A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications
Abstract
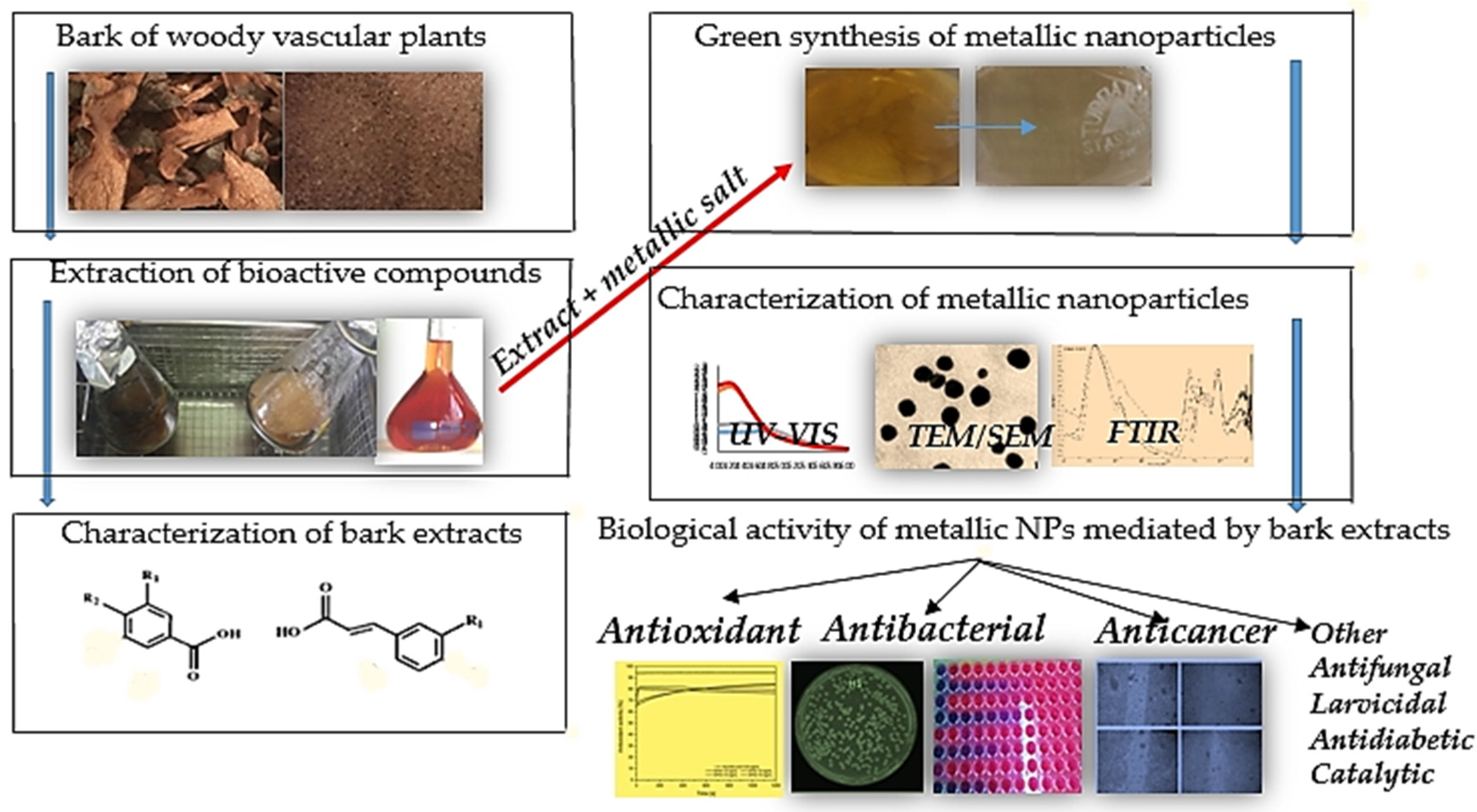
:1. Introduction
2. The Bark of Woody Vascular Plants—A Source of Phytoconstituents Responsible for Reduction of Metallic Ions in Nanoparticle Synthesis
3. Characteristics of Metallic Nanoparticles Mediated by the Bark Extracts of Woody Plants
| Source of Bark: Scientific Name (Family)—Common Name | NP Type | Size (nm) | Shape | Phytoconstituents Responsible for the Reduction | Reference |
|---|---|---|---|---|---|
| Acacia leucophloea Roxb. (Fabaceae)—White kabesak | Ag | 17–29 | Spherical | Aldehyde/ketone, aromatic, azo, and nitro compounds | [20] |
| Afzelia quanzensis Welw. (Fabaceae)—Pod mahogany | Ag | 10–80 | Spherical | Phytochemical functional groups (carboxyl, amine) | [21] |
| Albizia chevalieri Harms. (Fabaceae) | Ag | ~30 | Spherical | Alkaloids, terpenoids, flavonoids, and phenols | [31] |
| Alstonia scholaris (L.) R.Br. (Apocynaceae)—Devil’s tree | Ag | 50 | FCC | - | [32] |
| Artocarpus elasticus Reinw. (Moraceae)—Benda | Ag | 19.74 ± 9.70 | FCC | Flavonoids, phenols | [33] |
| Azadirachta indica A. Juss (Meliaceae)—Nimtree or Indian lillac | Ag | 19.22 | Spherical | - | [34,35] |
| Berberis lycium Royle. (Berberidaceae) | Ag | 10–100 | Spherical | - | [36] |
| Butea monosperma (Lam.) Laum. (Fabaceae)—Flame-of-the-forest | Ag | 35 | FCC | Carboxylic acid group | [37] |
| Cassia fistula L. (Fabaceae)—Golden tree | Au | 55.2–98.4 | - | Reducing sugars and terpenoids, secondary metabolites, such as lupeol, β-sitosterol, and hexacosanol | [38] |
| Cinnamomum cassia L. J. Presl (Lauraceae)—Chinese cinnamon | Ag | 25–55 | Spherical | Phenol, aldehydes, ketones, carboxylic acids, alkyl halides, aromatic groups | [39] |
| Cinnamomum zeylanicum J. Presl (Lauraceae)—True cinnamon | Ag Au | ~11.77 ~46.48 | Spherical | - | [40] |
| Coccinia grandis L.Voigt (Curcubitaceae)—ivy gourd | Au | 20 | Spherical | - | [41] |
| Cochlospermum religiosum (L.) Alston (Bixaceae)—Silk-cotton tree | Ag | 20–35 | Spherical | Carbohydrate, polyphenols, and protein molecules | [42] |
| Crataeva nurvala Buch.-Ham (Capparaceae)—Varuna | Ag | 15.2 ± 1.01 | spherical | Lupeol, lupenone, hexadecanoic ester, methyl ester | [43] |
| Dillenia indica L. (Dilleniaceae)—Elephant apple | Ag | 15–35 | Spherical | flavonol, flavonoids, phenolic compounds, stigmasterol, glycosides, and sulfates of flavonoid | [44] |
| Diospyros montana Roxb. (Ebenaceae)—Bombay ebony | Ag | 28 | -- | Amides, phenol, nitrogen, and aromatic compounds | [45,46] |
| Elaeodendron croceum Thunb. DC. (Celastraceae)—Saffron wood | Ag | 12.6–41.4 | spherical | Amino acids, proteins, polysaccharides, alkaloids, polyphenols, terpenoids or triterpenes, tannins, saponins, and vitamins | [4,47,48,49] |
| Eucalyptus globulus Labill. (Myrtiaceae)—Southern blue gum | Ag Au | 21 ± 4 52 ± 16 | FCC | Phenolic compounds, particularly galloyl derivatives, glucose and fructose, hydrolyzable tannins | [19] |
| Eucommia ulmoides Oliv. (Eucommiaceae)—Hardy rubber tree | Au | 15–40 | Spherical FCC | - | [50] |
| Pd | 12.6 | Spherical and quasi-spherical with FCC | Polyphenols, phytosterol, flavonoids, alkaloids, triterpenoids, aminoacids, and proteins | [51] | |
| Eysenhardtia polystachya Ort. Sarg. (Fabaceae)—Kidneywood tree | Ag | 10–12 | Spherical | Arylnaphthalenes, chalcones, flavonoids, and dihydrochalcones | [52] |
| Fagus sylvatica L. (Fagaceae)—Beech | Ag | 32 | spherical | Tannins and polyphenols | [9,11] |
| Ficus benghalensis var. krishnae (Moraceae)—Krishna butter cup | Ag | 15–28 | Spherical | Phenols, flavonoids, tannins, terpenoids, proteins, alkaloids, saponins, and vitamines | [53,54] |
| Ficus benghalensis (Moraceae)—Banyan tree Azadirachta indica A. Juss (Meliaceae)—Nimtree or Indian lilac | Ag | 40–50 | Spherical | Flavonoids, terpenoids, and phenols | [55] |
| Garcinia mangostana L. (Clusiaceae)—Mangosteen | Ag | 12–15 | Spherical | Polyphenols | [56] |
| Guazuma ulmifolia Lam. (Malvaceae)—Bay cedar | Ag Au Ag–Au | 10–15 20–25 10–20 | Spherical | Tannins | [57] |
| Holarrhena antidysenterica L. (Aponycaceae) Wall.—Tellicherry bark or conessi | Ag | 32 | Spherical | Terpenoids, alkaloids, flavonoids, and phenols | [58] |
| Melia azedarach L. (Meliaceae)—Indian lilac | Ag | 30–45 | Spherical | Phenolic compounds | [59,60,61] |
