Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities
Abstract
:1. Introduction
2. Ethnomedicinal Uses of Anonna Genus
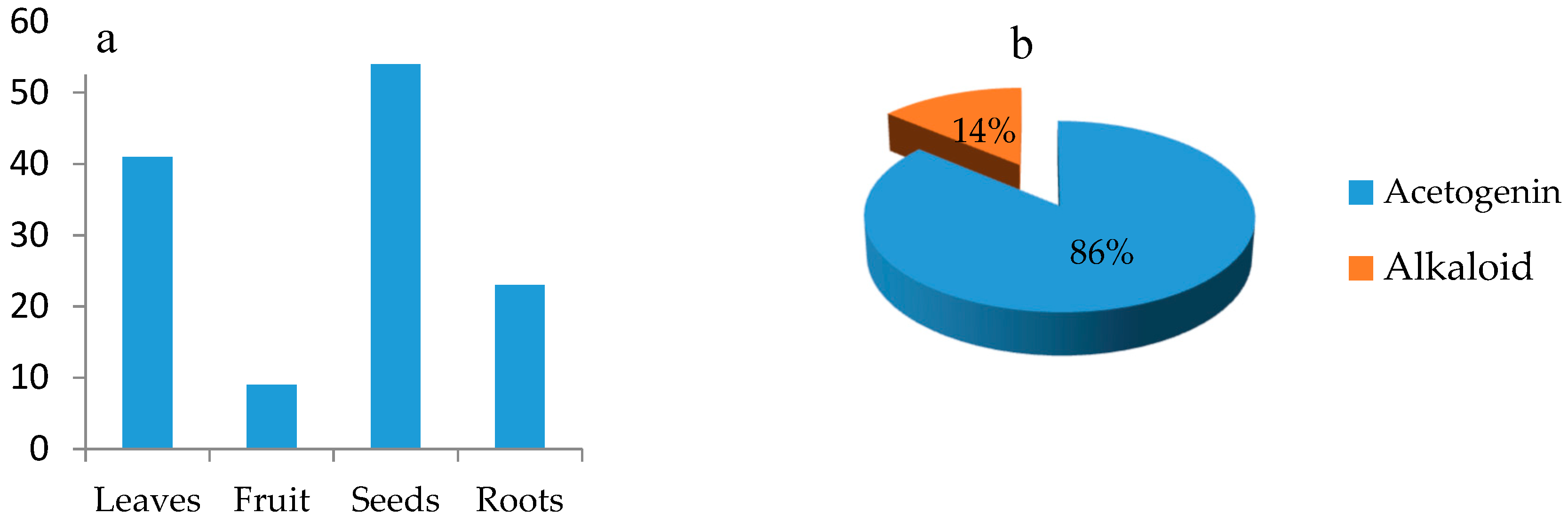
3. Phytochemical Studies of Secondary Metabolites of Annona Genus
4. Anti-Infective Alkaloids from the Genus Annona
4.1. Antiprotozoal Activities
4.2. Antimicrobial Activities
5. Anticancer Alkaloids Present in the Genus Annona
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The Plant List Version 1. Available online: http://www.theplantlist.org/ (accessed on 5 December 2018).
- Badrie, N.; Schauss, A.G. Soursop (Annona muricata L.): Composition, nutritional value, medicinal uses, and toxicology. In Bioactive Foods in Promoting Health; Watson, R.R., Preedy, V.R., Eds.; Elsevier Inc.: London, UK, 2010. [Google Scholar]
- Mishra, S.; Ahmad, S.; Kumar, N.; Sharma, B.K. Annona muricata (the cancer killer): A Review. Glob. J. Pharm. Res. 2013, 2, 1613–1618. [Google Scholar]
- Oliveira, B.H.; Sant’Ana, A.E.G.; Bastos, D.Z.L. Determination of the diterpenoid, kaurenoic acid, in Annona glabra by HPLC. Phytochem. Anal. 2002, 13, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.; de Goulart, R.; Vasques Farinazzi-Machado, F.; da Soares de Souza, M.; Santos Bueno, P.; Guiguer, E.; Araujo, A.; Groppo, M. Annona sp: Plants with Multiple Applications as Alternative Medicine - A Review. Curr. Bioact. Compd. 2012, 8, 277–286. [Google Scholar] [CrossRef]
- Asare, G.A.; Afriyie, D.; Ngala, R.A.; Abutiate, H.; Doku, D.; Mahmood, S.A.; Rahman, H. Antiproliferative activity of aqueous leaf extract of Annona muricata L. on the prostate, BPH-1 cells, and some target genes. Integr. Cancer Ther. 2015, 14, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270. [Google Scholar] [CrossRef] [PubMed]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef] [Green Version]
- Morton, J.F.; Dowling, C.F. Fruits of Warm Climates; Wipf and Stock Publishers: Miami, FL, USA, 1987. [Google Scholar]
- Jansen, P.C.M.; Jukema, J.; Oyen, L.P.A.; van Lingen, T.G. Annona reticulata L. In Plant Resources of South-East Asia No. 2: Edible fruits and nuts; Verheij, E.W.M., Coronel, R.E., Eds.; Pudoc: Wageningen, The Netherlands, 1991; p. 316. [Google Scholar]
- Karou, S.D.; Tchacondo, T.; Djikpo Tchibozo, M.A.; Abdoul-Rahaman, S.; Anani, K.; Koudouvo, K.; Batawila, K.; Agbonon, A.; Simpore, J.; de Souza, C. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus and hypertension in the Central Region of Togo. Pharm. Biol. 2011, 49, 1286–1297. [Google Scholar] [CrossRef]
- Dos, S.A.F.; Sant’Ana, A.E. The molluscicidal activity of plants used in Brazilian folk medicine. Phytomedicine 2000, 6, 431–438. [Google Scholar]
- Syamsuhidayat, S.; Hutapea, J.R. Inventaris Tanaman Obat Indonesia; Departemen Kesehatan RI, Badan Penelitian dan Pengembangan Kesehatan: Jakarta, Indonesia, 1991.
