Validation of an HPLC Method for the Simultaneous Quantification of Metabolic Reaction Products Catalysed by CYP2C11 Enzymes in Rat Liver Microsomes: In Vitro Inhibitory Effect of Salicylic Acid on CYP2C11 Enzyme
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Analytical Wavelength: UV-VIS (Ultraviolet-Visible) Spectroscopy (CYP2C11 Assay)
2.2. Method Validation
2.2.1. Linearity and Range
2.2.2. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
2.2.3. Specificity and Selectivity
2.2.4. Precision
Intra-Assay Variation of Testosterone and 16α-hydroxytestosterone
Inter-Assay Variation of Testosterone and 16α-hydroxytestosterone
2.2.5. Stability Test
Stability Test of Testosterone
Stability Test of 16α-hydroxytestosterone
2.2.6. Robustness Test
Changing the Percentage of the Mobile Phase
Changing the Column Temperature
2.3. Effects of Salicylic acid on CYP2C11 Activity
3. Materials and Methods
3.1. Materials
3.2. Instrument
3.3. CYP450 Assay
3.3.1. CYP2C11 Substrate and Its Metabolite
3.3.2. Microsomal Incubations and Treatment Protocol
3.4. Selection of Analytical Wavelength
CYP2C11 Assay
3.5. Preparation of Mobile Phase
CYP2C11 Assay
3.6. Preparation of Standard and Sample Solutions
3.6.1. CYP2C11 Assay
Analytes Standard Solution Preparation
Metabolite Standard Solution Preparation
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pacific J. Cancer Prev. 2018, 19, 2057–2070. [Google Scholar]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Furge, L.L.; Guengerich, F.P. Cytochrome P450 enzymes in drug metabolism and chemical toxicology: An introduction. Biochem. Mol. Biol. Educ. 2006, 34, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Curioni, O.A.; de Carvalho, M.B.; Dedivitis, R.A.; Rapoport, A.; Gattas, G.J.F. The Influence of Gene Polymorphisms on Tobacco and Alcohol-Induced Oral Cancer Risk. J. Cancer Ther. 2013, 04, 978–988. [Google Scholar] [CrossRef]
- McDonnell, A.; Dang, C. Basic Review of the Cytochrome P450 System. J. Adv. Practitioner Oncol. 2013, 4, 263–268. [Google Scholar]
- Wójcikowski, J.; Haduch, A.; Daniel, W.A. Effect of antidepressant drugs on cytochrome P450 2C11 (CYP2C11) in rat liver. Pharmacol. Rep. 2013, 65, 1247–1255. [Google Scholar] [CrossRef]
- Bigler, J.; Whitton, J.; Lampe, J.W.; Fosdick, L.; Bostick, R.M.; Potter, J.D. CYP2C9 and UGT1A6 genotypes modulate the protective effect of aspirin on colon adenoma risk. Cancer Res. 2001, 61, 3566–3569. [Google Scholar] [PubMed]
- Doi, H.; Horie, T. Salicylic acid-induced hepatotoxicity triggered by oxidative stress. Chem. Biol. Interact. 2010, 183, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Guo, J.M.; Shang, E.X.; Zhu, Z.H.; Liu, Y.; Zhao, B.C.; Zhao, J.; Tang, Z.S.; Duan, J.A. Quantitative determination of five metabolites of aspirin by UHPLC–MS/MS coupled with enzymatic reaction and its application to evaluate the effects of aspirin dosage on the metabolic profile. J. Pharm. Biomed. Anal. 2017, 138, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, F.; Kazumi, M.; Tatsuki, T.; Suzuki, R. Effect of salicylic acid and diclofenac on the medium-chain and long-chain acyl-CoA formation in the liver and brain of mouse. J. Appl. Toxicol. 2009, 29, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Gupta, N.V. A review on quality by design approach (QBD) for pharmaceuticals. Int. J. Drug Dev. Res. 2015, 7, 52–60. [Google Scholar]
