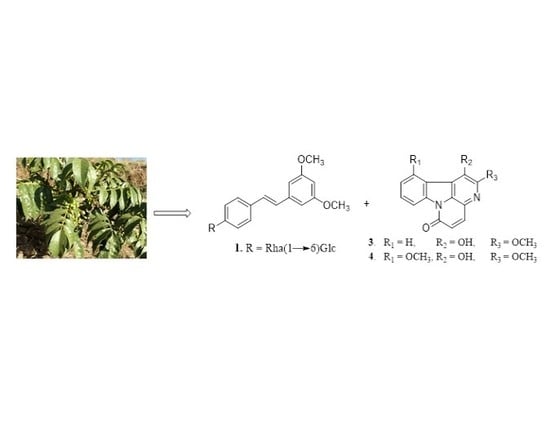
Cytotoxic Stilbenes and Canthinone Alkaloids from Brucea antidysenterica (Simaroubaceae)
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Bruceanoside A (1)
3.3.2. Bruceacanthinone A (3)
3.3.3. Bruceacanthinone B (4)
3.4. Cell Lines, Cell Cultures, and the MTT Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.-H.; Jin, H.-Z.; Zhang, W.-D.; Yan, S.-K.; Shen, Y.-H. Chemical constituents of plants from the genus Brucea. Chem. Biodiver. 2009, 6, 57–70. [Google Scholar] [CrossRef]
- Wright, C.W.; O’Neill, M.J.; Phillipson, J.D.; Warhurst, D.C. Use of microdilution to assess in vitro antiamoebic activities of Brucea javanica fruits, Simarouba amara stem, and a number of quassinoids. Antimicrob. Agents Chemother. 1988, 32, 1725–1729. [Google Scholar] [CrossRef] [Green Version]
- Lau, F.Y.; Chui, C.H.; Gambari, R.; Kok, S.H.L.; Kan, K.L.; Cheng, G.M.Y.; Wong, R.S.M.; Teo, I.T.N.; Cheng, C.H.; Wan, T.S.K.; et al. Antiproliferative and apoptosis-inducing activity of Brucea javanica extract on human carcinoma cells. Int. J. Mol. Med. 2005, 16, 1157–1162. [Google Scholar] [CrossRef]
- Okano, M.; Fukamiya, N.; Aratani, T.; Juichi, M.; Lee, K.H. Antitumor agents, 74. Bruceanol-A and -B, Two new antileukemic quassinoids from Brucea antidysenterica. J. Nat. Prod. 1985, 48, 972–975. [Google Scholar] [CrossRef]
- Fukamiya, N.; Okano, M.; Miyamoto, M.; Tagahara, K.; Lee, K.H. Antitumor agents, 127. Bruceoside C, a new cytotoxic quassinoid glucoside, and related compounds from Brucea javanica. J. Nat. Prod. 1992, 55, 468–475. [Google Scholar] [CrossRef]
- Kitagawa, I.; Mahmud, T.; Simanjuntak, P.; Hori, K.; Uji, T.; Shibuya, H. Indonesian medicinal plants. VIII. Chemical structures of three new triterpenoids, bruceajavanin A, dihydrobruceajavanin A, and bruceajavanin B, and a new alkaloidal glycoside, bruceacanthinoside, from the stems of Brucea javanica (Simaroubaceae). Chem. Pharm. Bull. 1994, 42, 1416–1421. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, M.J.; Bray, D.H.; Boardman, P.; Chan, K.L.; Phillipson, J.D.; Warhurst, D.C.; Peters, W. Plants as sources of antimalarial drugs, Part 4: Activity of Brucea javanica fruits against chloroquine-resistant Plasmodium falciparum in vitro and against Plasmodium berghei in vivo. J. Nat. Prod. 1987, 50, 41–48. [Google Scholar] [CrossRef]
- Rahman, S.; Fukamiya, N.; Okano, M.; Tagahara, K.; Lee, K.H. Anti-tuberculosis activity of quassinoids. Chem. Pharm. Bull. 1997, 45, 1527–1529. [Google Scholar] [CrossRef] [Green Version]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa, 2nd ed.; E. and S. Livingstone: London, UK, 1962. [Google Scholar]
- Grace, O.M.; Fowler, D.G. Brucea Antidysenterica, Medicinal Plants/Plantes Medicinales; Mill, J.F., Ed.; PROTA: Wageningen, The Netherlands, 2008. [Google Scholar]
- Cuendet, M.; Pezzuto, J.M. Antitumor activity of bruceantin. An old drug with new promise. J. Nat. Prod. 2004, 67, 269–272. [Google Scholar]
