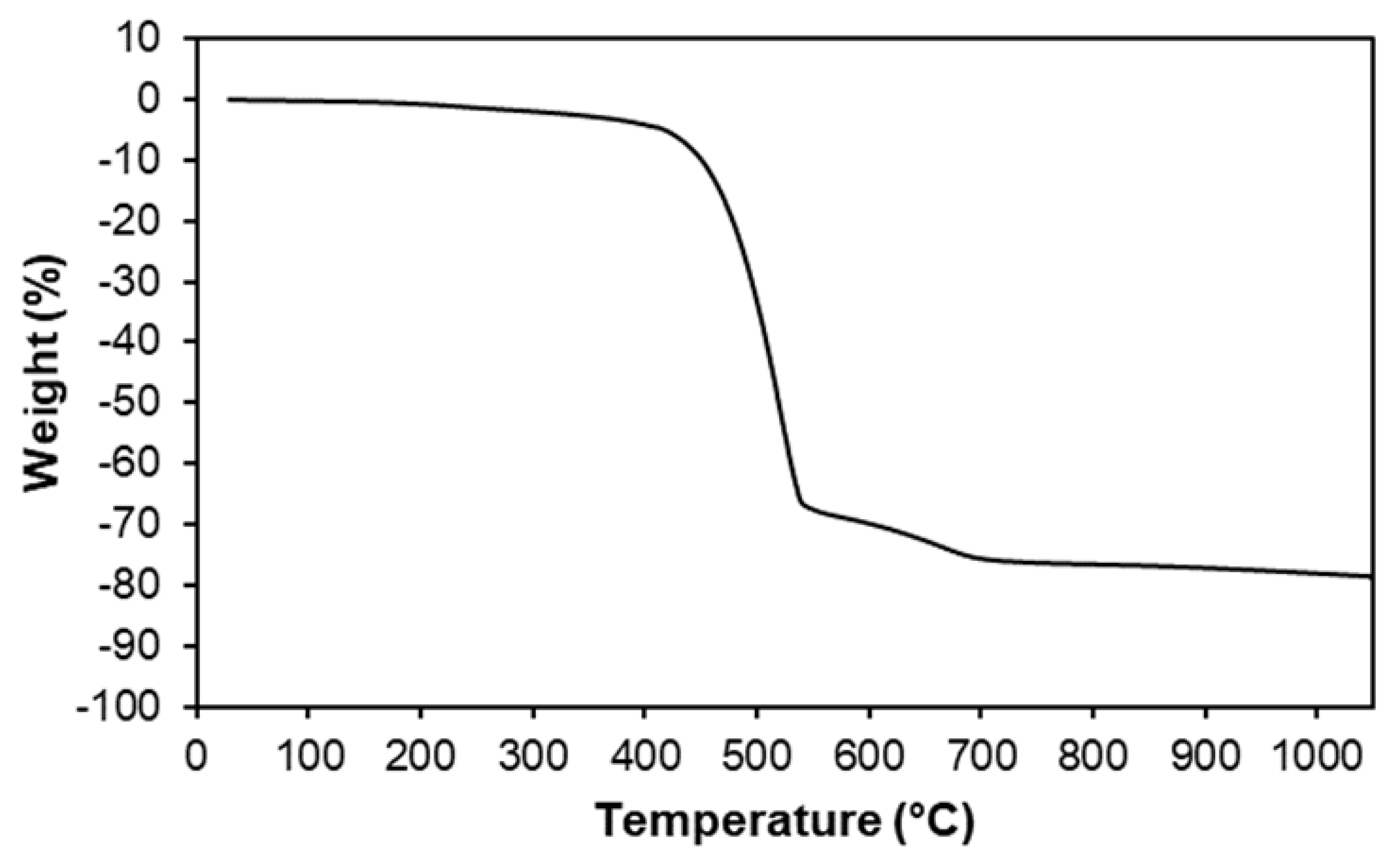
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Unsymmetrical T8D2, 3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Soares, S.F.; Fernandes, T.; Trindade, T.; Daniel-da-Silva, A.L. Trimethyl Chitosan/Siloxane-Hybrid Coated Fe3O4 Nanoparticles for the Uptake of Sulfamethoxazole from Water. Molecules 2019, 24, 1958. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Xu, K.; Zhang, N.; Lu, C.; Zhang, Q.; Yu, L.; Feng, F.; Li, X. In Situ Incorporation of Diamino Silane Group into Waterborne Polyurethane for Enhancing Surface Hydrophobicity of Coating. Molecules 2019, 24, 1667. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Alphonse, V.D.; Trigg, D.B.; Harrigan, T.P.; Paulson, J.M.; Luong, Q.T.; Lloyd, E.P.; Barbee, M.H.; Craig, S.L. ‘Seeing’ Strain in Soft Materials. Molecules 2019, 24, 542. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-W.; Hsiao, Y.-H.; Chen, C.-C.; Yet, S.-F.; Hsu, C.-H. A PDMS-Based Microfluidic Hanging Drop Chip for Embryoid Body Formation. Molecules 2016, 21, 882. [Google Scholar] [CrossRef]
- Islamova, R.M.; Dobrynin, M.V.; Ivanov, D.M.; Vlasov, A.V.; Kaganova, E.V.; Grigoryan, G.V.; Kukushkin, V.Y. bis-Nitrile and bis-Dialkylcyanamide Platinum(II) Complexes as Efficient Catalysts for Hydrosilylation Cross-Linking of Siloxane Polymers. Molecules 2016, 21, 311. [Google Scholar] [CrossRef]
- Cintra, G.A.S.; Pinto, L.A.; Calixto, G.M.F.; Soares, C.P.; Von Zuben, E.D.S.; Scarpa, M.V.; Gremião, M.P.D.; Chorilli, M. Bioadhesive Surfactant Systems for Methotrexate Skin Delivery. Molecules 2016, 21, 231. [Google Scholar] [CrossRef]
- Kim, C.; Hong, J.H. Carbosilane and Carbosiloxane Dendrimers. Molecules 2009, 14, 3719–3730. [Google Scholar] [CrossRef]
- Kunthom, R.; Jaroentomeechai, T.; Ervithayasuporn, V. Polyhedral oligomeric silsesquioxane (POSS) containing sulfonic acid groups as a metal-free catalyst to prepare polycaprolactone. Polymer 2017, 108, 173–178. [Google Scholar] [CrossRef]
- Li, J.; Dong, F.; Lu, L.; Li, H.; Xiong, Y.; Ha, C.-S. Raspberry-Like Polysilsesquioxane Particles with Hollow-Spheres-on-Sphere Structure: Rational Design, Controllable Synthesis, and Catalytic Application. Polymers 2019, 11, 1350. [Google Scholar] [CrossRef]
- Sodkhomkhum, R.; Ervithayasuporn, V. Synthesis of poly(siloxane/double-decker silsesquioxane) via dehydrocarbonative condensation reaction and its functionalization. Polymer 2016, 86, 113–119. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, B.; Wei, K.; Zheng, S. Organic-inorganic polyurethanes with double decker silsesquioxanes in the main chains: Morphologies, surface hydrophobicity, and shape memory properties. J. Polym. Sci. Phys. 2018, 56, 893–906. [Google Scholar] [CrossRef]