- DeFilipps, R.A.; Maina, S.L.; Crepin, J. Medicinal Plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2004. [Google Scholar]
- González-Trujano, M.E.; Navarrete, A.; Reyes, B.; Hong, E. Some pharmacological effects of the ethanol extract of leaves of Annona diversifolia on the central nervous system in mice. Phyther. Res. 1998, 12, 600–602. [Google Scholar] [CrossRef]
- Oliveira da Cruz, P.E.; Costa, E.V.; de S. Moraes, V.R.; de L. Nogueira, P.C.; Vendramin, M.E.; Barison, A.; Ferreira, A.G.; do N. Prata, A.P. Chemical constituents from the bark of Annona salzmannii (Annonaceae). Biochem. Syst. Ecol. 2011, 39, 872–875. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro da Silva, L.M.; Teixeira de Figueiredo, E.A.; Ricardo, N.M.P.S.; Vieira, I.G.P.; Wilane de Figueiredo, R.; Brasil, I.M.; Gomes, C.L. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014, 143, 398–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nugraha, A.S.; Haritakun, R.; Lambert, J.M.; Dillon, C.T.; Keller, P.A. Alkaloids from the root of Indonesian Annona muricata L. Nat. Prod. Res. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, J.K.; Hui, Y.-H.; McLaughlin, J.L. Annonaceous Acetogenins: A Review. J. Nat. Prod. 1990, 53, 237–278. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-P.; Rieser, M.J.; Gu, Z.-M.; Zhao, G.-X.; McLaughlin, J.L. Annonaceous acetogenins: An updated review. Phytochem. Anal. 1993, 4, 27–48. [Google Scholar] [CrossRef]
- Feras, Q.A.; Liu, A.; McLaughlin, J.L. Annonaceous Acetogenins: Recent Progress. J. Nat. Prod. 1999, 62, 504–540. [Google Scholar]
- Zeng, L.; Ye, Q.; Oberlies, H.; Shi, G.; Gu, Z.-M.; He, K.; McLaughlin, J.L. Recent advances in Annonaceous acetogenins. Nat. Prod. Rep. 1996, 13, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Abdul Kadir, H. Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef]
- Reyes, F.R.; Santos, A.C. Isolation of anonaine from Anona squamosa Linn. Philipp. J. Sci. 1931, 44, 409–410. [Google Scholar]
- De Oliveira, A.B.; De Oliveira, G.G.; Carazza, F.; Maia, J.G.S. Geovanine, a new azaanthracene alkaloid from Annona ambotay Aubl. Phytochemistry 1987, 26, 2650–2651. [Google Scholar] [CrossRef]
- Rabelo, S.V.; Costa, E.V.; Barison, A.; Dutra, L.M.; Nunes, X.P.; Tomaz, J.C.; Oliveira, G.G.; Lopes, N.P.; de F.C. Santos, M.; da Silva Almeida, J.R.G. Alkaloids isolated from the leaves of atemoya (Annona cherimola × Annona squamosa). Rev. Bras. Farmacogn. 2015, 25, 419–421. [Google Scholar] [CrossRef] [Green Version]
- Raju, D.U.; Babu, K.S.; Ravada, S.C.R.; Golakoti, T. Isoquinoline alkaloid, flavonoids and a triol from leaves of Annona cherimola. J. Appl. Chem. (Lumami, India) 2015, 4, 120–126. [Google Scholar]
- Villar, A.; Mares, M.; Rios, J.L.; Cortes, D. Alkaloids from Annona cherimolia leaves. J. Nat. Prod. 1985, 48, 151–152. [Google Scholar] [CrossRef]
- Rios, J.L.; Cortes, D.; Valverde, S. Acetogenins, aporphinoids, and azaanthraquinone from Annona cherimolia seeds. Planta Med. 1989, 55, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Villar del Fresno, A.; Rios Canavate, J.L. Alkaloids from Annona cherimolia seed. J. Nat. Prod. 1983, 46, 438. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chang, F.-R.; Wu, Y.-C. Cherimoline, a novel alkaloid from the stems of Annona cherimola. Tetrahedron Lett. 1997, 38, 6247–6248. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Pan, W.B.; Wu, Y.C. Four alkaloids from Annona cherimola. Phytochemistry 2001, 56, 753–757. [Google Scholar] [CrossRef]
- Simeon, S.; Rios, J.L.; Villar, A. Alkaloids from Annona cherimolia (Mill.) stem bark. Plant. Med. Phytother. 1989, 23, 159–161. [Google Scholar]
- Martinez-Vazquez, M.; De la Cueva Lozano, D.G.; Estrada-Reyes, R.; Gonzalez-Lugo, N.M.; Ramirez Apan, T.; Heinze, G. Bio-guided isolation of the cytotoxic corytenchine and isocoreximine from roots of Annona cherimolia. Fitoterapia 2005, 76, 733–736. [Google Scholar] [CrossRef]
- de la Cruz Chacon, I.; Gonzalez-Esquinca, A.R. Liriodenine alkaloid in Annona diversifolia during early development. Nat. Prod. Res. 2012, 26, 42–49. [Google Scholar] [CrossRef]
- Chang, F.-R.; Chen, C.-Y.; Hsieh, T.-J.; Cho, C.-P.; Wu, Y.-C. Chemical constituents from Annona glabra III. J. Chin. Chem. Soc. 2000, 47, 913–920. [Google Scholar] [CrossRef]
- Riley-Saldana, C.A.; del R. Cruz-Ortega, M.; Martinez Vazquez, M.; De-la-Cruz-Chacon, I.; Castro-Moreno, M.; Gonzalez-Esquinca, A.R. Acetogenins and alkaloids during the initial development of Annona muricata L. (Annonaceae). Zeitschrift fuer Naturforschung C 2017, 72, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-H.; Chen, C.-M.; Kuan, S.-S. Alkaloids of Annona glabra. I. Isolation of (−)-N-methylactinodaphnine. J. Chin. Chem. Soc. 1971, 18, 133–136. [Google Scholar] [CrossRef]
- Yang, T.-H.; Chen, C.-M. Studies on the alkaloids of Anona glabra. II. T’ai-wan Yao Hsueh Tsa Chih 1973, 25, 1–7. [Google Scholar]
- Yang, T.-H.; Chen, C.-M. Studies on the alkaloids of Anona glabra L. II. Proc. Natl. Sci. Counc., Part. 2 1974, 7, 177–184. [Google Scholar]
- Wu, Y.C.; Chang, G.Y.; Duh, C.Y.; Wang, S.K. Cytotoxic alkaloids of Annona montana. Phytochemistry 1993, 33, 497–500. [Google Scholar] [CrossRef]
- Leboeuf, M.; Cave, A.; Forgacs, P.; Tiberghien, R.; Provost, J.; Touche, A.; Jacquemin, H. Alkaloids of the genus Annona. XL. Chemical and pharmacological study of alkaloids from Annona montana Macf. Plant. Med. Phytother. 1982, 16, 169–184. [Google Scholar]