- Li, X.F.; Ma, M.; Cheng, A.; Zheng, J.; Tam, Y.K. Determination of testosterone and its metabolites using liquid chromatography with elevated column temperature and flow-rate gradient. Anal. Chim. Acta 2002, 457, 165–171. [Google Scholar] [CrossRef]
- Eagling, V.A.; Tjia, J.F.; Back, D.J. Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br. J. Clin. Pharmacol. 1998, 45, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Alvi, S.N.; al Dgither, S.; Hammami, M.M. Development and validation of LC-MS/MS method for determination of testosterone level in human saliva using lovastatin as internal standard. J. Bioequiv. Bioavailab. 2013, 5, 228–232. [Google Scholar]
- Purdon, M.P.; Lehman-McKeeman, L.D. Improved high-performance liquid chromatographic procedure for the separation and quantification of hydrotestosterone metabolites. J. Pharmacol. Toxicol. Methods 1997, 37, 67–73. [Google Scholar] [CrossRef]
- Mulholland, J.W. SCCNFP, SCCNFP052201 (salicylic acid). Perfusion 2008, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Tang, Y.; Ding, T.; Liu, M.; Wang, X. Inhibitory effects of celastrol on rat liver cytochrome P450 1A2, 2C11, 2D6, 2E1 and 3A2 activity. Fitoterapia 2014, 92, 1–8. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
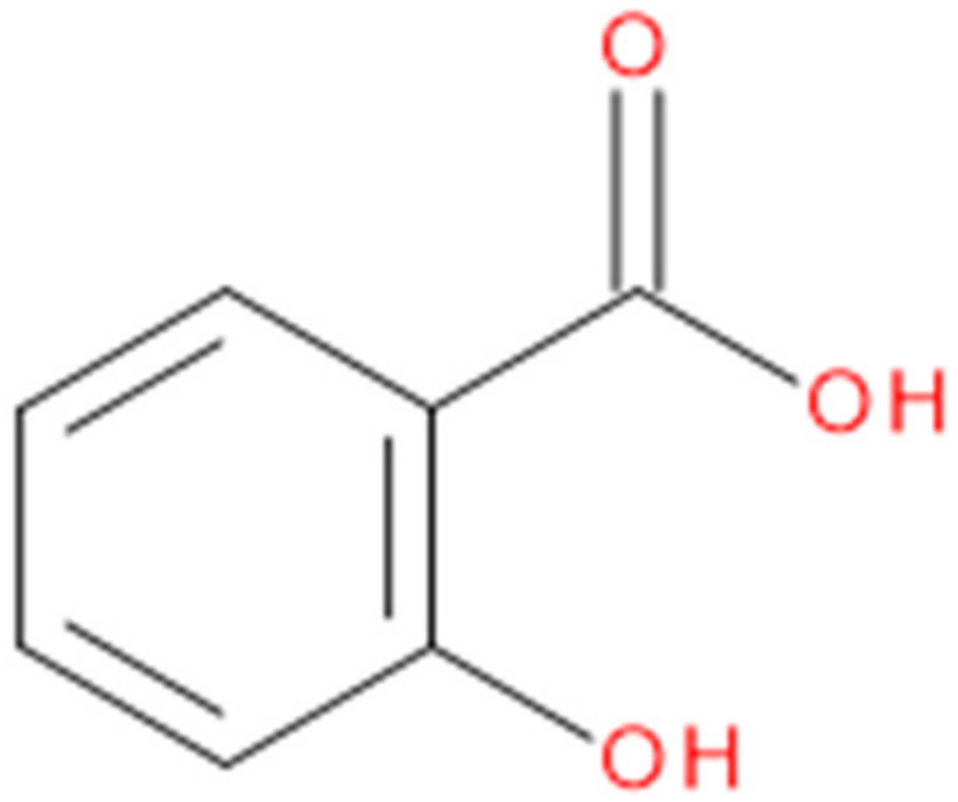




| Standards | Testosterone | 16α-hydroxytestosterone |
|---|---|---|
| Regression equation | Y = 0.0189X − 0.0203 | Y = 0.0204X − 0.0036 |
| r2 | 0.9999 | 0.9998 |
| Linear range | 10–400 µM | 10–100 µM |
| Standards | Testosterone | 16α-hydroxytestosterone |
|---|---|---|
| Limit of Detection (LOD) | 3.276 µM | 1.350 µM |
| Limit of Quantitation (LOQ) | 9.927 µM | 4.914 µM |
| Testosterone Standard | Mean Activity a (µM) | Standard Deviation | Relative Standard Deviation (%) |
|---|---|---|---|
| Low-activity standard (C = 25 µM) | 22.8856 | 0.4851 | 2.1198 |
| Medium-activity standard (C = 100 µM) | 102.7028 | 0.6444 | 0.6275 |
| High-activity standard (C = 200 µM) | 201.2282 | 4.9342 | 2.4520 |
| 16α-hydroxytestosterone Standard | Mean Activity a (µM) | Standard Deviation | Relative Standard Deviation (%) |
|---|---|---|---|
| Low-activity standard (C = 10 µM) | 8.5631 | 0.3848 | 4.4946 |
| Medium-activity standard (C = 40 µM) | 31.0608 | 0.3516 | 1.1322 |
| High-activity standard (C = 80 µM) | 67.9271 | 0.2154 | 0.3172 |
| Testosterone Standard (µM) | Mean Area Peak (n = 3 each Level) | Mean a Activity (µM) | Standard Deviation | Relative Standard Deviation (%) | |
|---|---|---|---|---|---|
| Low-activity standard (C = 25 µM) | Day 1 | 0.7057 | 24.3194 | 0.6826 | 2.8068 |