- Kunthom, R.; Piyanuch, P.; Wanichacheva, N.; Ervithayasuporn, V. Cage-like silsesequioxanes bearing rhodamines as fluorescence Hg2+ sensors. J. Photochem. Photobiol. 2018, 356, 248–255. [Google Scholar] [CrossRef]
- Mohapatra, S.; Chaiprasert, T.; Sodkhomkhum, R.; Kunthom, R.; Hanprasit, S.; Sangtrirutnugul, P.; Ervithayasuporn, V. Solid-state Synthesis of Polyhedral Oligomeric Silsesquioxane-Supported N-Heterocyclic Carbenes/Imidazolium salts on Palladium Nanoparticles: Highly Active and Recyclable Catalyst. Chem. Select 2016, 1, 5353–5357. [Google Scholar] [CrossRef]
- Walczak, M.; Januszewski, R.; Majchrzak, M.; Kubicki, M.; Dudziec, B.; Marciniec, B. Unusual cis and trans architecture of dihydrofunctional double-decker shaped silsesquioxane and synthesis of its ethyl bridged π-conjugated arene derivatives. New J. Chem. 2017, 41, 3290–3296. [Google Scholar] [CrossRef]
- Chanmungkalakul, S.; Ervithayasuporn, V.; Boonkitti, P.; Phuekphong, A.; Prigyai, N.; Kladsomboon, S.; Kiatkamjornwong, S. Anion identification using silsesquioxane cages. Chem. Sci. 2018, 9, 7753–7765. [Google Scholar] [CrossRef]
- Aziz, E.Y.; Taylor, G.P.; Bassindale, R.A.; Coles, J.S.; Pitak, B.M. Synthesis and Structures of Novel Molecular Ionic Compounds Based on Encapsulation of Anions and Cations. Organometallics 2016, 35, 4004–4013. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H. Selective dye adsorption and metal ion detection using multifunctional silsesquioxane-based tetraphenylethene-linked nanoporous polymers. J. Mater. Chem. A 2017, 5, 9156–9162. [Google Scholar] [CrossRef]
- Endo, H.; Takeda, N.; Unno, M. Synthesis and Properties of Phenylsilsesquioxanes with Ladder and Double-Decker Structures. Organometallics 2014, 33, 4148–4151. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Sodkhomkhum, R.; Teerawatananond, T.; Phurat, C.; Phinyocheep, P.; Somsook, E.; Osotchan, T. Unprecedented Formation of cis- and trans-Di[(3-chloropropyl)isopropoxysilyl]-Bridged Double-Decker Octaphenylsilsesquioxanes. Eur. J. Inorg. Chem. 2013, 3292–3296. [Google Scholar] [CrossRef]
- Kucuk, A.C.; Matsui, J.; Miyashita, T. Proton-conducting electrolyte film of double-decker-shaped polyhedral silsesquioxane containing covalently bonded phosphonic acid groups. J. Mater. Chem. 2012, 22, 3853–3858. [Google Scholar] [CrossRef]
- Lee, D.W.; Kawakami, Y. Incompletely Condensed Silsesquioxanes: Formation and Reactivity. Polym. J. 2007, 39, 230–238. [Google Scholar] [CrossRef]
