Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities
Abstract
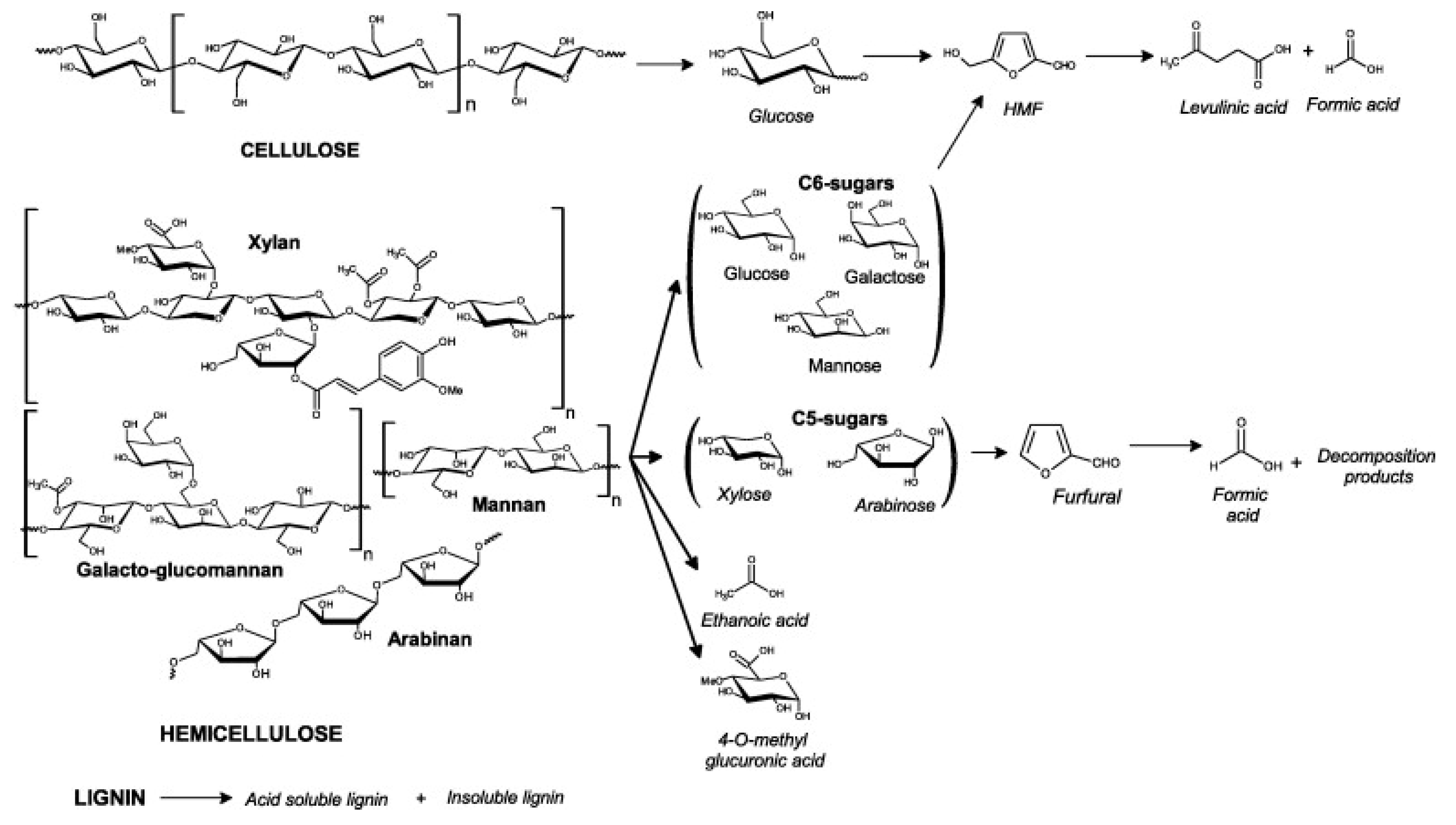
1. Introduction
2. Mineral Acid Fractionation
2.1. Effect and Mechanism
2.2. Nature of the Acid
2.3. Conditions and Yields
2.3.1. Low Concentrations of Acid and High Temperatures
2.3.2. High Concentrations of Acid and Low Temperatures
2.4. Industrial Applications
3. Purification Routes Applied to Lignocellulosic Acid Hydrolysates
3.1. Alkalinization/Overliming
3.2. Evaporation
3.3. Liquid/Liquid Extraction
3.4. Adsorption
3.4.1. Activated Charcoal
3.4.2. Resin
3.5. Low Pressure Chromatography
3.6. Cross-Flow Membrane Filtration
3.7. Electrodialysis
3.8. Combination of Different Techniques
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, H.-J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.J. An examination of biorefining processes, catalysts and challenges. Catal. Today 2009, 145, 138–151. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Petersen, G.; Aden, A.; Bozell, J.; Holladay, J.; White, J.; Manheim, A.; Eliot, D.; Lasure, L.; Jones, S. Top Value Added Chemicals From Biomass. Volume I—Results of Screening for Potential Candidates From Sugars and Synthesis Gas; National Renewable Energy Laboratory: Golden, CO, USA, 2004.