| Ag Ag–Au | 4–30 15–80 | Spherical, hexagonal, elliptical | Triterpenoids, flavonoids, glycosides steroids, and carbohydrates | [62] | |
| Mimusops elengi L. (Sapotaceae)—Bullet wood | Au | 9–14 | Spherical | Gallic acid, pinocembrin, quercetin, chlorogenic acid | [14] |
| Moringa oleifera Lam. (Moringaceae)—Moringa | Ag | 40 | Spherical, pentagon | Terpenoids, flavonoids, and polysaccharides | [26] |
| Nerium oleander L. (Apocynaceae)—Karabi | Au | 20–40 | Spherical | Flavonoids, steroids, and other secondary metabolites | [63] |
| Picea abies L. (Pinaceae)—Spruce | Ag | 44 | spherical or rarely polygonal | Catechin, vanillic and gallic acids | [10,64] |
| Pinus eldarica (Pinaceae)—Eldarica pine | Ag | 10–40 | Spherical | Catechin, taxifolin, procyanidins, and phenolic acids | [65] |
| Pongamia pinnata (L.) Pierre (Fabaceae)—Karum tree | Ag | 5–55 | Spherical | Phenolic amides, piperine, polysaccharides, and other reducing sugars | [66] |
| Prosopis juliflora Sw.DC. (Fabaceae)—Mesquite | Ag | 10–50 | Spherical | Flavonoids, alkaloids, and other phenolic compounds | [67] |
| Quercus sp. (Fagaceae)—Oak | Ag | - | - | Tannic acid, glucose, gallic acid | [68] |
| Salix alba L. (Salicaceae)—Willow tree | Au | ~15 | Spherical | Tannins, alkanoids, flavonoids | [69] |
| Saraca indica L. (Fabaceae)—Asoka tree | Au | 15–23 | Triangular, polygonal, spherical | Quercetin, epicatechin, catechin, leucopelargoni- din-3-O-p-D-glucoside, gallic acid, leucocyanidin | [28] |
| Salvadora persica L. (Salvadoraceae)—Toothbrush tree | Ag | 50 | Spherical | Tannins, flavonoids, alkaloids, | [23] |
| Shorea roxburghii D. Don (Dipterocarpaceae) | Ag | 4–50 | Spherical | Phenolic compounds | [70] |
| Stereospermum suaveolens Roxb. DC (Bignoniaceae) | Ag Au | 11.11 12.67 | Spherical | Lignans, polyphenols | [71] |
| Syzygium alternifolium (Wt.) Walp (Myrtiaceae) | Ag | 4–48 | Spherical | Ascorbic acid | [72] |
| Syzygium cumini L. (Myrtiaceae)—Black plum | Ag | 20–60 | Spherical | Phenols, tannins, alkaloids, glycosides, amino acids, and flavones | [73] |
| Syzygium jambos (L.) Alston (Myrtaceae) | Ag Au | 3–10 4–11 | Spherical, ellipsoidal | Saccharides and phenolics | [29] |
| Terminalia arjuna Wigh and Arn (Combretaceae)—Arjuna tree | Ag | 30–50 | Spherical | Polyphenols and proteins | [74] |
| Au | 3–70 | Spherical, triangular FCC | Catechin, gallic acid, ellagic acid | [30] | |
| Cu | ~23 | Spherical | Polyphenols (flavonoids), terpenoids, ketones, aldehydes | [75] | |
| Cu–Ag | ~20–30 | Spherical | Polyphenols, flavonoids, terpenoids, and reducing sugars | [17] | |
| Terminalia cuneata Roth. (Combretaceae)—White murdah | Ag | 20–50 | Spherical | Hydrolyzable tannins, gallic acid, polyphenols | [76] |
| Toxicodendron vernicifluum (Stokes) F. Barkley (Anacardiaceae)—Chinese Lacquer tree | Ag | 2–40 | Spherical, oval | Amine, amide, phenolic, and alcoholic aromatics | [27] |
| Zizyphus xylopyrus Retz. Willd (Rhamnaceae)—Kath ber | Ag | 60–70 | Spherical | - | [77] |
4. Applications of Metallic NPs Mediated by Plant Bark Extracts
4.1. Antioxidant Activity
4.2. Antibacterial Activity
4.3. Anticancer Activity
4.4. Other Activities
| Source of Bark: Scientific Name (Family)—Common Name | NP Type | Activity | Reference |
|---|---|---|---|
| Acacia leucophloea Roxb. (Fabaceae)—White kabesak | Ag | Antibacterial activity against the common pathogens, such as Staphylococcus aureus, Bacillus cereus, Listeria monocytogenes, and Shigella flexneri | [20] |
| Afzelia quanzensis Welw. (Fabaceae)—pod mahogany | Ag | Antibacterial against Escherichia coli, Staphylococcus aureus | [21] |
| Albizia chevalieri Harms. (Fabaceae) | Ag | Antibacterial against Escherichia coli, Staphylococcus aureus; Anticancer against MDA-MB231, MCF-7 breast cancer cell line, and HepG2 liver cancer cell line | [31] |
| Alstonia scholaris (L.) R.Br. (Apocynaceae)—Devil’s tree | Ag | Antimicrobial activity against fungal species, and Gram-positive and Gram-negative bacteria | [32] |
| Azadirachta indica A. Juss (Meliaceae)—Nimtree or Indian lillac | Ag | Larvicidal against the larvae, pupae, and adults of malaria vector Anopheles stephensi and filariasis vector Culex quinquefasciatus | [35] |
| Berberis lycium Royle. (Berberidaceae) | Ag | Antimicrobial activities against both Gram-negative bacteria (Escherichia coli, Klebseilla pneumoniae, Pseudomonas aeruginosa) and Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) | [36] |