- Yokomori, Y.; Sekido, K.; Wu, T.S.; Tien, H.J.; Hirokawa, S. The crystal and molecular structure of 1-(2-amino-4-pyrimidinyl)-β-carboline. Bull. Chem. Soc. Jpn. 1982, 55, 2236–2238. [Google Scholar] [CrossRef] [Green Version]
- Magadula, J.J.; Innocent, E.; Otieno, J.N. Mosquito larvicidal and cytotoxic activities of 3 Annona species and isolation of active principles. J. Med. Plants Res. 2009, 3, 674–680. [Google Scholar]
- Matsushige, A.; Kotake, Y.; Matsunami, K.; Otsuka, H.; Ohta, S.; Takeda, Y. Annonamine, a new aporphine alkaloid from the leaves of Annona muricata. Chem. Pharm. Bull. 2012, 60, 257–259. [Google Scholar] [CrossRef] [Green Version]
- Fofana, S.; Keita, A.; Balde, S.; Ziyaev, R.; Aripova, S.F. Alkaloids from leaves of Annona muricata. Chem. Nat. Compd. 2012, 48, 714. [Google Scholar] [CrossRef] [Green Version]
- Fofana, S.; Ziyaev, R.; Abdusamatov, A.; Zakirov, S.K. Alkaloids from Annona muricata leaves. Chem. Nat. Compd. 2011, 47, 321. [Google Scholar] [CrossRef]
- Leboeuf, M.; Legueut, C.; Cave, A.; Desconclois, J.F.; Forgacs, P.; Jacquemin, H. [Alkaloids of Annonaceae. XXIX. Alkaloids of Annona muricata]. Planta Med. 1981, 42, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Laprevote, O.; Leboeuf, M.; Cave, A.; Provost, J.; Forgacs, P.; Jacquemin, H. Alkaloids of the Annonaceae. 88. Alkaloids of Annona paludosa Aublet. Plant. Med. Phytother. 1988, 22, 159–164. [Google Scholar]
- Chang, F.-R.; Chen, K.-S.; Ko, F.-N.; Teng, C.-M.; Wu, Y.-C. Bioactive alkaloids from Annona reticulata. Chin. Pharm. J. 1995, 47, 483–491. [Google Scholar]
- Yang, T.H.; Cheng, M.Y. The alkaloids of Annona reticulata L. II. T’ai-wan Yao Hsueh Tsa Chih 1987, 39, 195–201. [Google Scholar]
- Xu, L.; Li, K.; Sun, N.; Kong, J. Alkaloids of Annona reticulata. Zhongguo Zhongyao Zazhi 1992, 17, 295–296. [Google Scholar]
- Campos, F.R.; Batista, R.L.; Batista, C.L.; Costa, E.V.; Barison, A.; dos Santos, A.G.; Pinheiro, M.L.B. Isoquinoline alkaloids from leaves of Annona sericea (Annonaceae). Biochem. Syst. Ecol. 2008, 36, 804–806. [Google Scholar] [CrossRef]
- Pinto, N.C.C.; Silva, J.B.; Menegati, L.M.; Guedes, M.C.M.R.; Scio, E.; Fabri, R.L.; Marques, L.B.; Souza-Fagundes, E.M.D.E.; Silva, T.P.D.A.; Melo, R.C.N.D.E.; et al. Cytotoxicity and bacterial membrane destabilization induced by Annona squamosa L. extracts. An. Acad. Bras. Cienc. 2017, 89, 2053–2073. [Google Scholar] [CrossRef]
- Philipov, S.; Kande, K.M.; Machev, K. Alkaloids of Annona senegalensis. Fitoterapia 1995, 66, 275–276. [Google Scholar]
- Fofana, S.; Ziyaev, R.; Diallo, S.K.; Camara, M.; Aripova, S.F. Alkaloids of Annona senegalensis. Chem. Nat. Compd. 2013, 49, 587–588. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Tewari, S.; Dhar, M.M. Aporphine alkaloids of Annona squamosa. Phytochemistry 1972, 11, 1819–1822. [Google Scholar] [CrossRef]
- Bhaumik, P.K.; Mukherjee, B.; Juneau, J.P.; Bhacca, N.S.; Mukerjee, R. Alkaloids from leaves of Annona squamosa. Phytochemistry 1979, 18, 1584–1586. [Google Scholar] [CrossRef]
- You, M.; Mahinda Wickramaratne, D.B.; Silva, G.L.; Chai, H.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Kinghorn, A.D.; Pezzuto, J.M. (-)-Roemerine, an aporphine alkaloid from Annona senegalensis that reverses the multidrug-resistance phenotype with cultured cells. J. Nat. Prod. 1995, 58, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Chang, F.-R.; Wu, Y.-C. Annosqualine: A novel alkaloid from the stems of Annona squamosa. Helv. Chim. Acta 2004, 87, 1392–1399. [Google Scholar] [CrossRef]
- Yang, T.-H.; Chen, C.-M. Constituents of Annona squamosa. J. Chin. Chem. Soc. 1970, 17, 243–250. [Google Scholar] [CrossRef]
- Pimenta, L.P.S.; Garcia, G.M.; do V. Goncalves, S.G.; Dionisio, B.L.; Braga, E.M.; Mosqueira, V.C.F. In vivo antimalarial efficacy of acetogenins, alkaloids and flavonoids enriched fractions from Annona crassiflora Mart. Nat. Prod. Res. 2014, 28, 1254–1259. [Google Scholar] [CrossRef]
- Kamaraj, C.; Kaushik, N.K.; Mohanakrishnan, D.; Elango, G.; Bagavan, A.; Zahir, A.A.; Rahuman, A.A.; Sahal, D. Antiplasmodial potential of medicinal plant extracts from Malaiyur and Javadhu hills of South India. Parasitol Res. 2012, 111, 703–715. [Google Scholar] [CrossRef]
- Somsak, V.; Polwiang, N.; Chachiyo, S. In vivo antimalarial activity of Annona muricata leaf extract in mice infected with Plasmodium berghei. J. Pathog. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Meira, C.S.; Guimaraes, E.T.; Macedo, T.S.; da Silva, T.B.; Menezes, L.R.A.; Costa, E.V.; Soares, M.B.P. Chemical composition of essential oils from Annona vepretorum Mart. and Annona squamosa L. (Annonaceae) leaves and their antimalarial and trypanocidal activities. J. Essent. Oil Res. 2015, 27, 160–168. [Google Scholar] [CrossRef]
- Kamaraj, C.; Bagavan, A.; Elango, G.; Zahir, A.A.; Rajakumar, G.; Marimuthu, S.; Santhoshkumar, T.; Abdul Rahuman, A. Larvicidal activity of medicinal plant extracts against Anopheles subpictus & Culex tritaeniorhynchus. Indian J. Med. Res. 2011, 134, 101–106. [Google Scholar]
- Kihampa, C.; Joseph, C.C.; Nkunya, M.H.H.; Magesa, S.M.; Hassanali, A.; Heydenreich, M.; Kleinpeter, E. Larvicidal and IGR activity of extract of Tanzanian plants against malaria vector mosquitoes. J. Vector Borne Dis. 2009, 46, 145–152. [Google Scholar]