| Day 2 | 0.5725 | ||||
| Day 3 | 0.8314 | ||||
| Medium-activity standard (C = 100 µM) | Day 1 | 2.9393 | 101.7997 | 2.0495 | 2.0133 |
| Day 2 | 2.3360 | ||||
| Day 3 | 3.4765 | ||||
| High-activity standard (C = 200 µM) | Day 1 | 5.9357 | 210.938 | 8.8989 | 4.2187 |
| Day 2 | 4.8596 | ||||
| Day 3 | 7.3105 | ||||
| 16α-hydroxytestosterone Standard (µM) | Mean Area Peak (n = 3 each level) | Mean a Activity (µM) | Standard Deviation | Relative Standard Deviation (%) | |
|---|---|---|---|---|---|
| Low-activity standard (C = 10 µM) | Day 1 | 0.1932 | 10.5557 | 0.7102 | 6.7286 |
| Day 2 | 0.3154 | ||||
| Day 3 | 0.1227 | ||||
| Medium-activity standard (C = 40 µM) | Day 1 | 0.7802 | 38.1011 | 2.6567 | 6.9730 |
| Day 2 | 1.2850 | ||||
| Day 3 | 0.4912 | ||||
| High-activity standard (C = 80 µM) | Day 1 | 1.6356 | 79.7765 | 2.4168 | 3.0295 |
| Day 2 | 2.6480 | ||||
| Day 3 | 1.1200 | ||||
| Nominal Level (Actual Concentration of Testosterone (µM)) | ||||
|---|---|---|---|---|
| 25 | 100 | 200 | ||
| Calculated concentration (µM) | 0 h | 20.0423 | 80.1101 | 159 |
| 24 h | 21.6355 | 80.1101 | 159 | |
| 48 h | 21.5520 | 86.0148 | 162.3135 | |
| 72 h | 21.4054 | 80 | 163.3444 | |
| % Recovery a | 24 h | 107.9492 | 100 | 100 |
| 48 h | 107.5323 | 107.3707 | 102.0839 | |
| 72 h | 106.8007 | 99.8624 | 102.7323 | |
| Accuracy b (%) | 0 h | 119.8305 | 119.8898 | 120.5 |
| 24 h | 113.4576 | 119.8898 | 120.5 | |
| 48 h | 113.7918 | 113.9851 | 118.8432 | |
| 72 h | 114.3783 | 120 | 118.3277 | |
| Nominal Level (Actual Concentration of 16-alfa Hydroxytestosterone (µM)) | ||||
|---|---|---|---|---|
| 10 | 40 | 80 | ||
| Calculated concentration (µM) | 0 h | 10.533 | 38.0055 | 77.5659 |
| 24 h | 10.9725 | 41.3021 | 88.5549 | |
| 48 h | 11.1923 | 44.2142 | 91.8516 | |
| 72 h | 10.4 | 35.0866 | 76.5 | |
| % Recovery a | 24 h | 109.725 | 103.2552 | 110.6936 |
| 48 h | 111.923 | 110.5355 | 114.8145 | |
| 72 h | 104 | 87.7165 | 95.625 | |
| Accuracy b (%) | 0 h | 94.67 | 104.9862 | 103.0426 |
| 24 h | 90.275 | 96.7448 | 89.3064 | |
| 48 h | 88.077 | 89.4645 | 85.1855 | |
| 72 h | 96 | 112.2835 | 104.375 | |
| Mobile Phase Composition | Compounds | Average Retention Time (n = 3) (min) | Average Area Peak (n = 3) (Arb units) | Resolution |
|---|---|---|---|---|
| 70% Methanol + 30% Phosphate buffer at pH = 3.36 | Salicylic acid (100 µM) | 3.468 | 63443 | 16α-hydroxytestosterone and phenacetin were well separated (good resolution) (Difference in retention time = 0.842 min). |
| Phenacetin (50 µM) | 4.512 | 143502 | ||
| Testosterone (200 µM) | 10.726 | 644120 | ||
| 16α- hydroxytestosterone (50 µM) | 5.354 | 222620 | ||
| 68% Methanol + 32% Phosphate buffer at pH = 3.36 | Salicylic acid (100 µM) | 3.572 | 67994 | 16α-hydroxytestosterone and phenacetin were well separated from each other (very good resolution) (Difference in retention time = 1.083 min). |
| Phenacetin (50 µM) | 4.730 | 141116 | ||
| Testosterone (200 µM) | 12.359 | 641325 | ||