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Silveira, M.H.L.; Morais, A.R.C.; da Costa Lopes, A.M.; Olekszyszen, D.N.; Bogel-Łukasik, R.; Andreaus, J.; Pereira Ramos, L. Current pretreatment technologies for the development of cellulosic ethanol and biorefineries. ChemSusChem 2015, 8, 3366–3390. [Google Scholar] [CrossRef]
- Viell, J.; Harwardt, A.; Seiler, J.; Marquardt, W. Is biomass fractionation by Organosolv-like processes economically viable? A conceptual design study. Bioresour. Technol. 2013, 150, 89–97. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Simmons, B.A.; Blanch, H.W. Techno-economic analysis of a lignocellulosic ethanol biorefinery with ionic liquid pre-treatment. Biofuelsbioproducts Biorefining 2011, 5, 562–569. [Google Scholar] [CrossRef]
- Da Costa Lopes, A.M.; Lins, R.M.G.; Rebelo, R.A.; Łukasik, R.M. Biorefinery approach for lignocellulosic biomass valorisation with an acidic ionic liquid. Green Chem. 2018, 20, 4043–4057. [Google Scholar] [CrossRef]
- Avignon, G.; Delmas, M. Method for Producing Paper Pulp, Lignins, Sugars and Acetic Acid by Fractionation of Lignocellulosic Vegetable Material in Formic/Acetic Acid Medium 2000. Available online: https://patentimages.storage.googleapis.com/56/5c/29/a999e754e0ddcd/WO2000068494A1.pdf (accessed on 1 November 2019).
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Abdelkafi, F.; Ammar, H.; Rousseau, B.; Tessier, M.; El Gharbi, R.; Fradet, A. Structural analysis of Alfa Grass (Stipa tenacissima L.) lignin obtained by acetic acid/formic acid delignification. Biomacromolecules 2011, 12, 3895–3902. [Google Scholar] [CrossRef] [PubMed]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Moe, S.T.; Janga, K.K.; Hertzberg, T.; Hägg, M.-B.; Øyaas, K.; Dyrset, N. Saccharification of lignocellulosic biomass for biofuel and biorefinery applications – a renaissance for the concentrated acid hydrolysis? Energy Procedia 2012, 20, 50–58. [Google Scholar] [CrossRef]
- Humbrid, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Clausen, E.C.; Gaddy, J.L. Concentrated Sulfuric Acid Process for Converting Lignocellulosic Materials to Sugars 1993. Available online: https://patentimages.storage.googleapis.com/82/98/bb/f47b60240f922c/US5188673.pdf (accessed on 1 November 2019).