- Mitula, K.; Duszczak, J.; Brzakalski, D.; Dudziec, B.; Kubicki, M.; Marciniec, B. Tetra-functional double-decker silsesquioxanes as anchors for reactive functional groups and potential synthons for hybrid materials. Chem. Commun. 2017, 53, 10370–10373. [Google Scholar] [CrossRef] [PubMed]
- Schoen, B.W.; Holmes, D.; Lee, A. Identification and quantification of cis and trans isomers in aminophenyl double-decker silsesquioxanes using 1H-29Si gHMBC-NMR. Magn. Reson. Chem. 2013, 51, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Żak, P.; Majchrzak, M.; Wilkowski, G.; Dudziec, B.; Dutkiewicz, M.; Marciniec, B. Synthesis and characterization of functionalized molecular and macromolecular double-decker silsesquioxane systems. RSC Adv. 2016, 6, 10054–10063. [Google Scholar] [CrossRef]
- Tanaka, T.; Hasegawa, Y.; Kawamori, T.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of Double-Decker Silsesquioxanes from Substituted Difluorosilane. Organometallics 2019, 38, 743–747. [Google Scholar] [CrossRef]
- Liu, Y.; Takeda, N.; Ouali, A.; Unno, M. Synthesis, Characterization, and Functionalization of Tetrafunctional Double-Decker Siloxanes. Inorg. Chem. 2019, 58, 4093–4098. [Google Scholar] [CrossRef]
- Chimjarn, S.; Kunthom, R.; Chancharone, P.; Sodkhomkhum, R.; Sangtrirutnugul, P.; Ervithayasuporn, V. Synthesis of aromatic functionalized cage-rearranged silsesquioxanes (T8, T10, and T12) via nucleophilic substitution reactions. Dalton. Trans. 2015, 44, 916–919. [Google Scholar] [CrossRef]
- Endo, H.; Takeda, N.; Takanashi, M.; Imai, T.; Unno, M. Refractive Indices of Silsesquioxanes with Various Structures. Silicon 2014, 7, 127–132. [Google Scholar] [CrossRef]
- Ervithayasuporn, V.; Tomeechai, T.; Takeda, N.; Unno, M.; Chaiyanurakkul, A.; Hamkool, R.; Osotchan, T. Synthesis and Characterization of Octakis(3-propyl ethanethioate)octasilsesquioxane. Organometallics 2011, 30, 4475–4478. [Google Scholar] [CrossRef]
- Asuncion, M.Z.; Ronchi, M.; Abu-Seir, H.; Laine, R.M. Synthesis, functionalization and properties of incompletely condensed “half cube” silsesquioxanes as a potential route to nanoscale Janus particles. C. R. Chim. 2010, 13, 270–281. [Google Scholar] [CrossRef]
- Sugiyama, T.; Shiba, H.; Yoshikawa, M.; Wada, H.; Shimojima, A.; Kuroda, K. Synthesis of Polycyclic and Cage Siloxanes by Hydrolysis and Intramolecular Condensation of Alkoxysilylated Cyclosiloxanes. Chem. Eur. J. 2019, 25, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Oguri, N.; Egawa, Y.; Takeda, N.; Unno, M. Janus-Cube Octasilsesquioxane: Facile Synthesis and Structure Elucidation. Angew. Chem. Int. Ed. 2016, 55, 9336–9339. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Egawa, Y.; Adachi, T.; Oguri, N.; Kobayashi, M.; Kudo, T.; Takeda, N.; Unno, M.; Tanaka, R. Synthesis, Structures, and Thermal Properties of Symmetric and Janus “Lantern Cage” Siloxanes. Chem. Eur.J. 2019, 25, 1683–1686. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang, D.F.; Dannatt, J.E.; Maleczka, R.E.; Lee, A. Separation of asymmetrically capped double-decker silsesquioxanes mixtures. Polyhedron 2018, 155, 189–193. [Google Scholar] [CrossRef]
- Barry, B.-D.; Dannatt, J.E.; King, A.K.; Lee, A.; Maleczka, R.E. A general diversity oriented synthesis of asymmetric double-decker shaped silsesquioxanes. Chem. Commun. 2019, 55, 8623–8626. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yang, S.; Bae, B.-S. Thermally Stable Transparent Sol-Gel Based Siloxane Hybrid Material with High Refractive Index for Light Emitting Diode (LED) Encapsulation. Chem. Mater. 2010, 22, 3549–3555. [Google Scholar] [CrossRef]
- Seki, H.; Abe, Y.; Gunji, T. Stereochemistry of the reaction of cis,trans,cis-2,4,6,8-tetraisocyanato-2,4,6,8-tetramethylcyclotetrasiloxane with triphenylsilanol and 1,1,3,3-tetraphenyldisiloxane-1,3-diol. J. Organomet. Chem. 2011, 696, 846–851. [Google Scholar] [CrossRef]
- Kunthom, R.; Adachi, T.; Liu, Y.; Takeda, N.; Unno, M.; Tanaka, R. Synthesis of ‘butterfly cage’ based on double-decker silsesquioxane. Chem. Asian. J. 2019, (in press). [CrossRef]
- Kozelj, M.; Orel, B. Synthesis of polyhedral phenylsilsesquioxanes with KF as the source of the fluoride ion. Dalton Trans. 2008, 5072. [Google Scholar] [CrossRef][Green Version]
- Chinnam, P.R.; Gau, M.R.; Schwab, J.; Zdilla, M.J.; Wunder, S.L. The polyoctahedral silsesquioxane (POSS) 1,3,5,7,9,11,13,15-octaphenylpentacyclo [9.5.1.13,9.15,15.17,13]octasiloxane (octaphenyl-POSS). Acta. Cryst. 2014, C70, 971–974. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunthom, R.; Takeda, N.; Unno, M. Synthesis and Characterization of Unsymmetrical Double-Decker Siloxane (Basket Cage). Molecules 2019, 24, 4252. https://doi.org/10.3390/molecules24234252
Kunthom R, Takeda N, Unno M. Synthesis and Characterization of Unsymmetrical Double-Decker Siloxane (Basket Cage). Molecules. 2019; 24(23):4252. https://doi.org/10.3390/molecules24234252
Chicago/Turabian StyleKunthom, Rungthip, Nobuhiro Takeda, and Masafumi Unno. 2019. "Synthesis and Characterization of Unsymmetrical Double-Decker Siloxane (Basket Cage)" Molecules 24, no. 23: 4252. https://doi.org/10.3390/molecules24234252
APA StyleKunthom, R., Takeda, N., & Unno, M. (2019). Synthesis and Characterization of Unsymmetrical Double-Decker Siloxane (Basket Cage). Molecules, 24(23), 4252. https://doi.org/10.3390/molecules24234252