- Dilnesa, A.; Mekonon, A.; Abebe, A. Phytochemical screening and antioxidant activity investigations on the crude extracts of Brucea antidysenterica leaves. Int. J. Res. Dev. 2016, 1, 131–144. [Google Scholar]
- Mekonnen, Z.; Amuamuta, A.; Abere, Y. Wound healing effect of aqueous extracts of Brucea antidysenterica and Croton marcostachys from Northwest Ethiopia in albino mice. J. Afr. Pharmacol. Ther. 2019, 8, 14–19. [Google Scholar]
- Kefe, A.; Giday, M.; Mamo, H.; Erko, B. Antimalarial properties of crude extracts of seeds of Brucea antidysenterica and leaves of Ocimum lamiifolium. BMC Compl. Altern. Med. 2016, 16, 118–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kupchan, S.M.; Britton, R.W.; Ziegler, M.F.; Sigel, C.W. Bruceantin, a new potent antileukemic simaroubolide from Brucea antidysenterica. J. Org. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Vangapandu, S.; Sindelar, R.W.; Walker, L.A.; Sindelar, R.D. Biologically active quassinoids and their chemistry: potential leads for drug design. Curr. Med. Chem. 2005, 12, 173–190. [Google Scholar] [CrossRef]
- Makong, Y.S.; Fotso, G.W.; Mouthe, G.H.; Lenta, B.; Rennert, R.; Sewald, N.; Arnold, N.; Wansi, J.D.; Ngadjui, B.T. Bruceadysentoside A, a new pregnane glycoside and others secondary metabolites with cytotoxic activity from Brucea antidysenterica JF Mill. (Simaroubaceae). Nat. Prod. Res. 2019, 1–7. [Google Scholar] [CrossRef]
- Aburjai, T.A. Anti-platelet stilbenes from aerial parts of Rheum palaestinum. Phytochemistry 2000, 55, 407–410. [Google Scholar] [CrossRef]
- Narihiko, F.; Masayoshi, O.; Aratani, T. Antitumor agents, 79. Cytotoxic antileukemic alkaloids from Brucea antidysenterica. J. Nat. Prod. 1986, 49, 428–434. [Google Scholar]
- Ohmoto, T.; Tanaka, R.; Nikaido, T. Studies on the constituents of Ailanthus altissima Swingle. The alkaloidal constituents. Chem. Pharm. Bull. 1976, 24, 1532–1536. [Google Scholar] [CrossRef] [Green Version]
- Njar, V.C.; Alao, T.O.; Okogun, J.I.; Holland, H.L. 2-Methoxycanthin-6-one: A new alkaloid from the stem wood of Quassia amara. Planta Med. 1993, 59, 259–261. [Google Scholar] [CrossRef]
- Harris, A.; Anderson, L.A.; Phillipson, J.D.; Brown, R.T. Canthin-6-one alkaloids from Brucea antidysenterica root bark. Planta Med. 1985, 51, 151–153. [Google Scholar] [CrossRef]
- Yishan, O.; Katsuyoshi, M.; Kazuo, K.; Taichi, O. Alkaloids and quassinoids of Brucea mollis var. tonkinensis. Phytochemistry 1995, 39, 911–913. [Google Scholar]
- Ray, A.B.; Chattopadhyay, K.S.; Kumar, S.; Konno, C.; Kiso, Y.; Hikino, H. Structures of cleomiscisins, coumarinolignoids of Cleome Viscosa seeds. Tetrahedron 1985, 41, 209–214. [Google Scholar] [CrossRef]