- Harmsen, P.F.H.; Huijen, W.J.J.; Bermúdez López, L.M.; Bakker, R.R.C. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass; Wageningen University: Wageningen, The Netherlands, 2010. [Google Scholar]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification and fermentation of wheat straw to ethanol. Process Biochem. 2005, 40, 3693–3700. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sun, R.; Sun, X.F.; Wang, S.Q.; Zhu, W.; Wang, X.Y. Ester and ether linkages between hydroxycinnamic acids and lignins from wheat, rice, rye, and barley straws, maize stems, and fast-growing poplar wood. Ind. Crop. Prod. 2002, 15, 179–188. [Google Scholar] [CrossRef]
- Sarkanen, S.; Teller, D.C.; Stevens, C.R.; McCarthy, J.L. Associative interactions between Kraft lignin components. Macromolecules 1984, 17, 2588–2597. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Shi, Y.; Yokoyama, T.; Akiyama, T.; Yashiro, M.; Matsumoto, Y. Degradation kinetics of monosaccharides in hydrochloric, sulfuric, and sulfurous acid. BioResources 2012, 7, 4085–4097. [Google Scholar]
- Svetlitchnyi, V.A.; Kensch, O.; Falkenhan, D.A.; Korseska, S.G.; Lippert, N.; Prinz, M.; Sassi, J.; Schickor, A.; Curvers, S. Single-step ethanol production from lignocellulose using novel extremely thermophilic bacteria. Biotechnol Biofuels 2013, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; Hospido, A.; Agnemo, R.; Svensson, P.; Selling, E.; Moreira, M.T.; Feijoo, G. Environmental life cycle assessment of a Swedish dissolving pulp mill integrated biorefinery. J. Ind. Ecol. 2011, 15, 568–583. [Google Scholar] [CrossRef]
- Kanchanalai, P. New Dehydration and Pretreatment Process for Ethanol Production from Biomass. Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2015. [Google Scholar]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, R.; Ramírez, J.A.; Garrote, G.; Vázquez, M. Kinetic study of the acid hydrolysis of sugar cane bagasse. J. Food Eng. 2002, 55, 309–318. [Google Scholar] [CrossRef]
- Bustos, G.; Ramirez, J.A.; Garrote, G.; Vazquez, M. Modeling of the hydrolysis of sugar cane bagasse with hydrochloric acid. Appl. Biochem. Biotechnol. 2003, 104, 51–68. [Google Scholar] [CrossRef]
- Rodríguez-Chong, A.; Alberto Ramírez, J.; Garrote, G.; Vázquez, M. Hydrolysis of sugar cane bagasse using nitric acid: A kinetic assessment. J. Food Eng. 2004, 61, 143–152. [Google Scholar] [CrossRef]
- Gámez, S.; González-Cabriales, J.J.; Ramírez, J.A.; Garrote, G.; Vázquez, M. Study of the hydrolysis of sugar cane bagasse using phosphoric acid. J. Food Eng. 2006, 74, 78–88. [Google Scholar] [CrossRef]
- Sindhu, R.; Kuttiraja, M.; Binod, P.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresour. Technol. 2011, 102, 10915–10921. [Google Scholar] [CrossRef]
- Lavarack, B.P.; Griffin, G.J.; Rodman, D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 2002, 23, 367–380. [Google Scholar] [CrossRef]
- Goldstein, I.S.; Pereira, H.; Pittman, J.L.; Strouse, B.A.; Scaringelli, F.P. Hydrolysis of cellulose with super concentrated hydrochloric acid. Biotechnol. Bioeng. Symp. (U. S.) 1983, 13. [Google Scholar]
- Israilides, C.J.; Grant, G.A.; Han, Y.W. Sugar level, fermentability, and acceptability of straw treated with different acids. Appl. Environ. Microbiol. 1978, 36, 43–46. [Google Scholar] [PubMed]
- Idrees, M.; Adnan, A.; Sheikh, S.; Qureshic, F.A. Optimization of dilute acid pretreatment of water hyacinth biomass for enzymatic hydrolysis and ethanol production. Excli J 2013, 12, 30–40. [Google Scholar] [PubMed]
- Lee, Y.Y.; Iyer, P.; Torget, R.W. Dilute-acid hydrolysis of lignocellulosic biomass. In Recent Progress in Bioconversion of Lignocellulosics; Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 1999; pp. 93–115. ISBN 978-3-540-65577-0. [Google Scholar]
- Chaturvedi, V.; Verma, P. An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products. 3 Biotech 2013, 3, 415–431. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Y.; Zhang, X. Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem 2015, 8, 24–51. [Google Scholar] [CrossRef]