- Osorio, E.; Arango, G.J.; Jimenez, N.; Alzate, F.; Ruiz, G.; Gutierrez, D.; Paco, M.A.; Gimenez, A.; Robledo, S. Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J. Ethnopharmacol 2007, 111, 630–635. [Google Scholar] [CrossRef]
- Yamthe, L.R.T.; Fokou, P.V.T.; Mbouna, C.D.J.; Keumoe, R.; Ndjakou, B.L.; Djouonzo, P.T.; Mfopa, A.N.; Legac, J.; Tsabang, N.; Gut, J.; et al. Extracts from Annona muricata L. and Annona reticulata L. (Annonaceae) potently and selectively inhibit Plasmodium falciparum. Medicines 2015, 2, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Garcia Diaz, J.; Tuenter, E.; Escalona Arranz, J.C.; Llaurado Maury, G.; Cos, P.; Pieters, L. Antimicrobial activity of leaf extracts and isolated constituents of Croton linearis. J. Ethnopharmacol. 2019, 236, 250–257. [Google Scholar] [CrossRef]
- Mollataghi, A.; Coudiere, E.; Hadi, A.H.A.; Mukhtar, M.R.; Awang, K.; Litaudon, M.; Ata, A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia 2012, 83, 298–302. [Google Scholar] [CrossRef]
- de Lima, J.P.S.; Pinheiro, M.L.B.; Santos, A.M.G.; Pereira, J.L.S.; Santos, D.M.F.; Barison, A.; Silva-Jardim, I.; Costa, E. V In vitro antileishmanial and cytotoxic activities of Annona mucosa (Annonaceae). Rev. Virtual Quim. 2012, 4, 692–702. [Google Scholar]
- de Omena, M.C.; Navarro, D.M.A.F.; de Paula, J.E.; Luna, J.S.; Ferreira de Lima, M.R.; Sant’Ana, A.E.G. Larvicidal activities against Aedes aegypti of some Brazilian medicinal plants. Bioresour. Technol. 2007, 98, 2549–2556. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; De Paula, J.E.; Degallier, N.; Molez, J.E.; Espindola, L.S. Larvicidal activity of some Cerrado plant extracts against Aedes aegypti. J. Am. Mosq Control. Assoc. 2006, 22, 314–317. [Google Scholar] [CrossRef]
- Hoe, P.K.; Yiu, P.H.; Eea, G.C.L.; Wong, S.C.; Rajan, A.; Bong, C.F.J. Biological Activity of Annona muricata Seed Extracts. Malaysian J. Sci. 2010, 29, 153–159. [Google Scholar] [CrossRef]
- Das, N.G.; Goswami, D.; Rabha, B. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J. Vector Borne Dis 2007, 44, 145–148. [Google Scholar]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.-H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lu, W.; Zhou, X. Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: Persimmon, guava, and sweetsop. Biomed. Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.-S.; Jong, T.-T.; Tien, H.-J.; Kuoh, C.-S.; Furukawa, H.; Lee, K.-H. Annoquinone-A, an antimicrobial and cytotoxic principle from Annona montana. Phytochemistry 1987, 26, 1623–1625. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Pereira, C.R.; Pimenta, L.P.S.; Boaventura, M.A.D.; Silva, L.G.F.E. Antibacterial activity of eight Brazilian annonaceae plants. Nat. Prod. Res. 2006, 20, 21–26. [Google Scholar] [CrossRef]
- Bories, C.; Loiseau, P.; Cortes, D.; Myint, S.H.; Hocquemiller, R.; Gayral, P.; Cave, A.; Laurens, A. Antiparasitic activity of Annona muricata and Annona cherimolia seeds. Planta Med. 1991, 57, 434–436. [Google Scholar] [CrossRef]
- Bento, E.B.; Matias, E.F.F.; Brito, F.E.; Oliveira, D.R.; Coutinho, H.D.M.; Costa, J.G.M.; Kerntopf, M.R.; Menezes, I.R.A. Association between food and drugs: Antimicrobial and synergistic activity of Annona muricata L. Int. J. Food Prop. 2012, 16, 738–744. [Google Scholar] [CrossRef]
- Tsobou, R.; Mapongmetsem, P.-M.; Voukeng, K.I.; Van Damme, P. Phytochemical screening and antibacterial activity of medicinal plants used to treat typhoid fever in Bamboutos division, West Cameroon. J. Appl. Pharm. Sci. 2015, 5, 34–49. [Google Scholar]
- Dzotam, J.K.; Touani, F.K.; Kuete, V. Antibacterial activities of the methanol extracts of Canarium schweinfurthii and four other Cameroonian dietary plants against multi-drug resistant Gram-negative bacteria. Saudi J. Biol. Sci. 2016, 23, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Essama, S.H.R.; Nyegue, M.A.; Foe, C.N.; Silihe, K.K.; Tamo, S.P.B.; Etoa, F.X. Antibacterial and antioxidant activities of hydro-ehanol extracts of barks, leaves and stems of Annona muricata. Am. J. Pharmacol. Sci. 2015, 3, 126–131. [Google Scholar]
- Darji, B.; Ratani, J.; Doshi, M.; Kothari, V. In vitro antimicrobial activity in certain plant products/seed extracts against selected phytopathogens. Res. Pharm. 2012, 2, 1–10. [Google Scholar]
- Mohamad, N.; Majid, E.-M.; Falah, A.; Layla, C.; Akram, H.; Ali, C.; Hassan, R. Antibacterial, antioxidant and antiproliferative activities of the hydroalcoholic extract of the Lebanese Annona squamosa L. seeds. Int. Res. J. Pharm. 2017, 8, 1–7. [Google Scholar] [CrossRef]
- More, G.; Tshikalange, T.E.; Lall, N.; Botha, F.; Meyer, J.J.M. Antimicrobial activity of medicinal plants against oral microorganisms. J. Ethnopharmacol 2008, 119, 473–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.V.; da Cruz, P.E.O.; de Lourenço, C.C.; de Souza Moraes, V.R.; de Lima Nogueira, P.C.; Salvador, M.J. Antioxidant and antimicrobial activities of aporphinoids and other alkaloids from the bark of Annona salzmannii A. DC. (Annonaceae). Nat. Prod. Res. 2012, 27, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Bettarini, F.; Borgonovi, G.E.; Fiorani, T.; Gagliardi, I.; Caprioli, V.; Massardo, P.; Ogoche, J.I.J.; Hassanali, A.; Nyandat, E.; Chapya, A. Antiparasitic compounds from East African plants: Isolation and biological activity of anonaine, matricarianol, canthin-6-one and caryophyllene oxide. Insect Sci. Its Appl. 1993, 14, 93–99. [Google Scholar] [CrossRef]