- Lee, K.-H.; Hayashi, N.; Okano, M.; Nazaki, H.; Ju-Ichi, M. Antitumor agents, 65. Brusatol and cleomiscosin-A, antileukemic principles from Brucea javanica. J. Nat. Prod. 1984, 47, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Chin, Y.-W.; Chai, H.-B.; Ninh, T.N.; Soejarto, D.D.; Kinghorn, A.D. Bioactivity-guided isolation of cytotoxic constituents of Brucea javanica collected in Vietnam. Bioorg. Med. Chem. 2009, 17, 2219–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanjala, C.C.W.; Majinda, R.R.T. A new stilbene glycoside from Elephantorrhiza goetzei. Fitoterapia 2001, 72, 649–655. [Google Scholar] [CrossRef]
- Fuendjiep, V.; Wandji, J.; Tillequin, F.; Mulholland, D.A.; Budzikiewicz, H.; Fomum, Z.T.; Nyemba, A.M.; Koch, M. Chalconoid and stilbenoid glycosides from Guibourtia tessmanii. Phytochemistry 2002, 60, 803–806. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C NMR spectra of pentacyclic triterpenoids-a compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- O’Donnell, G.; Gibbons, S. Antibacterial activity of two canthin-6-one alkaloids from Allium neapolitanum. Phytother. Res. 2007, 21, 653–657. [Google Scholar] [CrossRef]
- López, C.; Pastrana, M.; Ríos, A.; Cogollo, A.; Pabón, A. Huberine, a new canthin-6-one alkaloid from the bark of Picrolemma huberi. Molecules 2018, 23, 934. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Basar, N.; Oridupa, O.A.; Ritchie, K.J.; Nahar, L.; Osman, N.M.; Stafford, A.; Kushiev, H.; Kan, A.; Sarker, S.D. Comparative cytotoxicity of Glycyrrhiza glabra roots from different geographical origins against immortal human keratinocyte (HaCaT), lung adenocarcinoma (A549) and liver carcinoma (HepG2) cells. Phytother. Res. 2015, 29, 944–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahsin, T.; Wansi, J.D.; Al-Groshi, A.; Evans, A.R.; Nahar, L.; Martin, C.; Sarker, S.D. Cytotoxic properties of the stem bark of Citrus reticulata Blanco (Rutaceae). Phytother. Res. 2017, 31, 1215–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, T.; Lupu, A.R.; Raditoiu, V.; Purcar, V.; Teodorescu, V.S. On the photocatalytic reduction of MTT tetrazolium salt on the surface of TiO2 nanoparticles: Formazan production kinetics and mechanism. J. Colloid Interface Sci. 2015, 457, 108–120. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–13 are available from the authors. |



| No | 1 | |
|---|---|---|
| δC | δH (mult., J in Hz) | |
| α | 126.4 | 6.99 (1H, d, 16.4) |
| β | 129.1 | 7.20 (1H, d, 16.4) |
| 1 | 129.9 | – |
| 2 | 105.6 | 6.82 (1H, brs) |
| 3 | 159.3 | – |
| 4 | 101.9 | 6.52 (1H, t, 2.2) |
| 5 | 160.2 | – |
| 6 | 107.6 | 6.78 (1H, brs) |
| 1′ | 139.7 | – |
| 2′/6′ | 128.4 | 7.58 (2H, d, 8.8) |
| 3′/5′ | 114.6 | 6.95 (2H, d, 8.8) |
| 4′ | 159.8 | – |
| OCH3-3/5 | 55.6 | 3.80 (6H, s) |
| sugar moieties | ||
| glc-1 | 101.0 | 4.89 (1H, d, 7.6) |
| 2 | 73.7 | 3.19 (1H, m) |
| 3 | 75.8 | 3.52 (1H. m) |
| 4 | 70.8 | 3.65 (1H, m) |
| 5 | 76.9 | 3.30 (1H, m) |
| 6 | 66.8 | 3.41 (1H, m) 3.87 (1H, m) |
| rha-1 | 101.0 | 4.56 (1H, d, 1.6) |
| 2 | 71.2 | 3.42 (1H, m) |
| 3 | 70.3 | 3.46 (1H, m) |
| 4 | 72.5 | 3.18 (1H, m) |
| 5 | 68.8 | 3.43 (1H, m) |
| 6 | 18.3 | 1.11 (3H, d, 6.1) |
| No | 3 | 4 | ||