| Butea monosperma (Lam.) Laum. (Fabaceae)—Flame-of-the-forest | Ag | Antibacterial activity against Gram-positive (Bacillus subtilis) and Gram-negative (Escherichia coli) | [37] |
| Cassia fistula L. (Fabaceae)—Golden tree | Au | Antidiabetic: reduces serum blood glucose concentrations, induces favorable changes in body weight, improves transaminase activity, achieves a better lipid profile, and reverses renal dysfunction to a greater extent | [38] |
| Cinnamomum cassia L. J. Presl (Lauraceae)—Chinese cinnamon | Ag | Non-toxic against Vero cells Antiviral activity against H7N3 influenza virus | [39] |
| Cinnamomum zeylanicum J.Presl (Lauraceae)—True cinnamon | Ag Au | Antibacterial: EC50 value of 11 ± 1.72 mg/L against Escherichia coli BL-21 strain | [40] |
| Coccinia grandis L.Voigt (Curcubitaceae)—ivy gourd | Au | Increasing biocompatibility and bioavailability of N-acetylcysteine drug molecule that is used for cataract treatment, which was successfully encapsulated into AuNPs | [41] |
| Cochlospermum religiosum (L.) Alston (Bixaceae)—Silk-cotton tree | Ag | Antibacterial activity against Staphylococcus aureus, followed by Pseudomonas, Escherichia coli, Bacillus, and lowest activity toward Proteus Antifungal against Aspergillus flavus, followed by Rhizopus, Fusarium, and Curvularia | [42] |
| Crataeva nurvala Buch.-Ham (Capparaceae)—Varuna | Ag | Antibiofilm properties in Pseudomonas aeruginosa | [43] |
| Dillenia indica L. (Dilleniaceae)—Elephant apple | Ag | Catalytic degradation of 4-Nitrophenol, methylene blue; radical scavenging activity | [44] |
| Diospyros montana Roxb. (Ebenaceae)—Bombay ebony | Ag | Antibacterial activity in vitro against Gram-positive (Bacillus subtilis, Staphylococcus aureus) and Gram-negative (Escherichia coli, Klebsiella aerogenes); antioxidant activity | [45,46] |
| Elaeodendron croceum Thunb. DC. (Celastraceae)—Saffron wood | Ag | Cytotoxic activity against the MDA-MB-231 cell line | [47] |
| Eucommia ulmoides Oliv. (Eucommiaceae)—Hardy rubber tree | Au | Excellent performance for the catalytic decoloration of reactive yellow 179 and Congo red by NaBH4 in aqueous solution | [50] |
| Pd | Catalytic activity for the electro-catalytic oxidation of hydrazine and the catalytic reducing degradation of p-Aminoazobenzene | [51] | |
| Eysenhardtia polystachya Ort. Sarg. (Fabaceae)—Kidneywood tree | Ag | Promote pancreatic β-cell survival, insulin secretion Enhances hyperglycemia and hyperlipidemia in glucose-induced diabetic zebrafish | [52] |
| Fagus sylvatica L. (Fagaceae)—Beech | Ag | Antioxidant and antibacterial against Gram-positive and Gram-negative bacteria | [9] |
| Ficus benghalensis var. krishnae (Moraceae)—Krishna butter cup | Ag | Antimicrobial activity against Staphylococcus aureus (ATCC 29122), Escherichia coli (MTCC 45) and Salmonella typhimurium (MTCC 98) Cytotoxicity on ovarian cancer cell lines (SKOV-3 cells) | [53,54] |
| Ficus benghalensis (Moraceae)—Banyan trees Azadirachta indica A. Juss (Meliaceae)—Nimtree or Indian lilac | Ag | Antibacterial against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, and Vibrio cholerae Antiproliferative against MG-63 ostheosarcoma cell line | [55] |
| Ficus racemosa L. (Moraceae)—Indian fig tree | Ag | The larvicidal activity results showed the highest mortality in synthesized AgNPs compared with the aqueous bark extract of F. racemosa | [104] |
| Garcinia mangostana L. (Clusiaceae)—Mangosteen | Ag | Anticancer activity in lung cancer cells (A549) | [56] |
| Guazuma ulmifolia Lam. (Malvaceae)—Bay cedar | Ag–Au | Anticancer activity against HeLa cells Antibacterial and antifungal activity Catalityc activity against Congo red and 4-nitrophenol | [57] |
| Holarrhena antidysenterica L. (Aponycaceae) Wall.—Tellicherry bark or conessi | Ag | Larvicidal activity against the larvae of Aedes aegypti and Culex quinquefasciatus | [58] |
| Melia azedarach L. (Meliaceae)—Indian lilac | Ag | Antibacterial against Esherichia coli, Klebsiella pneumoniae | [59] |
| Ag Ag–Au | Antibacterial against Bacillus cereus, Cronobacter sakazakii, Salmonella enterica, Escherichia coli, Listeria monocytogenes, Candida albicans | [62] | |