- Girisuta, B.; Dussan, K.; Haverty, D.; Leahy, J.J.; Hayes, M.H.B. A kinetic study of acid catalysed hydrolysis of sugar cane bagasse to levulinic acid. Chem. Eng. J. 2013, 217, 61–70. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 472, 499. [Google Scholar]
- Almeida, J.R.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Chum, H.L.; Johnson, D.K.; Black, S.K.; Overend, R.P. Pretreatment-catalyst effects and the combined severity parameter. Appl. Biochem. Biotechnol. 1990, 24, 1. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, J.Y.; Jang, H.M.; Lee, M.W.; Park, J.M. Sequential dilute acid and alkali pretreatment of corn stover: Sugar recovery efficiency and structural characterization. Bioresour. Technol. 2015, 182, 296–301. [Google Scholar] [CrossRef]
- Pattra, S.; Sangyoka, S.; Boonmee, M.; Reungsang, A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int. J. Hydrog. Energy 2008, 33, 5256–5265. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute acid pretreatment, enzymatic saccharification, and fermentation of rice hulls to ethanol. Biotechnol Prog. 2005, 21, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J.J. Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.V.P.; Rodrigues, T.H.S.; de Albuquerque, T.L.; Gonçalves, L.R.B.; de Macedo, G.R. Evaluation of dilute acid pretreatment on cashew apple bagasse for ethanol and xylitol production. Chem. Eng. J. 2014, 243, 234–243. [Google Scholar] [CrossRef]
- Sun, R.; Lawther, J.M.; Banks, W.B. Influence of alkaline pre-treatments on the cell wall components of wheat straw. Ind. Crop. Prod. 1995, 4, 127–145. [Google Scholar] [CrossRef]
- Sun, R.; Fang, J.; Rowlands, P. Physico-chemical and thermal characterization of alkali-soluble lignins from wheat straw. Polym J 1998, 30, 289–294. [Google Scholar] [CrossRef]
- Chandel, A.K.; Antunes, F.A.; Silva, M.B.; da Silva, S.S. Unraveling the structure of sugarcane bagasse after soaking in concentrated aqueous ammonia (SCAA) and ethanol production by Scheffersomyces (Pichia) stipitis. Biotechnol. Biofuels 2013, 6, 102. [Google Scholar] [CrossRef]
- Fitzpatrick, S.W. Lignocellulose Degradation to Furfural and Levulinic Acid 1990. Available online: https://patentimages.storage.googleapis.com/71/6f/25/bd0134fcae464a/US4897497.pdf (accessed on 1 November 2019).
- Fitzpatrick, S.W. Production of Levulinic Acid from Carbohydrate-Containing Materials 1997. Available online: https://patentimages.storage.googleapis.com/b7/56/9b/36a230b4cdeb60/US5608105.pdf (accessed on 1 November 2019).
- Canilha, L.; Santos, V.T.O.; Rocha, G.J.M.; Almeida e Silva, J.B.; Giulietti, M.; Silva, S.S.; Felipe, M.G.A.; Ferraz, A.; Milagres, A.M.F.; Carvalho, W. A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J. Ind. Microbiol. Biotechnol. 2011, 38, 1467–1475. [Google Scholar] [CrossRef]
- Wu, L.; Arakane, M.; Ike, M.; Wada, M.; Takai, T.; Gau, M.; Tokuyasu, K. Low temperature alkali pretreatment for improving enzymatic digestibility of sweet sorghum bagasse for ethanol production. Bioresour. Technol. 2011, 102, 4793–4799. [Google Scholar] [CrossRef]
- Liu, Z.-S.; Wu, X.-L.; Kida, K.; Tang, Y.-Q. Corn stover saccharification with concentrated sulfuric acid: Effects of saccharification conditions on sugar recovery and by-product generation. Bioresour. Technol. 2012, 119, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.-K.; Cai, B.-Y.; Zhang, J.-A.; Ling, H.-Z.; Zhou, Y.-J.; Ge, J.-P.; Xu, J.-M. Sugarcane bagasse hemicellulose hydrolysate for ethanol production by acid recovery process. Biochem. Eng. J. 2008, 38, 105–109. [Google Scholar] [CrossRef]
- van Groenestijn, J.; Hazewinkel, O.; Bakker, R. Pretreatment of lignocellulose with biological acid recycling (Biosulfurol process). Sugar Ind. 2006, 639–641. [Google Scholar]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Farone, W.A.; Cuzens, J.E. Method of Producing Sugars Using Strong Acid Hydrolysis of Cellulosic and Hemicellulosic Materials 1993. Available online: https://patentimages.storage.googleapis.com/1a/85/31/da3874d969b337/US5562777.pdf (accessed on 1 November 2019).