|---|---|---|---|---|
| δC | δH (mult., J in Hz) | δC | δH (mult., J in Hz) | |
| 1 | 140.0 | – | 140.4 | – |
| 2 | 152.6 | – | 151.5 | – |
| 4 | 139.0 | 8.08 (1H, d, 9.7) | 139.9 | 8.04 (1H, d, 9.8) |
| 5 | 127.3 | 6.90 (1H, d, 9.7) | 125.9 | 6.90 (1H, d, 9.8) |
| 6 | 159.6 | – | 160.0 | – |
| 8 | 117.1 | 8.70 (1H, dd, 1.2, 7.8) | 112.9 | 8.40 (1H, dd, 0.8, 8.2) |
| 9 | 130.7 | 7.70 (1H, td, 1.0, 7.6, 7.8) | 132.3 | 7.65 (1H, t, 8.2) |
| 10 | 127.5 | 7.58 (1H, td, 1.2, 7.6, 7.8) | 108.0 | 7.01 (1H, dd, 0.8, 8.2) |
| 11 | 125.0 | 8.26 (1H, dd, 1.0, 7.6) | 156.1 | – |
| 12 | 123.1 | – | 126.8 | – |
| 13 | 136.4 | – | 139.9 | – |
| 14 | 130.7 | – | 132.9 | – |
| 15 | 128.7 | – | 132.3 | – |
| 16 | 126.1 | – | 126.8 | – |
| OMe-2 | 57.2 | 4.29 (3H, s) | 56.3 | 4.09 (3H, s) |
| OMe-11 | – | – | 57.8 | 4.28 (3H, s) |
| OH-1 | – | 8.52 (1H, s) | – | 8.50 (1H, s) |
| Samples | IC50 in μg/mL | ||
|---|---|---|---|
| A-549 | MCF-7 | PC-3 | |
| Root extract | 75.2 ± 3.2 | 80.5 ± 1.8 | 77.7 ± 2.5 |
| Bark extract | 65.1 ± 1.5 | 72.3 ± 3.5 | 70.2 ± 4.2 |
| DCM | 50.0 ± 5.2 | 55.1 ± 4.2 | 57.0 ± 5.3 |
| EA | 61.5 ± 3.2 | 58.1 ± 1.9 | 55.8 ± 2.4 |
| 3 | >250 | >250 | 195.5 ± 9.5 |
| 4 | 150.3 ± 8.5 | 157.5 ± 7.5 | 160.5 ± 5.5 |
| 5 | >250 | >250 | >250 |
| 6 | 175.6 ± 6.1 | 170.3 ± 5.8 | 177.3 ± 5.9 |
| 7 | 125.8 ± 7.2 | 152.1 ± 11.2 | 155.1 ± 5.3 |
| 8 | 121.5 ± 8.2 | 126.2 ± 5.5 | 130.9 ± 7.4 |
| 9 | 138.1 ± 9.7 | 145.2 ± 9.9 | 151.5 ± 8.3 |
| 10 | >250 | >250 | >250 |
| 11 | >250 | >250 | >250 |
| 12 | 130.8 ± 7.4 | 132.1 ± 11.2 | 130.1 ± 5.3 |
| 13 | >250 | >250 | >250 |
| Etoposide | 3.5 ± 0.5 | 10.2 ± 1.2 | 7.9 ± 1.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makong, Y.S.; Mouthé Happi, G.; Djouaka Bavoua, J.L.; Wansi, J.D.; Nahar, L.; Kamdem Waffo, A.F.; Martin, C.; Sewald, N.; Sarker, S.D. Cytotoxic Stilbenes and Canthinone Alkaloids from Brucea antidysenterica (Simaroubaceae). Molecules 2019, 24, 4412. https://doi.org/10.3390/molecules24234412
Makong YS, Mouthé Happi G, Djouaka Bavoua JL, Wansi JD, Nahar L, Kamdem Waffo AF, Martin C, Sewald N, Sarker SD. Cytotoxic Stilbenes and Canthinone Alkaloids from Brucea antidysenterica (Simaroubaceae). Molecules. 2019; 24(23):4412. https://doi.org/10.3390/molecules24234412
Chicago/Turabian StyleMakong, Yves Salomon, Gervais Mouthé Happi, Judith Liliane Djouaka Bavoua, Jean Duplex Wansi, Lutfun Nahar, Alain François Kamdem Waffo, Claire Martin, Norbert Sewald, and Satyajit Dey Sarker. 2019. "Cytotoxic Stilbenes and Canthinone Alkaloids from Brucea antidysenterica (Simaroubaceae)" Molecules 24, no. 23: 4412. https://doi.org/10.3390/molecules24234412
APA StyleMakong, Y. S., Mouthé Happi, G., Djouaka Bavoua, J. L., Wansi, J. D., Nahar, L., Kamdem Waffo, A. F., Martin, C., Sewald, N., & Sarker, S. D. (2019). Cytotoxic Stilbenes and Canthinone Alkaloids from Brucea antidysenterica (Simaroubaceae). Molecules, 24(23), 4412. https://doi.org/10.3390/molecules24234412