| Mimusops elengi L. (Sapotaceae)—Bullet wood | Au | Efficient catalyst for the reduction of 3-nitrophenol and 4-nitrophenol to their corresponding aminophenols in water at room temperature | [14] |
| Moringa oleifera Lam. (Moringaceae)—Moringa | Ag | Anticancer activity against HeLa cell type (human cervical carcinoma) | [26] |
| Nerium oleander L. (Apocynaceae)—Karabi | Au | In vitro anticancer activity of the stabilized AuNPs on MCF-7 cell lines; catalytic activities demonstrated for borohydride reduction of 3- and 4-nitrophenols | [60] |
| Picea abies L. (Pinaceae)—Spruce | Ag | Antioxidant and Antibacterial against Gram-positive and Gram-negative bacteria | [10] |
| Pongamia pinnata (L.) Pierre (Fabaceae)—Karum tree | Ag | Antibacterial activity against Klebsiella planticola and Staphylococcus aureus | [66] |
| Prosopis juliflora Sw.DC. (Fabaceae)—Mesquite | Ag | Antibacterial activity against Escherichia coli and Pseudomonas Aeruginosa; Anticancer activity against A549 cells (adenocarcinomic human alveolar basal epithelial cells); photocatalytic degradation of 4-nitrophenol | [67] |
| Quercus sp. (Fagaceae)—Oak | Ag | Antibacterial effect against Staphylococcus aureus ATCC 25923, Listeria monocytogenes ATCC 19111, Bacillus cereus ATCC 11778, Escherichia coli ATCC 25922, Salmonella enterica subsp. enterica Serovar typhimurium ATCC 13076 reference strains —cultures were isolated from food products | [68] |
| Salix alba L. (Salicaceae)—Willow tree | Au | Used in colorimetric detection of cysteine | [69] |
| Saraca indica L. (Fabaceae)—Asoka tree | Au | Catalyst for the reduction of 4-nitrophenol to 4-aminophenol | [28] |
| Salvadora persica L. (Salvadoraceae)—Toothbrush tree | Ag | Antibacterial activity against Escherichia coli and Staphylococcus aureus | [23] |
| Stereospermum suaveolens Roxb. DC (Bignoniaceae) | Ag Au | Anticancer activity against lung carcinoma cell lines A549 Antibacterial against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis Antifungal against Aspergillus flavus, Aspergillus nidulans | [71] |
| Syzygium alternifolium (Wt.) Walp (Myrtiaceae) | Ag | Antibacterial activity against Salmonella typhimurium, Proteus vulgaris, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis Antifungal: highest inhibition zones are observed in Aspergillus flavus followed by Penicillium chrysogenum, Trichoderma harzianum, Alternaria solani, and Aspergillus Niger | [72] |
| Syzygium cumini L.(Myrtiaceae)—Black plum | Ag | Antibacterial against Escherichia coli, Staphylococcus aureus, Bacillus licheniformis | [73] |
| Syzygium jambos (L.) Alston (Myrtaceae) | Ag Au | Antiplasmodial effect (AgNPs > AuNPs) against both chloroquine sensitive (3D7) and resistant (Dd2) strain of Plasmodium falciparum | [29] |
| Terminalia arjuna Wigh and Arn (Combretaceae)—Arjuna tree | Ag | Antibacterial against Escherichia coli | [74] |
| Au | Reducing and capping agent; acetylcholinesterase and Butyrylcholinesterase inhibitory activities; excellent free radical scavenging and metal chelating activity, suitable for Alzheimer’s disease therapy. | [30] | |
| Cu | Antioxidant properties; Antibacterial activity against Escherichia coli and Staphylococcus aureus and less effective against both Pseudomonas aeruginosa and Salmonella typhium; Effective against Candida albicans, Trichophyton rubrum, Chrisosporium indicum | [75] | |
| Cu–Ag | Cytotoxic effect of biohybrid nanomaterials on different cell lines, MDA-MB-231 (poorly differentiated triple-negative breast cancer), HeLa (cervical cancer cells), SiHa (squamous cell carcinoma), and He-G2 (liver cancer cells), and non-toxic against Vero (normal epithelial cells); antibacterial activity against bacterial strains Escherichia coli, Staphylococcus aureus | [17] | |
| Terminalia cuneata Roth. (Combretaceae)—White murdah | Ag | Catalytic activity in the reduction of direct yellow-12 | [76] |
| Toxicodendron vernicifluum (Stokes) F.Barkley (Anacardiaceae)—Chinese Lacquer tree | Ag | Anticancer activity in human lung cancer A549 cells Antibacterial activity against STEC (Shiga Toxina Escherichia Coli) and Helicobacter pylori | [27] |
| Zizyphus xylopyrus Retz. Willd (Rhamnaceae)—Kath ber | Ag | Antimicrobial agents in water purification systems | [77] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Endophytes: Toward a Vision in Synthesis of Nanoparticle for Future Therapeutic Agents. Int. J. Bio-Inorg. Hybrid. Nanomater. 2012, 1, 67–77.