- Farone, W.A.; Cuzens, J.E. Strong Acid Hydrolysis of Cellulosic and Hemicellulosic Materials 1997. Available online: https://patentimages.storage.googleapis.com/3a/cb/1f/99b277398627bf/US5597714.pdf (accessed on 1 November 2019).
- Sun, Z.-Y.; Tang, Y.-Q.; Iwanaga, T.; Sho, T.; Kida, K. Production of fuel ethanol from bamboo by concentrated sulfuric acid hydrolysis followed by continuous ethanol fermentation. Bioresour. Technol. 2011, 102, 10929–10935. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Weil, J.R.; Dien, B.; Bothast, R.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Removal of fermentation inhibitors formed during pretreatment of biomass by polymeric adsorbents. Ind. Eng. Chem. Res. 2002, 41, 6132–6138. [Google Scholar] [CrossRef]
- Alriksson, B.; Sjöde, A.; Nilvebrant, N.-O.; Jönsson, L.J. Optimal conditions for alkaline detoxification of dilute-acid lignocellulose hydrolysates. Appl. Biochem. Biotech. 2006, 130, 599–611. [Google Scholar] [CrossRef]
- Lemaire, J.; Blanc, C.-L.; Duval, F.; Théoleyre, M.-A.; Pareau, D. Purification of pentoses from hemicellulosic hydrolysates with sulfuric acid recovery by using electrodialysis. Sep. Purif. Technol. 2016, 166, 181–186. [Google Scholar] [CrossRef]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef]
- Mateo, S.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Detoxification of hemicellulosic hydrolyzate from olive tree pruning residue. Ind. Crop. Prod. 2013, 49, 196–203. [Google Scholar] [CrossRef]
- Wilson, J.J.; Deschatelets, L.; Nishikawa, N.K. Comparative fermentability of enzymatic and acid hydrolysates of steam-pretreated aspenwood hemicellulose by Pichia stipitis CBS 5776. Appl. Microbiol. Biotechnol. 1989, 31, 592–596. [Google Scholar] [CrossRef]
- Villarreal, M.L.M.; Prata, A.M.R.; Felipe, M.G.A.; Almeida E Silva, J.B. Detoxification procedures of eucalyptus hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Enzym. Microb. Technol. 2006, 40, 17–24. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J.M. Study of charcoal adsorption for improving the production of xylitol from wood hydrolysates. Bioprocess Eng. 1996, 16, 39–43. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J.M. Charcoal adsorption of wood hydrolysates for improving their fermentability: Influence of the operational conditions. Bioresour. Technol. 1996, 57, 179–185. [Google Scholar] [CrossRef]
- Soto, M.L.; Moure, A.; Domínguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Sainio, T.; Turku, I.; Heinonen, J. Adsorptive removal of fermentation inhibitors from concentrated acid hydrolyzates of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6048–6057. [Google Scholar] [CrossRef]
- Nilvebrant, N.-O.; Reimann, A.; Larsson, S.; Jönsson, L.J. Detoxification of lignocellulose hydrolysates with ion-exchange resins. Appl. Biochem. Biotechnol. 2001, 91–93, 35–49. [Google Scholar] [CrossRef]
- Schwartz, T.J.; Lawoko, M. Removal of acid-soluble lignin from biomass extracts using Amberlite XAD-4 resin. BioResources 2010, 5, 2337–2347. [Google Scholar]