- Rao, G.-X.; Zhang, S.; Wang, H.-M.; Li, Z.-M.; Gao, S.; Xu, G.-L. Antifungal alkaloids from the fresh rattan stem of Fibraurea recisa Pierre. J. Ethnopharmacol. 2009, 123, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Du, F.; Yan, L.; He, G.; He, J.; Wang, C.; Rao, G.; Jiang, Y.; Xu, G. Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules 2015, 20, 17913–17928. [Google Scholar] [CrossRef] [Green Version]
- Morteza-Semnani, K.; Amin, G.; Shidfar, M.R.; Hadizadeh, H.; Shafiee, A. Antifungal activity of the methanolic extract and alkaloids of Glaucium oxylobum. Fitoterapia 2003, 74, 493–496. [Google Scholar] [CrossRef]
- Wu, C.-C.; Wu, C.-L.; Huang, S.-L.; Chang, H.-T. Antifungal activity of Liriodenine from Michelia formosana heartwood against wood-rotting fungi. Wood Sci. Technol. 2012, 46, 737–747. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, J.; Liu, X.; Qiu, J.; Min, H.; Zheng, R.; Xu, H.; Li, H.; Zhan, R.; Chen, W. Antibacterial constituents from roots of Zanthoxylum nitidum. Zhongcaoyao 2013, 44, 1546–1551. [Google Scholar]
- Li, C.; Lee, D.; Graf, T.N.; Phifer, S.S.; Nakanishi, Y.; Riswan, S.; Setyowati, F.M.; Saribi, A.M.; Soejarto, D.D.; Farnsworth, N.R.; et al. Bioactive constituents of the stem bark of Mitrephora glabra. J. Nat. Prod. 2009, 72, 1949–1953. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, S.R.M.; Mohamed, G.A.; Zayed, M.F.; Sayed, H.M. Ingenines A and B, Two new alkaloids from the Indonesian sponge Acanthostrongylophora ingens. Drug Res. 2015, 65, 361–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, E.V.; Marques, F. de A.; Pinheiro, M.L.B.; Braga, R.M.; Delarmelina, C.; Duarte, M.C.T.; Ruiz, A.L.T.G.; Ernesto de Carvalho, J.; Maia, B.H.L.N.S. Chemical constituents isolated from the bark of Guatteria blepharophylla (Annonaceae) and their antiproliferative and antimicrobial activities. J. Braz. Chem. Soc. 2011, 22, 1111–1117. [Google Scholar]
- Liu, C.-M.; Kao, C.-L.; Wu, H.-M.; Li, W.-J.; Huang, C.-T.; Li, H.-T.; Chen, C.-Y. Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. Molecules 2014, 19, 17829–17838. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Liu, T.-Z.; Tseng, W.-C.; Lu, F.-J.; Hung, R.-P.; Chen, C.-H.; Chen, C.-H. (-)-Anonaine induces apoptosis through Bax- and caspase-dependent pathways in human cervical cancer (HeLa) cells. Food Chem. Toxicol. 2008, 46, 2694–2702. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Ebel, R.; Ebel, R.; Proksch, P. Acanthomine A, a new pyrimidine-β-carboline alkaloid from the sponge Acanthostrongylophora ingens. Nat. Prod. Commun. 2008, 3, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, T.-J.; Chang, F.-R.; Chia, Y.-C.; Chen, C.-Y.; Lin, H.-C.; Chiu, H.-F.; Wu, Y.-C. The alkaloids of Artabotrys uncinatus. J. Nat. Prod. 2001, 64, 1157–1161. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Chang, F.-R.; Chia, Y.-C.; Chen, C.-Y.; Chiu, H.-F.; Wu, Y.-C. Cytotoxic Constituents of the Fruits of Cananga odorata. J. Nat. Prod. 2001, 64, 616–619. [Google Scholar] [CrossRef]
- Nordin, N.; Majid, N.A.; Mohan, S.; Dehghan, F.; Karimian, H.; Rahman, M.A.; Ali, H.M.; Hashim, N.M. Cleistopholine isolated from Enicosanthellum pulchrum exhibits apoptogenic properties in human ovarian cancer cells. Phytomedicine 2016, 23, 406–416. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.-Y.; Han, C.-R.; Yuan, Y.; Yang, B.; Zhang, Y.; Wang, J.; Zhong, X.-Q.; Huang, X. Two novel alkaloids from the stem of Saprosma hainanense and their cytotoxic activities in vitro. Chem. Pharm. Bull. 2011, 59, 338–340. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; He, Q.; Deng, Z.; Geng, Z.; Jiang, H.; Zhang, W.; Yang, K.; Du, S.; Wang, C.; Fan, L.; et al. Cytotoxicity of Aporphine, Protoberberine, and Protopine Alkaloids from Dicranostigma leptopodum (Maxim.) Fedde. Evid. Based. Complement. Alternat. Med. 2014, 2014, 1–6. [Google Scholar]
- Del Rayo Camacho, M.; Kirby, G.C.; Warhurst, D.C.; Croft, S.L.; Phillipson, J.D. Oxoaporphine alkaloids and quinones from Stephania dinklagei and evaluation of their antiprotozoal activities. Planta Med. 2000, 66, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Goeren, A.C.; Zhou, B.; Kingston, D.G.I. Cytotoxic and DNA damaging activity of some aporphine alkaloids from Stephania dinklagei. Planta Med. 2003, 69, 867–868. [Google Scholar]
- Rong, L.; Hu, D.; Wang, W.; Zhao, R.; Xu, X.; Jing, W. Alkaloids from root tubers of Stephania kwangsiensis H.S.Lo and their effects on proliferation and apoptosis of lung NCI-H446 cells. Biomed. Res. 2016, 27, 893–896. [Google Scholar]
- Chen, J.J.; Ishikawa, T.; Duh, C.Y.; Tsai, I.L.; Chen, I.S. New dimeric aporphine alkaloids and cytotoxic constituents of Hernandia nymphaefolia. Planta Med. 1996, 62, 528–533. [Google Scholar] [CrossRef]
- Soonthornchareonnon, N.; Suwanborirux, K.; Bavovada, R.; Patarapanich, C.; Cassady, J.M. New cytotoxic 1-azaanthraquinones and 3-aminonaphthoquinone from the stem bark of Goniothalamus marcanii. J. Nat. Prod. 1999, 62, 1390–1394. [Google Scholar] [CrossRef]
- Hoet, S.; Stevigny, C.; Block, S.; Opperdoes, F.; Colson, P.; Baldeyrou, B.; Lansiaux, A.; Bailly, C.; Quetin-Leclercq, J. Alkaloids from Cassytha filiformis and related aporphines: Antitrypanosomal activity, cytotoxicity, and interaction with DNA and topoisomerases. Planta Med. 2004, 70, 407–413. [Google Scholar]
- Hassan, E.M.; Hassan, R.A.; Salib, J.Y.; Mohamed, S.M.; El-Toumy, S.A. Chemical constituents and cytotoxic activity of Codiaeum variegatum CV. petra. J. Appl. Sci. Res. 2013, 9, 4884–4888. [Google Scholar]