| 16α- hydroxytestosterone (50 µM) | 5.813 | 216239 |
| Mobile Phase Composition | Compounds | Average Retention Time (n = 3) (min) | Average Area Peak (n = 3) | Resolution |
|---|---|---|---|---|
| 68% Methanol + 32% Phosphate buffer at pH = 3.36 at a flow rate = 0.8 mL/min and T = 25 °C | Salicylic acid (100 µM) | 3.549 | 50965 | All compounds were well separated from each other. Difference in retention time between salicylic acid and phenacetin was 1.287 min. |
| Phenacetin (50 µM) | 4.836 | 135948 | ||
| Testosterone (200 µM) | 13.287 | 597882 | ||
| 16α- hydroxytestosterone (50 µM) | 6.025 | 184493 | ||
| 68% Methanol + 32% Phosphate buffer at pH = 3.36 at a flow rate= 0.8 mL/min and T = 30 °C | Salicylic acid(100 µM) | 3.492 | 50536 | All components were well separated from each other. Difference in retention time between salicylic acid and phenacetin was 1.26 min. |
| Phenacetin (50 µM) | 4.752 | 136505 | ||
| Testosterone (200 µM) | 12.472 | 598229 | ||
| 16α- hydroxytestosterone (50 µM) | 5.854 | 175528 |
| Pharmacokinetic Parameters | No Inhibitor | 50 µM Salicylic Acid | 100 µM Salicylic Acid | 200 µM Salicylic Acid |
|---|---|---|---|---|
| Km(µM) | 87.5613 ± 3.0516 | 86.5999 ± 3.0855 | 83.3333 ± 3.2064 | 80.8335 ± 3.3056 |
| Vmax (µM−1∙min−1) | 0.7668 ± 0.1445 | 0.6581 ± 0.1684 | 0.5000 ± 0.2216 | 0.5094 ± 0.2175 |
| Clint (µM−2∙min−1) | 0.0087 ± 0.1228 | 0.0075 ± 0.1425 | 0.0060 ± 0.1782 | 0.0063 ± 0.1697 |
| α’ | - | 1.1651 ± 0.1437 | 1.5336 ± 0.1092 | 1.5052 ± 0.1112 |
| % inhibition | - | 14.1766 ± 0.6661 | 34.7967 ± 0.2714 | 33.5642 ± 0.2814 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salhab, H.; Naughton, D.P.; Barker, J. Validation of an HPLC Method for the Simultaneous Quantification of Metabolic Reaction Products Catalysed by CYP2C11 Enzymes in Rat Liver Microsomes: In Vitro Inhibitory Effect of Salicylic Acid on CYP2C11 Enzyme. Molecules 2019, 24, 4294. https://doi.org/10.3390/molecules24234294
Salhab H, Naughton DP, Barker J. Validation of an HPLC Method for the Simultaneous Quantification of Metabolic Reaction Products Catalysed by CYP2C11 Enzymes in Rat Liver Microsomes: In Vitro Inhibitory Effect of Salicylic Acid on CYP2C11 Enzyme. Molecules. 2019; 24(23):4294. https://doi.org/10.3390/molecules24234294
Chicago/Turabian StyleSalhab, Hassan, Declan P. Naughton, and James Barker. 2019. "Validation of an HPLC Method for the Simultaneous Quantification of Metabolic Reaction Products Catalysed by CYP2C11 Enzymes in Rat Liver Microsomes: In Vitro Inhibitory Effect of Salicylic Acid on CYP2C11 Enzyme" Molecules 24, no. 23: 4294. https://doi.org/10.3390/molecules24234294
APA StyleSalhab, H., Naughton, D. P., & Barker, J. (2019). Validation of an HPLC Method for the Simultaneous Quantification of Metabolic Reaction Products Catalysed by CYP2C11 Enzymes in Rat Liver Microsomes: In Vitro Inhibitory Effect of Salicylic Acid on CYP2C11 Enzyme. Molecules, 24(23), 4294. https://doi.org/10.3390/molecules24234294