- Ladisch, M.R. Bioseparations Engineering: Principles, Practice, and Economics; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-24476-9. [Google Scholar]
- Ruckenstein, E.; Prieve, D.C. Adsorption and desorption of particles and their chromatographic separation. Aiche J. 1976, 22, 276–283. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-treatment and extraction techniques for recovery of added value compounds from wastes throughout the agri-food chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef]
- Heinonen, J.; Sainio, T. Chromatographic recovery of monosaccharides for the production of bioethanol from wood. Ind. Eng. Chem. Res. 2010, 49, 2907–2915. [Google Scholar] [CrossRef]
- Heinonen, J.; Sainio, T. Modelling and performance evaluation of chromatographic monosaccharide recovery from concentrated acid lignocellulosic hydrolysates. J. Chem. Technol. Biotechnol. 2012, 87, 1676–1686. [Google Scholar] [CrossRef]
- Wooley, R.; Ma, Z.; Wang, N.-H.L. A nine-zone simulating moving bed for the recovery of glucose and xylose from biomass hydrolyzate. Ind. Eng. Chem. Res. 1998, 37, 3699–3709. [Google Scholar] [CrossRef]
- Xie, Y.; Phelps, D.; Lee, C.-H.; Sedlak, M.; Ho, N.; Wang, N.-H.L. Comparison of two adsorbents for sugar recovery from biomass hydrolyzate. Ind. Eng. Chem. Res. 2005, 44, 6816–6823. [Google Scholar] [CrossRef]
- Lei, H.; Bao, Z.; Xing, H.; Yang, Y.; Ren, Q.; Zhao, M.; Huang, H. Adsorption behavior of glucose, xylose, and arabinose on five different cation exchange resins. J. Chem. Eng. Data 2010, 55, 735–738. [Google Scholar] [CrossRef]
- Chen, K.; Luo, G.; Lei, Z.; Zhang, Z.; Zhang, S.; Chen, J. Chromatographic separation of glucose, xylose and arabinose from lignocellulosic hydrolysates using cation exchange resin. Sep. Purif. Technol. 2018, 195, 288–294. [Google Scholar] [CrossRef]
- Jönsson, A.-S.; Wallberg, O. Cost estimates of kraft lignin recovery by ultrafiltration. Desalination 2009, 237, 254–267. [Google Scholar] [CrossRef]
- Blanc, C.-L.; Lemaire, J.; Duval, F.; Théoleyre, M.-A.; Pareau, D. Purification of pentoses from hemicellulosic hydrolysates without neutralization for sulfuric acid recovery. Sep. Purif. Technol. 2017, 174, 513–519. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Wei, H.-J.; Tsai, T.-Y.; Lin, T.-H.; Wei, T.-Y.; Guo, G.-L.; Huang, C.-P. Separation of furans and carboxylic acids from sugars in dilute acid rice straw hydrolyzates by nanofiltration. Bioresour. Technol. 2010, 101, 4889–4894. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Wei, H.-J.; Tsai, T.-Y.; Chen, W.-H.; Wei, T.-Y.; Hwang, W.-S.; Wang, C.-P.; Huang, C.-P. Separation of acetic acid from xylose by nanofiltration. Sep. Purif. Technol. 2009, 67, 95–102. [Google Scholar] [CrossRef]
| Biomass | Variable | Optimized Conditions | Monomer Yield | Reference |
|---|---|---|---|---|
| Sugarcane bagasse | 0.25–7% H2SO4 (v/v) No variation of S:L ratio No variation of temperature Duration: 15–240 min | 0.5% H2SO4 (v/v) S:L ratio 1:15 (w/v) 121 °C 60 min | 44% glucose 74% hemicelluloses | [49] |