- Hasna, A.S.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Sangeetha, G.; Rajeshwari, S. Plants: Green Route for Nanoparticle Synthesis. Int. Res. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.-S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Kavitha, K.S.; Syed, B.; Rakshith, D.; Kavitha, H.U.; Yashwantha Rao, H.C.; Harini, B.P.; Satish, S. Plants as Green Source towards Synthesis of Nanoparticles. Int. Res. J. Biol. Sci. 2013, 2, 66–76. [Google Scholar]
- Rajan, R.; Chandran, K.; Harper, S.L.; Yun, S.-I.; Kalaichelvan, P.T. Plant Extract Synthesized Silver Nanoparticles: An Ongoing Source of Novel Biocompatible Materials. Ind. Crop. Prod. 2015, 70, 356–373. [Google Scholar] [CrossRef]
- Ferreres, F.; Gomes, N.G.M.; Valentão, P.; Pereira, D.M.; Gil-Izquierdo, A.; Araújo, L.; Silva, T.C.; Andrade, P.B. Leaves and Stem Bark from Allophylus Africanus P. Beauv.: An Approach to Anti-Inflammatory Properties and Characterization of Their Flavonoid Profile. Food Chem. Toxicol. 2018, 118, 430–438. [Google Scholar] [CrossRef]
- Tanase, C.; Berta, L.; Coman, A.N.; Roșca, I.; Man, A.; Toma, F.; Mocan, A.; Jakab-Farkas, L.; Biró, D.; Mare, A. Investigation of In Vitro Antioxidant and Antibacterial Potential of Silver Nanoparticles Obtained by Biosynthesis Using Beech Bark Extract. Antioxidants 2019, 8, 459. [Google Scholar] [CrossRef] [PubMed]
- Tanase, C.; Berta, L.; Coman, A.N.; Roșca, I.; Man, A.; Toma, F.; Mocan, A.; Nicolescu, A.; Jakab-Farkas, L.; Biró, D.; et al. Antibacterial and Antioxidant Potential of Silver Nanoparticles Biosynthesized Using the Spruce Bark Extract. Nanomaterials 2019, 9, 1541. [Google Scholar] [CrossRef]
- Tanase, C.; Mocan, A.; Coșarcă, S.; Gavan, A.; Nicolescu, A.; Gheldiu, A.-M.; Vodnar, C.D.; Muntean, D.-L.; Crișan, O. Biological and Chemical Insights of Beech (Fagus sylvatica L.) Bark: A Source of Bioactive Compounds with Functional Properties. Antioxidants 2019, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Alfredsen, G.; Solheim, H.; Slimestad, R. Antifungal Effect of Bark Extracts from Some European Tree Species. Eur. J. Res. 2008, 127, 387. [Google Scholar] [CrossRef]
- Verica, D.-U.; Levaj, B.; Mrkic, V.; Bursać Kovačević, D.; Boras, M. The Content of Polyphenols and Carotenoids in Three Apricot Cultivars Depending on Stage of Maturity and Geographical Region. Food Chem. 2007, 102, 966–975. [Google Scholar] [CrossRef]
- Majumdar, R.; Bag, B.G.; Ghosh, P. Mimusops Elengi Bark Extract Mediated Green Synthesis of Gold Nanoparticles and Study of Its Catalytic Activity. Appl. Nanosci. 2016, 6, 521–528. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant Terpenes: Defense Responses, Phylogenetic Analysis, Regulation and Clinical Applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Prakash, D.; Kumar, N. Cost Effective Natural Antioxidants. In Nutrients, Dietary Supplements, and Nutriceuticals: Cost Analysis Versus Clinical Benefits; Gerald, J.K., Watson, R.R., Preedy, V.R., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 163–187. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L.; Vishwanatha, U.; Ravishankar, B.; Gururaj, H.; Niranjana, P.; Hungund, B.S. Phytochemically Functionalized Cu and Ag Nanoparticles Embedded in MWCNTs for Enhanced Antimicrobial and Anticancer Properties. Nano-Micro Lett. 2016, 8, 120–130. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, M.S.; Granby, K. Current Issues in Dietary Acrylamide: Formation, Mitigation and Risk Assessment. J. Sci. Food Agric. 2014, 94, 9–20. [Google Scholar] [CrossRef]
- Santos, S.; Pinto, R.; Rocha, S.; Marques, P.; Neto, C.; Silvestre, A.; Freire, C. Unveiling the Chemistry behind the Green Synthesis of Metal Nanoparticles. ChemSusChem 2014, 7, 2704–2711. [Google Scholar] [CrossRef]
- Murugan, K.; Senthilkumar, B.; Senbagam, D.; Al-Sohaibani, S. Biosynthesis of Silver Nanoparticles Using Acacia leucophloea Extract and Their Antibacterial Activity. Int. J. Nanomed. 2014, 9, 2431–2438. [Google Scholar] [CrossRef]
- Moyo, M.; Gomba, M.; Nharingo, T. Afzelia quanzensis Bark Extract for Green Synthesis of Silver Nanoparticles and Study of Their Antibacterial Activity. Int. J. Ind. Chem. 2015, 6, 329–338. [Google Scholar] [CrossRef]
- Lin, Z.; Jianming, W.; Xue, R.; Yang, Y. Spectroscopic Characterization of Au3+ Biosorption by Waste Biomass of Saccharomyces cerevisiae. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.; Dorani, N.; Darroudi, M.; Sarani, M. Green Synthesis of Silver Nanoparticles Using Salvadora persica L. and Its Antibacterial Activity. Cell. Mol. Biol. 2016, 62, 46–50. [Google Scholar] [CrossRef]
- Kabera, J. Plant Secondary Metabolites: Biosynthesis, Classification, Function and Pharmacological Classification, Function and Pharmacological Properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Geethalakshmi, R.; Sarada, D.V.L. Characterization and Antimicrobial Activity of Gold and Silver Nanoparticles Synthesized Using Saponin Isolated from Trianthema decandra L. Ind. Crop. Prod. 2013, 51, 107–115. [Google Scholar] [CrossRef]
- Vasanth, K.; Ilango, K.; Ramasamy, M.; Agrawal, A.; Dubey, G.P. Anticancer Activity of Moringa oleifera Mediated Silver Nanoparticles on Human Cervical Carcinoma Cells by Apoptosis Induction. Colloids Surf. B Biointerfaces 2014, 117, 354–359. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Oh, D.-H.; Kathiresan, K.; Wang, M.-H. Unveiling the Potentials of Biocompatible Silver Nanoparticles on Human Lung Carcinoma A549 Cells and Helicobacter pylori. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Dash, S.S.; Majumdar, R.; Sikder, A.K.; Bag, B.G.; Patra, B.K. Saraca indica Bark Extract Mediated Green Synthesis of Polyshaped Gold Nanoparticles and Its Application in Catalytic Reduction. Appl. Nanosci. 2014, 4, 485–490. [Google Scholar] [CrossRef]
- Dutta, P.P.; Bordoloi, M.; Gogoi, K.; Roy, S.; Narzary, B.; Bhattacharyya, D.R.; Mohapatra, P.K.; Mazumder, B. Antimalarial Silver and Gold Nanoparticles: Green Synthesis, Characterization and In Vitro Study. Biomed. Pharm. 2017, 91, 567–580. [Google Scholar] [CrossRef]