- Kim, K.H.; Piao, C.J.; Choi, S.U.; Son, M.W.; Lee, K.R. New cytotoxic tetrahydroprotoberberine-aporphine dimeric and aporphine alkaloids from Corydalis turtschaninovii. Planta Med. 2010, 76, 1732–1738. [Google Scholar] [CrossRef] [Green Version]
- Demirgan, R.; Karagoz, A.; Pekmez, M.; Onay-Ucar, E.; Artun, F.T.; Gurer, C.; Mat, A. In vitro anticancer activity and cytotoxicity of some papaver alkaloids on cancer and normal cell lines. African J. Tradit. Complement. Altern. Med. 2016, 13, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.M.; Hassan, E.M.; Ibrahim, N.A. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat. Prod. Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef]
- Ubonopas, L.; Wongsinkongman, P.; Chuakul, W.; Suwanborirux, K.; Lee, K.H.; Soonthornchareonnon, N. Bioactive flavonoids and alkaloids from Anomianthus dulcis (Dunal) J. Sinclair stem bark. Mahidol Univ. J. Pharm. Sci. 2014, 41, 13–22. [Google Scholar]
- Pang, S.-Q.; Wang, G.-Q.; Lin, J.; Diao, Y.; Xu, R. Cytotoxic activity of the alkaloids from Broussonetia papyrifera fruits. Pharm. Biol. 2014, 52, 1315–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Lopa, S.S.; Sadik, G.; Harun-or-Rashid; Islam, R.; Khondkar, P.; Alam, A.H.M.K.; Rashid, M.A. Antibacterial and cytotoxic compounds from the bark of Cananga odorata. Fitoterapia 2005, 76, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Khamis, S.; Bibby, M.C.; Brown, J.E.; Cooper, P.A.; Scowen, I.; Wright, C.W. Phytochemistry and preliminary biological evaluation of Cyathostemma argenteum, a Malaysian plant used traditionally for the treatment of breast cancer. Phyther. Res. 2004, 18, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jian, L.; Chen, G.; Song, X.; Han, C.; Wang, J. Chemical constituents and in vitro anticancer cytotoxic activities of Polyalthia plagioneura. Chem. Nat. Compd. 2014, 49, 1172–1174. [Google Scholar] [CrossRef]
- Nordin, N.; Abdul Majid, N.; Hashim, N.M.; Abd Rahman, M.; Hassan, Z.; Ali, H.M. Liriodenine, an aporphine alkaloid from Enicosanthellum pulchrum, inhibits proliferation of human ovarian cancer cells through induction of apoptosis via the mitochondrial signaling pathway and blocking cell cycle progression. Drug Des. Devel. Ther. 2015, 9, 1437–1448. [Google Scholar]
- Macabeo, A.P.G.; Lopez, A.D.A.; Schmidt, S.; Heilmann, J.; Dahse, H.-M.; Alejandro, G.J.D.; Franzblau, S.G. Antitubercular and cytotoxic constituents from Goniothalamus gitingensis. Rec. Nat. Prod. 2014, 8, 41–45. [Google Scholar]
- Costa, E.V.; Pinheiro, M.L.B.; Maia, B.H.L.N.S.; Marques, F.A.; Ruiz, A.L.T.G.; Marchetti, G.M.; de Carvalho, J.E.; Soares, M.B.P.; Costa, C.O.S.; Galvao, A.F.C.; et al. 7,7-Dimethylaporphine and Other Alkaloids from the Bark of Guatteria friesiana. J. Nat. Prod. 2016, 79, 1524–1531. [Google Scholar] [CrossRef]
- Dong, X.; Mondranondra, I.O.; Che, C.T.; Fong, H.H.S.; Farnsworth, N.R. Kmeriol and other aromatic constituents of Kmeria duperreana. Pharm. Res. 1989, 6, 637–640. [Google Scholar] [CrossRef]
- Mondranondra, I.O.; Che, C.T.; Rimando, A.M.; Vajrodaya, S.; Fong, H.H.S.; Farnsworth, N.R. Sesquiterpene lactones and other constituents from a cytotoxic extract of Michelia floribunda. Pharm. Res. 1990, 7, 1269–1272. [Google Scholar] [CrossRef]
- Chan, Y.-Y.; Juang, S.-H.; Huang, G.-J.; Liao, Y.-R.; Chen, Y.-F.; Wu, C.-C.; Chang, H.-T.; Wu, T.-S. The constituents of Michelia compressa var. formosana and their bioactivities. Int. J. Mol. Sci. 2014, 15, 10926–10935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.-W.; Liu, C.-M.; Chung, M.-I.; Chen, C.-Y. Biofunctional constituents from Michelia compressa var. lanyuensis with anti-melanogenic properties. Molecules 2015, 20, 12166–12174. [Google Scholar] [CrossRef] [Green Version]
- Still, P.C.; Yi, B.; Gonzalez-Cestari, T.F.; Pan, L.; Pavlovicz, R.E.; Chai, H.-B.; Ninh, T.N.; Li, C.; Soejarto, D.D.; McKay, D.B.; et al. Alkaloids from Microcos paniculata with Cytotoxic and Nicotinic Receptor Antagonistic Activities. J. Nat. Prod. 2013, 76, 243–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thuy, T.T.T.; Quan, T.D.; Nguyen, T.H.A.; Sung, T.V. A new hydrochalcone from Miliusa sinensis. Nat. Prod. Res. 2011, 25, 1361–1365. [Google Scholar] [CrossRef]
- Chang, F.-R.; Hwang, T.-L.; Yang, Y.-L.; Li, C.-E.; Wu, C.-C.; Issa, H.H.; Hsieh, W.-B.; Wu, Y.-C. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med. 2006, 72, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Wirasathien, L.; Boonarkart, C.; Pengsuparp, T.; Suttisri, R. Biological activities of alkaloids from Pseuduvaria setosa. Pharm. Biol. 2006, 44, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Waechter, A.-I.; Cave, A.; Hocquemiller, R.; Bories, C.; Munoz, V.; Fournet, A. Antiprotozoal activity of aporphine alkaloids isolated from Unonopsis buchtienii (Annonaceae). Phyther. Res. 1999, 13, 175–177. [Google Scholar] [CrossRef]
- Zhao, L.-N.; Wang, J.; Wang, Z.; Tan, N.-H. Chemical and cytotoxic constituents of Zanthoxylum nitidum. Zhongguo Zhong Yao Za Zhi 2018, 43, 4659–4664. [Google Scholar]
- Yang, C.-H.; Cheng, M.-J.; Lee, S.-J.; Yang, C.-W.; Chang, H.-S.; Chen, I.-S. Secondary metabolites and cytotoxic activities from the stem bark of Zanthoxylum nitidum. Chem. Biodivers. 2009, 6, 846–857. [Google Scholar] [CrossRef]