| Sugarcane bagasse | 0–3% H2SO4 (w/v) No variation of S:L ratio 112.5–157.5 °C 5–35 min | 1.5% H2SO4 (w/v) S:L ratio 1:6.7 (w/v) 135 °C 20 min | 62% xylose | [59] |
| Sugarcane bagasse | 0.25–8% H2SO4 (w/w) S:L ratio 1:5–1:20 (w/w) DS 80–200 °C 10–2000 min | 4% H2SO4 (w/v) S:L ratio 1:20 120 °C 60 min | 80% xylose | [37] |
| Sugarcane bagasse | 2–6% H2SO4 (w/w) No variation of S:L ratio 100–128 °C 0–300 min | 2% H2SO4 (w/w) S:L ratio 1:10 (w/w) 122 °C 24.1 min | 5% glucose 92% xylose | [32] |
| Sugarcane bagasse | 2–6% HCl (w/w) No variation of S:L ratio 100–128 °C 0–300 min | 2% HCl (w/w) S:L ratio 1:10 (w/w) 128 °C 51.1 min | 8% glucose 100% xylose 36% arabinose | [33] |
| Sugarcane bagasse | 2–6% HNO3 (w/w) No variation of S:L ratio 100–128 °C 0–300 min | 6% HNO3 (w/w) S:L ratio 1:10 (w/w) DS 122 °C 9.3 min | 7% glucose 85% xylose 32% arabinose | [34] |
| Sugarcane bagasse | 2–6% H3PO4 (w/w) No variation of S:L ratio 100–128 °C 0–300 min | 4% H3PO4 (w/w) S:L ratio 1:8 (w/w) DS 122 °C 300 min | 6% glucose 60% xylose 33% arabinose | [35] |
| Rye straw | 0.6–1.5% H2SO4 (w/w) No variation of S:L ratio No variation of temperature 30–90 min | 1.5% H2SO4 (w/w) S:L ratio 1:10 (w/v) DS 121 °C 90 min | 10% glucose 65% xylose 67% arabinose | [52] |
| Bermudagrass | 0.6–1.5% H2SO4 (w/w) No variation of S:L ratio No variation of temperature 30–90 min | 1.5% H2SO4 (w/w) S:L ratio 1:10 (w/v) DS 121 °C 90 min | 33% glucose 59% xylose 65% arabinose | [52] |
| Sweet sorghum bagasse | No variation of acid concentration No variation of S:L ratio No variation of temperature No variation of duration | 0.5% H2SO4 (w/w) S:L ratio 1:20 (w/v) 170 °C 20 min | 85% xylose | [60] |
| Reaction | Reactant | % Converted to Product |
|---|---|---|
| (Glucan)n + H2O → n Glucose | Glucan | 9.9% |
| (Glucan)n + H2O → n Glucose Oligomer a | Glucan | 0.3% |
| (Glucan)n → n HMF + 2n H2O | Glucan | 0.3% |
| Sucrose → HMF + Glucose + 2 H2O | Sucrose | 100% |
| (Xylan)n + n H2O → n Xylose | Xylan | 90.0% |
| (Xylan)n + m H2O → m Xylose Oligomer a | Xylan | 2.4% |
| (Xylan)n → n Furfural + 2n H2O | Xylan | 5.0% |
| Acetate → Acetic Acid | Acetate | 100% |
| (Lignin)n → n Soluble Lignin | Lignin | 5.0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Molecules 2019, 24, 4273. https://doi.org/10.3390/molecules24234273
Oriez V, Peydecastaing J, Pontalier P-Y. Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Molecules. 2019; 24(23):4273. https://doi.org/10.3390/molecules24234273
Chicago/Turabian StyleOriez, Vincent, Jérôme Peydecastaing, and Pierre-Yves Pontalier. 2019. "Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities" Molecules 24, no. 23: 4273. https://doi.org/10.3390/molecules24234273
APA StyleOriez, V., Peydecastaing, J., & Pontalier, P.-Y. (2019). Lignocellulosic Biomass Fractionation by Mineral Acids and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Molecules, 24(23), 4273. https://doi.org/10.3390/molecules24234273