- Sivakumar, S.; Vijayan, S.R.; Pugazhendhi, A.; Benelli, G.; Archunan, G. Biogenic Synthesis of Gold Nanoparticles from Terminalia arjuna Bark Extract: Assessment of Safety Aspects and Neuroprotective Potential via Antioxidant, Anticholinesterase, and Antiamyloidogenic Effects. Environ. Sci. Pollut. Res. 2017, 25, 1–16. [Google Scholar] [CrossRef]
- Khan, S.; Bello, B.; Khan, J.; Anwar, Y.; Mirza, M.; Qadri, F.; Farooq, A.; Adam, I.K.; Asiri, A.M.; Khan, S. Albizia chevalier Based Ag Nanoparticles: Anti-Proliferation, Bactericidal and Pollutants Degradation Performance. J. Photochem. Photobiol. B 2018, 182, 62–70. [Google Scholar] [CrossRef]
- Shetty, P.; Supraja, N.; Garud, M.; Prasad, T.N.V.K.V. Synthesis, Characterization and Antimicrobial Activity of Alstonia Scholaris Bark-Extract-Mediated Silver Nanoparticles. J. Nanostructure Chem. 2014, 4, 161–170. [Google Scholar] [CrossRef]
- Abdullah, N.; Ahmad, M.; Kamyar, S. Biosynthesis of Silver Nanoparticles Using Artocarpus elasticus Stem Bark Extract. Chem. Cent. J. 2015, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Nahak, G.; Sahu, R. In Vitro Antioxidative Acitivity of Azadirachta indica and Melia azedarach Leaves by DPPH Scavenging Assay. Nat. Sci. 2010, 8, 22–28. [Google Scholar]
- Soni, N.; Prakash, S. Silver Nanoparticles: A Possibility for Malarial and Filarial Vector Control Technology. Parasitol. Res. 2014, 113, 4015–4022. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Murtaza, G.; Bhatti, T.; Kausar, R.; Ahmed, M. Biosynthesis, Characterization and Antimicrobial Action of Silver Nanoparticles from Root Bark Extract of Berberis lycium Royle. Pak. J. Pharm. Sci. 2016, 29, 131–136. [Google Scholar]
- Pattanayak, S.; Mollick, M.M.R.; Maity, D.; Chakraborty, S.; Dash, S.K.; Chattopadhyay, S.; Roy, S.; Chattopadhyay, D.; Chakraborty, M. Butea monosperma Bark Extract Mediated Green Synthesis of Silver Nanoparticles: Characterization and Biomedical Applications. J. Saudi Chem. Soc. 2017, 21, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Daisy, P.; Saipriya, K. Biochemical Analysis of Cassia Fistula Aqueous Extract and Phytochemically Synthesized Gold Nanoparticles as Hypoglycemic Treatment for Diabetes Mellitus. Int. J. Nanomed. 2012, 7, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- Fatima, M.; Zaidi, N.-U.-S.; Amraiz, D.; Afzal, F. In Vitro Antiviral Activity of Cinnamomum cassia and Its Nanoparticles Against H7N3 Influenza A Virus. J. Microbiol. Biotechnol. 2015, 26, 151–159. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Green Nanoparticles for Mosquito Control. Sci. World J. 2014, 2014, 496362. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xia, R.; Hu, H.; Peng, T. Biosynthesis, Characterization and Cytotoxicity of Gold Nanoparticles and Their Loading with N-Acetylcarnosine for Cataract Treatment. J. Photochem. Photobiol. B 2018, 187, 180–183. [Google Scholar] [CrossRef]
- Sasikala, A.; Linga Rao, M.; Savithramma, N.; Prasad, T.N.V.K.V. Synthesis of Silver Nanoparticles from Stem Bark of Cochlospermum religiosum (L.) Alston: An Important Medicinal Plant and Evaluation of Their Antimicrobial Efficacy. Appl. Nanosci. 2015, 5, 827–835. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Ansari, M.; Khan, H.; Jalal, M.; Mahdi, A.A.; Cameotra, S. Crataeva nurvala Nanoparticles Inhibit Virulence Factors and Biofilm Formation in Clinical Isolates of Pseudomonas Aeruginosa: Nanoparticles as an Antivirulent. J. Basic Microbiol. 2016, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Jena, B.S. Innate Catalytic and Free Radical Scavenging Activities of Silver Nanoparticles Synthesized Using Dillenia indica Bark Extract. J. Colloid Interface Sci. 2017, 496, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, B.; Josebin, M.; Seerangaraj, V.; Veluswamy, B. Biosynthesis of Silver Nanoparticles Using Stem Bark Extracts of Diospyros montana and Their Antioxidant and Antibacterial Activities. J. Nanostructure Chem. 2018, 8, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Vasantharaj, S.; Sripriya, N.; Shanmugavel, M.; Manikandan, E.; Gnanamani, A.; Senthilkumar, P. Surface Active Gold Nanoparticles Biosynthesis by New Approach for Bionanocatalytic Activity. J. Photochem. Photobiol. B 2018, 179, 119–125. [Google Scholar] [CrossRef]
- Odeyemi, S.; Mare, J.-A.; Edkins, A.; Afolayan, A. In Vitro and in Vivo Toxicity Assessment of Biologically Synthesized Silver Nanoparticles from Elaeodendron Croceum. J. Complement. Integr. Med. 2019, 16, 1–14. [Google Scholar] [CrossRef]
- Kulkarni, N.; Muddapur, U. Biosynthesis of Metal Nanoparticles: A Review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef] [Green Version]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of Silver Nanoparticles Using Plants Extract and Analysis of Their Antimicrobial Property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Li, W.; Yang, F.; Liu, H. Controllable Biosynthesis of Gold Nanoparticles from a Eucommia ulmoides Bark Aqueous Extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 73–79. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Liu, H. Biosynthesised Palladium Nanoparticles Using Eucommia ulmoides Bark Aqueous Extract and Their Catalytic Activity. IET Nanobiotechnol. 2015, 9, 349–354. [Google Scholar] [CrossRef]
- Garcia Campoy, A.H.; Perez Gutierrez, R.M.; Manriquez-Alvirde, G.; Muñiz Ramirez, A. Protection of Silver Nanoparticles Using Eysenhardtia polystachya in Peroxide-Induced Pancreatic β-Cell Damage and Their Antidiabetic Properties in Zebrafish. Int. J. Nanomed. 2018, 13, 2601–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanjikar, A.; Lingappa Hugar, A.; Londonkar, R. Characterization of Phyto-Nanoparticles from Ficus krishnae for Their Antibacterial and Anticancer Activities. Drug Dev. Ind. Pharm. 2017, 44, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, V.; Melchias, G.; Kumaravel, P. Ficus benghalensis Mediates Synthesis of Silver Nanoparticles: The Green Approach Yields NPs that Are Its Anti-Bacterial and Anti-Oxidant. World J. Pharm. Sci. 2016, 4, 1–12. [Google Scholar]