- Amna, U.; Hasnan, M.H.H.; Ahmad, K.; Abdul Manaf, A.; Awang, K.; Nafiah, M.A. In vitro cytotoxic of aporphine and proaporphine alkaloids from phoebe grandis (Ness) merr. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 15–20. [Google Scholar]
- Chang, Y.-C.; Chang, F.-R.; Khalil, A.T.; Hsieh, P.-W.; Wu, Y.-C. Cytotoxic benzophenanthridine and benzylisoquinoline alkaloids from Argemone mexicana. Z. Naturforsch. C. 2003, 58, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahari, A.; Cheah, F.K.; Mohamad, J.; Sulaiman, S.N.; Litaudon, M.; Leong, K.H.; Awang, K. Antiplasmodial and antioxidant isoquinoline alkaloids from Dehaasia longipedicellata. Planta Med. 2014, 80, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chen, J.J.; Duh, C.Y.; Tsai, J.L.; Chang, C.T. New aporphine alkaloids and cytotoxic constituents of Hernandia nymphaefolia. Planta Med. 1997, 63, 154–157. [Google Scholar] [CrossRef] [PubMed]







| Plant Parts | Location | Isolated Alkaloids |
|---|---|---|
| Annona ambotay | ||
| Wood | Brazil | benzene-EtOH: cleistopholine 1, dielsinol 2, dielsiquinone 3, geovanine 4, kinabaline 5, 6-methoxyonychine 6, onychine 7 [25] |
| Annona cherimola | ||
| Leaves | Brazil | (−)-anonaine 8, (−)-asimilobine 9, lanuginosine 10, liriodenine 11, lysicamine 12, pronuciferine 13, (+)-stepharine 14 [26] |
| Leaves | India | Phanostenine 15 [27] |
| Leaves | Spain | (−)-anonaine 8, (+)-corytuberine 16, (+)-isoboldine 17, lanuginosine 10, liriodenine 11, (+)-nornantenine 18, (+)-reticuline 19, (−)-stepholidine 20 [28] |
| Seeds | Spain | (−)-anonaine 8, cleistopholine 1, lanuginosine 10, liriodenine or xoushinsunine 11 [29,30] |
| Stem | Taiwan | (+)-annocherine A 21, (+)-annocherine B 22, (−)-artabonatine B 23, cherianoine 24, cherimoline 25, (−)-romucosine H 26 [31,32] |
| Stem | Spain | (−)-anolobine 27, (+)-anonaine 28, (−)-asimilobine 9, (−)-corydine 29, (−)-corypalmine 30, (−)-discretamine 31, (+)-glaziovine 32, (+)-isoboldine 17, lanuginosine 10, liriodenine 11, lysicamine 12, (−)-N-methylasimilobine 33, (−)-norushinsunine 34, (−)-nuciferine 35, (−)-stepholidine 20, (−)-tetrahydropalmatine 36, (−)-xylopine 37, (+)-reticuline 19 [33] |
| Root | Mexico | (−)-corytenchine 38, (−)-isocoreximine 39 [34] |
| Annona diversifolia | ||
| Roots | Mexico | Liriodenine 11 [35] |
| Annona glabra | ||
| Fruit-stem | Taiwan | (−)-anonaine 8, annobraine 40, (−)-asimilobine 9, 1-aza-4-methyl-2-oxo-1,2-dihydro-9,10-anthracenedione 41, dehydrocorydalmine 42, (−)-N-formylanonaine 43, (−)-kikemanine 44, liriodenine 11, lysicamine 12, (−)-nornuciferine or (−)-N-methylasimilobine 33, (+)-nordomesticine 45, (+)-stepharine 14 [36] |
| Leaves | Mexico | (−)-anonaine 8, asimilobine 9, coreximine 46, (+)-reticuline 19 [37] |
| Leaves | Taiwan | (−)-N-methyl-actinodaphnine 47, (+)-reticuline 19 [38] |
| Root | Mexico | (−)-anonaine 8, (−)-asimilobine 9, (−)-coreximine 46, (−)-nornuciferine or (−)-N-methylasimilobine 33, (+)-reticuline 19 [37] |
| Stem | Mexico | (−)-anonaine 8, (−)-asimilobine 9, (−)-nornuciferine or (−)-N-methylasimilobine 33, (+)-reticuline 19 [37] |
| Stem | Taiwan | (−)-anolobine 27, (−)-anonaine 8, (−)-asimilobine 9, (+)-isoboldine 17, liriodenine (or oxoushinsunine) 11, (−)-N-nornuciferine 48, (−)-norushinsunine (or michelalbine) 34, (+)-reticuline 19, (−)-roemerine 49 [39,40] |
| Annona montana Macf (wild soursop) | ||
| Leaves | Taiwan | annolatine 50, annoretine 51, argentinine 52, liriodenine 11 [41] |
| Stem-Root bark | Guinea | Annomontine 53, (−)-anonaine 8, atherosperminine 54, (−)-asimilobine 9, (−)-coclaurine 55, (−)-coreximine 46, methoxyannomontine 56, oxoushinsunine or liriodenine 11, (+)-reticuline 19, (−)-xylopine 37 [42] |
| Stem bark | Japan | Annomontine 53 [43] |
| Annona muricata L. (soursop) | ||
| Leaves | Tanzania | (−)-anonaine 8, (−)-roemerine 49 [44] |
| Japan | (−)-anonaine 8, (−)-annonamine 57, (+)-O,O-dimethylcoclaurine 58, (+)-4′-O-methylcoclaurine 59, (+)-norcorydine 60 [45] | |
| Leaves | Guinea | (−)-anonaine 8, (−)-coclaurine 55, isolaureline 61, isoboldine 17, liriodenine 11, (+)-N-methylcoclaurine 62, norisolaurelin or (−)-xylopine 37, (−)-roemerine 49 [46,47] |
| Stem (bark) | Guinea | Anomurine 63, anomuricine 64, atherosperminine 54, (−)-coclaurine 55, (−)-coreximine 46, (+)-reticuline 19, (+)-stepharine 14 [48] |
| Roots | Indonesia | (−)-coclaurine 55, (+)-reticuline 19, argentinine 52, atherosperminine 54, (+)-xylopine 65 [18] |
| Annona paludosa Aubl. | ||
| Root bark | Guiena | (−)-anonaine 8, (−)-asimilobine 9, (−)-coreximine 46, dihydropalmatine 66, (+)-reticuline 19, (−)-scoulerine or (−)-discretamine 31, (−)-roemerine 49, (±)-tetrahydropalmatine 36 [49] |
| Annona reticulata | ||
| Leaves | Taiwan | (−)-asimilobine 9, (+)-corydine 67, (+)-glaucine 68, liriodenine 11, (+)-norcorydine 60, oxonantenine 69, oxoxylopine or lanuginosine 10, (−)-xylopine 37 [50] |
| Roots | Taiwan | (−)-aequaline or (−)-discretamine 31, (+/-)-annomontine 53, (−)-anonaine 8, (−)-asimilobine 9, (−)-3-hydroxynornuciferine 70, liriodenine 11, methoxyannomontine 56, (−)-michelalbine or (−)-norushinsunine 34, oxoushinsunine or liriodenine 11, (+)-reticuline 19 [51,52] |
| Annona salzmanii A. DC | ||
| Bark | Brazil | (−)-anonaine 8, (−)-asimilobine 9, cleistopholine 1, liriodenine 11, oxolaureline or 10-methoxyliriodenine 71, (+)- reticuline 19, (−)-xylopine 37 [16] |
| Annona sericea | ||