- Nayak, D.; Ashe, S.; Rauta, P.; Kumari, M.; Nayak, B. Bark Extract Mediated Green Synthesis of Silver Nanoparticles: Evaluation of Antimicrobial Activity and Antiproliferative Response against Osteosarcoma. Mater. Sci. Eng. C 2015, 58, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, C. Biofabrication of Silver Nanoparticles and Their Combined Effect with Low Intensity Ultrasound for Treatment of Lung Cancer. J. Photochem. Photobiol. B 2018, 181, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Karthika, V.; Arumugam, A.; Gopinath, K.; Periyannan, K.; Govindarajan, M.; Alharbi, N.; Km, S.; Khaled, J.; Benelli, G. Guazuma ulmifolia Bark-Synthesized Ag, Au and Ag/Au Alloy Nanoparticles: Photocatalytic Potential, DNA/Protein Interactions, Anticancer Activity and Toxicity against 14 Species of Microbial Pathogens. J. Photochem. Photobiol. B 2017, 167, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, G.; Agrawal, V. Green Synthesis of Silver Nanoparticles Using Holarrhena antidysenterica (L.) Wall.Bark Extract and Their Larvicidal Activity against Dengue and Filariasis Vectors. Parasitol. Res. 2017, 117, 377–389. [Google Scholar] [CrossRef]
- Mehmood, A. Antibacterial Efficacy of Silver Nanoparticles Synthesized by a Green Method Using Bark Extract of Melia azedarach L. J. Pharm. Innov. 2014, 9, 238–245. [Google Scholar] [CrossRef]
- Li, S.; Deng, J.; Zhao, S. Minor phenolic constituents of chinaberry-tree (Melia azedarach). Zhong Cao Yao Chin. Tradit. Herb. Drugs 2000, 31, 86–89. [Google Scholar]
- Kumar, S.V.; Sanghai, D.B.; Rao, C.M.; Shreedhara, C.S. Histological and Physiochemical Standardization of Melia azedarach. Linn Bark. Asian Pac. J. Trop. Biomed. 2012, 2, S284–S289. [Google Scholar] [CrossRef]
- Pani, A.; Lee, J.; Yun, S.-I. Autoclave Mediated One-Pot-One-Minute Synthesis of AgNPs and Au–Ag Nanocomposite from Melia azedarach Bark Extract with Antimicrobial Activity against Food Pathogens. Chem. Cent. J. 2016, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barai, A.; Paul, K.; Dey, A.; Manna, S.; Roy, S.; Bag, B.; Mukhopadhyay, C. Green Synthesis of Nerium oleander-Conjugated Gold Nanoparticles and Study of Its in Vitro Anticancer Activity on MCF-7 Cell Lines and Catalytic Activity. Nano Converg. 2018, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Radu, D.; Volf, I.; Pag, A.; Popa, V. Antioxidant and Antibacterial Activities of Some Natural Polyphenols. Cellul. Chem. Technol. 2013, 47, 387–399. [Google Scholar]
- Iravani, S.; Zolfaghari, B. Green Synthesis of Silver Nanoparticles Using Pinus eldarica Bark Extract. Biomed. Res. Int. 2013, 2013, 639725. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, R. Synthesis of Silver Nanoparticles Using Fresh Bark of Pongamia pinnata and Characterization of Its Antibacterial Activity against Gram Positive and Gram Negative Pathogens. Resour.-Effic. Technol. 2016, 2, 30–35. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, M.; Gupta, N.; Kumar, A.; Chandra, R.; Nimesh, S. Artificial Cells, Nanomedicine, and Biotechnology Green Synthesis of Silver Nanoparticles Using Prosopis juliflora Bark Extract: Reaction Optimization, Antimicrobial and Catalytic Activities. Artif. Cells Nanomed. Biotechnol. 2017, 46, 985–993. [Google Scholar] [CrossRef] [Green Version]
- Puiso, J.; Mačionienė, I.; Jonkuvienė, D.; Šalomskienė, J. Antimicrobial Activity of Silver Nanoparticles Synthesized Using Plant Extracts. Vet. Ir Zootech. 2014, 65, 61–67. [Google Scholar]
- Bahram, M.; Mohammadzadeh, E. Green Synthesis of Gold Nanoparticles with Willow Tree Bark Extract: A Sensitive Colourimetric Sensor for Cysteine Detection. Anal. Methods 2014, 6, 6916–6924. [Google Scholar] [CrossRef]
- Subramanian, R.; Subbramaniyan, P.; Raj, V. Antioxidant Activity of the Stem Bark of Shorea roxburghii and Its Silver Reducing Power. SpringerPlus 2013, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Francis, S.; Koshy, E.; Mathew, B. Green Synthesis of Stereospermum suaveolens Capped Silver and Gold Nanoparticles and Assessment of Their Innate Antioxidant, Antimicrobial and Antiproliferative Activities. Bioprocess. Biosyst. Eng. 2018, 41, 939–951. [Google Scholar] [CrossRef]
- Yugandhar, P.; Haribabu, R.; Savithramma, N. Synthesis, Characterization and Antimicrobial Properties of Green-Synthesised Silver Nanoparticles from Stem Bark Extract of Syzygium alternifolium (Wt.). Walp. 3 Biotech 2015, 5, 1031–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Satyanarayana Swamy, V. Antibacterial Activity of Silver Nanoparticles Synthesized by Bark Extract of Syzygium cumini. J. Nanoparticles 2013, 2013, 431218. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Q.; Gupta, N.; Kumar, A.; Nimesh, S. Antibacterial Efficacy of Silver Nanoparticles Synthesized Employing Terminalia arjuna Bark Extract. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yallappa, S.; Manjanna, J.; Sindhe, M.A.; Satyanarayan, N.D.; Pramod, S.N.; Nagaraja, K. Microwave Assisted Rapid Synthesis and Biological Evaluation of Stable Copper Nanoparticles Using T. arjuna Bark Extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 110, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.N.J.I.; Lee, Y.R.; Sethuraman, M.G. Green Synthesis of Silver Nanoparticles Using Terminalia cuneata and Its Catalytic Action in Reduction of Direct Yellow-12 Dye. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 161, 122–129. [Google Scholar] [CrossRef]
- Babu, S.; Devadiga, A.; Shetty, K.V.; Saidutta, M.B. Synthesis of Silver Nanoparticles Using Medicinal Zizyphus xylopyrus Bark Extract. Appl. Nanosci. 2014, 5, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Prashanth, G.K.; Prashanth, P.A.; Bora, U.; Gadewar, M.; Nagabhushana, B.M.; Ananda, S.; Krishnaiah, G.M.; Sathyananda, H.M. In Vitro Antibacterial and Cytotoxicity Studies of ZnO Nanopowders Prepared by Combustion Assisted Facile Green Synthesis. Karbala Int. J. Mod. Sci. 2015, 1, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Guo, J.-J.; Hsieh, H.-Y.; Hu, C.-H. Chain-Breaking Activity of Carotenes in Lipid Peroxidation: A Theoretical Study. J. Phys. Chem. B 2009, 113, 15699–15708. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Chou, C.-C. Antioxidative Activities of Water-Soluble Disaccharide Chitosan Derivatives. Food Res. Int. 2004, 37, 883–889. [Google Scholar] [CrossRef]
- Phull, A.-R.; Abbas, Q.; Ali, A.; Raza, H.; Kim, S.J.; Zia, M.; Haq, I. Antioxidant, Cytotoxic and Antimicrobial Activities of Green Synthesized Silver Nanoparticles from Crude Extract of Bergenia ciliata. Future J. Pharm. Sci. 2016, 2, 31–36. [Google Scholar] [CrossRef]
- Ramamurthy, C.; Padma, M.; Samadanam, I.; Ramachandran, M.; Suyavaran, A.; Kumar, M.; Premkumar, K.; Thirunavukkarasu, C. The Extra Cellular Synthesis of Gold and Silver Nanoparticles and Their Free Radical Scavenging and Antibacterial Properties. Colloids Surf. B Biointerfaces 2012, 102C, 808–815. [Google Scholar] [CrossRef]
- Wang, Y.; He, X.; Wang, K.; Zhang, X.; Tan, W. Barbated Skullcup Herb Extract-Mediated Biosynthesis of Gold Nanoparticles and Its Primary Application in Electrochemistry. Colloids Surf. B Biointerfaces 2009, 73, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yudha, S.S.; Notriawan, D.; Angasa, E.; Suharto, T.; Hendri, J.; Nisina, Y. Green Synthesis of Silver Nanoparticles Using Aqueous Rinds Extract of Brucea javanica (L.) Merr at Ambient Temperature. Mater. Lett. 2013, 97, 181–183. [Google Scholar] [CrossRef]
- Wijnhoven, S.; Peijnenburg, W.; Herberts, C.; Hagens, W.; Oomen, A.; Heugens, E.; Roszek, B.; Bisschops, J.; Gosens, I.; Van de meent, D.; et al. Nano-Silver—A Review of Available Data and Knowledge Gaps in Human and Environmental Risk Assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Yang, P.; Yang, W. Biomimetic Synthesis of Gold Nanoparticles and Their Aggregates Using a Polypeptide Sequence. Appl. Organomet. Chem. 2007, 21, 645–651. [Google Scholar] [CrossRef]
- Mirzajani, F.; Ghassempour, A.; Aliahmadi, A.; Esmaeili, M.A. Antibacterial Effect of Silver Nanoparticles on Staphylococcus Aureus. Res. Microbiol. 2011, 162, 542–549. [Google Scholar] [CrossRef]
- Savithramma, N.; Lingarao, M.; Ankanna, S.; Venkateswarlu, P. Screening of Medicinal Plants for Effective Biogenesis of Silver Nanoparticles and Efficient Antimicrobial Activity. Int. J. Pharm. Sci. Res. 2012, 3, 1141–1148. [Google Scholar]
- Dibrov, P.; Dzioba, J.; Gosink, K.; Häse, C. Chemiosmotic Mechanism of Antimicrobial Activity of Ag+ in Vibrio Cholerae. Antimicrob. Agents Chemother. 2002, 46, 2668–2670. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Wu, J.; Chen, G.-Q.; Cui, F.-Z.; Kim, T.; Kim, J. A Mechanistic Study of the Antibacterial Effect of Silver Ions On Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of Bactericidal Action of Silver Zeolite and Its Comparison with That of Silver Nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazeruddin, G.M.; Prasad, N.R.; Prasad, S.R.; Shaikh, Y.I.; Waghmare, S.R.; Adhyapak, P. Coriandrum sativum Seed Extract Assisted in Situ Green Synthesis of Silver Nanoparticle and Its Anti-Microbial Activity. Ind. Crop. Prod. 2014, 60, 212–216. [Google Scholar] [CrossRef]
- Cooper, G. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Singh, R.; Shedbalkar, U.; Wadhwani, S.; Chopade, P.B. Bacteriagenic Silver Nanoparticles: Synthesis, Mechanism, and Applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. coli as a Model for Gram-Negative Bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Asharani, P.V.; Hande, M.P.; Valiyaveettil, S. Anti-Proliferative Activity of Silver Nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-F.; Choi, Y.-J.; Han, J.W.; Kim, E.; Park, J.H.; Gurunathan, S.; Kim, J.-H. Differential Nanoreprotoxicity of Silver Nanoparticles in Male Somatic Cells and Spermatogonial Stem Cells. Int. J. Nanomed. 2015, 10, 1335–1357. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Medhat, D.; Hussein, J.; El-naggar, M.; Attia, M.; Anwar, M.; Latif, Y.; Booles, H.; Morsy, S.; Farrag, A.R.; Khalil, W.; et al. Effect of Au-Dextran NPs as Anti-Tumor Agent against EAC and Solid Tumor in Mice by Biochemical Evaluations and Histopathological Investigations. Biomed. Pharm. 2017, 91, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Melián, J.A.; Martín-Rodríguez, A.J.; Ortega-Méndez, A.; Araña, J.; Doña-Rodríguez, J.M.; Pérez-Peña, J. Degradation and Detoxification of 4-Nitrophenol by Advanced Oxidation Technologies and Bench-Scale Constructed Wetlands. J. Environ. Manag. 2012, 105, 53–60. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Xu, Z.; Liu, M. A Graphene Oxide-Based Electrochemical Sensor for Sensitive Determination of 4-Nitrophenol. J. Hazard. Mater. 2012, 201–202, 250–259. [Google Scholar] [CrossRef]
- Wunder, S.; Lu, Y.; Albrecht, M.; Ballauff, M. Catalytic Activity of Faceted Gold Nanoparticles Studied by a Model Reaction: Evidence for Substrate-Induced Surface Restructuring. ACS Catal. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Velayutham, K.; Rahuman, A.A.; Rajakumar, G.; Roopan, S.M.; Elango, G.; Kamaraj, C.; Marimuthu, S.; Santhoshkumar, T.; Iyappan, M.; Siva, C. Larvicidal Activity of Green Synthesized Silver Nanoparticles Using Bark Aqueous Extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus. Asian Pac. J. Trop. Med. 2013, 6, 95–101. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlacu, E.; Tanase, C.; Coman, N.-A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. https://doi.org/10.3390/molecules24234354
Burlacu E, Tanase C, Coman N-A, Berta L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules. 2019; 24(23):4354. https://doi.org/10.3390/molecules24234354
Chicago/Turabian StyleBurlacu, Ema, Corneliu Tanase, Năstaca-Alina Coman, and Lavinia Berta. 2019. "A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications" Molecules 24, no. 23: 4354. https://doi.org/10.3390/molecules24234354
APA StyleBurlacu, E., Tanase, C., Coman, N.-A., & Berta, L. (2019). A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules, 24(23), 4354. https://doi.org/10.3390/molecules24234354