| Leaves | (−)-3-hydroxynornuciferine 70, (+)-isoboldine 17, (+)-N-methylcoclaurine 62, (+)-nornantenine 18, (−)-nornuciferine or (−)-N-methylasimilobine 33, oxonuciferine or lysicamine 12, (+)-reticuline 19 [53] | |
| Annona squamosa | ||
| Leaves | Brazil | (−)-anonaine 8, asimilobine 9, liriodenine 11, (−)-nornuciferine or (−)-N-methylasimilobine 33, (+)-reticuline 19 [54] |
| Leaves- stem bark | Guinea | (−)-anonaine 8, (+)-coclaurine 72, (+)-isoboldine 17, liriodenine 11, (+)-nornuciferine 73, (−)-roemerine 49 [55,56] |
| Leaves | India | (−)-anonaine 8, (+)-corydine 67, (+)-glaucine 68, (+)-isocorydine 74, lanuginosine 10, (+)-O-methylarmepavine 75, (+)-norcorydine 60, norisocorydine 76, (−)-roemerine 49, (−)-xylopine 7 [57,58] |
| Leaves | Tanzania | (−)-anonaine 8,(−)-roemerine 49 [44] |
| Leaves | Zimbabwe | (−)-isocorydine 77, (−)-roemerine 49 [59] |
| Seeds | Brazil | (−)-anonaine 8, asimilobine 9, corypalmine 30, (−)-nornuciferine or (−)-N-methylasimilobine 33, (+)-reticuline 19 [54] |
| Stem | Taiwan | Annobraine 40, annosqualine 78, demethylsonodione 79, dihydroferuloyltyramine 80, dihydrosinapoyltyramine 81, liriodenine 11, squamolone 82, thalifoline 83 [60] |
| Roots | Taiwan | (−)-anolobine 27, (−)-anonaine 8, (−)-norushinsunine (or michelalbine) 34, oxoushinsuine (liriodenine) 11, (+)-reticuline 19 [61] |
| Species | Part of Plant (Extract) | Anti-Protozoal Activity (IC50, µg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Leishmania species | Trypanosoma cruzi | P. falciparum | |||||
| PH8 | M2903 | PP75 | F32 | W2 | |||
| A. muricata | LF (Hexane) | 100.0 | >100.0 | >100.0 | 100.0 | 7.2 a | 38.6 a |
| LF (EtOAc) | 25.0 | 25.0 | 25.0 | 25.0 | 8.5 a | 10.4 a | |
| LF (MeOH) | >100.0 | >100 | >100.0 | 100.0 | 9.2 a | 36.8 a | |
| SD (Hexane) | 98.6 | 76.3 | 83.1 | 74.9 | 11.4 a | 38.2 a | |
| SD (EtOAc) | 63.2 | 63.2 | 63.2 | 63.2 | 40.2 a | 34.7 a | |
| SD (MeOH) | 98.6 | 98.6 | 98.6 | 98.6 | 32.5 a | 26.3 a | |
| PC (EtOH) | 1.01 | ||||||
| PC (H2O) | >10 | ||||||
| PC (CH2Cl2) | 0.94 | ||||||
| RT (EtOH) | 0.79 | ||||||
| RT (H2O) | >10 | ||||||
| RT (CH2Cl2) | 0.19 | ||||||
| ST(EtOH) | 1.45 | ||||||
| ST (H2O) | >10 | ||||||
| ST (CH2Cl2) | 3.32 | ||||||
| A. reticulata | LF(EtOH) | >10 | |||||
| LF (H2O) | >10 | ||||||
| LF (CH2Cl2) | >10 | ||||||
| TW (EtOH) | >10 | ||||||
| TW (H2O) | >10 | ||||||
| TW (CH2Cl2) | 0.88 | ||||||
| ST(EtOH) | 0.29 | ||||||
| ST (H2O) | >10 | ||||||
| ST (CH2Cl2) | 0.82 | ||||||
| RT (EtOH) | 1.90 | ||||||
| RT (H2O) | >10 | ||||||
| RT (CH2Cl2) | 0.38 | ||||||
| FR (EtOH) | 0.67 | ||||||
| RF (H2O) | >10 | ||||||
| RF (CH2Cl2) | 0.42 | ||||||
| Standard drug | Pentamidine | 10.0 | 10.0 | 10.0 | |||
| Amphotericin B | 0.2 | 0.2 | 0.2 | ||||
| Bensoidazole | 2.0 | ||||||
| Chloroquine | 0.01 | 0.9 | |||||
| Artemisisn | 0.005 | ||||||
| Plant Name | Plant Extract | LC50 (µg/mL) | |||
|---|---|---|---|---|---|
| Aedes aegypti | Aedes albopictus | Culex quinquefasciatus | Culex tritaeniorhynchus | ||
| A. crassiflora | SB (hexane) | 192.57 | |||
| RW (hexane) | 154.02 | ||||
| RB (hexane) | 264.15 | ||||
| RB (EtOH) | 0.71 | ||||
| RW (EtOH) | 8.94 | ||||
| ST (EtOH) | 16.1 | ||||
| A. glabra | SD (EtOH) | 0.06 | |||
| A. muricata | RT (EtOH) | 42.3 | |||
| SD (hexane) | 122.77 | ||||
| SD (CHCl3) | 0.90 | ||||
| SD (MeOH) | 85.91 | ||||
| LF (MeOH) | 56.47 | ||||
| A. senegalensis | LF (MeOH) | 23.42 | |||
| A. squamosal | RT (EtOH) | 31.9 | |||
| LF (EtOH) | 169 | 20.70 | |||
| SD (EtOH) | 5.12 | 6.96 | |||
| LF (MeOH) | 20.26 | 17.70 | |||
| SB (MeOH) | 104.94 | ||||
| Plant Name/Standards | Plant Extract | MIC (µg/mL) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ST | PA | KP | BC | EC | SA | PS | XC | AT | PM | PC | EH | TV | NB a | MD a | BC b | AN c | AI c | SM c | PI c | PG c | ||
| A. ambotay [80] | LF (EtOH) | 9 b | 10 b | |||||||||||||||||||
| A. cherimola [81] | SD (MeOH) | >100 | 15 | 5 | 8 | |||||||||||||||||
| A. cherimola [80] | LF (EtOH) | 11b | 14b | |||||||||||||||||||
| A. muricata [82,83,84] | LF (H2O) | 4096 | 1024 | 512 | >1024 | >1024 | ||||||||||||||||
| A. muricata [81] | SD (MeOH) | >100 | 30 | 26 | 25 | |||||||||||||||||
| A. muricata [85] | SB (EtOH) | 6.25 | 6.25 | 12.5 | ||||||||||||||||||
| A. muricata [80] | STm (EtOH) | |||||||||||||||||||||
| A. muricata [18] | RT (MeOH) | >32 | >32 | >32 | ||||||||||||||||||
| A. squamosa [86] | SD (EtOH) | >771 | >771 | >771 | >771 | >771 | ||||||||||||||||
| A. squamosa [86] | SD (Acetone) | >475 | >475 | >475 | >475 | >475 | ||||||||||||||||
| A. squamosa [87] | SD (MeOH) | 50 * | 50 * | 50 * | ||||||||||||||||||
| A. squamosa [78] | FR (MeOH) | 1250 * | 1250 * | 1250 * | ||||||||||||||||||
| A. Senegalensis [88] | BK (MeOH) | 4.5 | 5.0 | 3.0 | 2.5 | 6.5 | ||||||||||||||||
| Streptomycin | 10 | 10 | 20 | 10 | ||||||||||||||||||
| Chloramfenicol | RST | 30 | ||||||||||||||||||||
| Metronidazole | 1.25 | 2.5 | ||||||||||||||||||||
| Ivermictine | 0.8 | 1.3 | ||||||||||||||||||||
| Neomycin | 312.5 | 312.5 | ||||||||||||||||||||
| Gentamycin | 0.06 | 0.06 | 0.01 | 0.12 | ||||||||||||||||||
| Compound | MIC (µg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KZ | SA | Sap | SE | Sep | EF | EC | PA | CA | CP | CD | CDb | Fm | |
| A. salzmannii [89] | - | ||||||||||||
| Liriodenine 11 | - | >500 | >500 | 50 | 50 | - | - | - | - | - | 50 | 100 | - |
| Anonaine 8 | 50 | >500 | 50 | 25 | 50 | - | - | - | - | - | 50 | 50 | - |
| Asimilobine 9 | 50 | >500 | 50 | 50 | 50 | 100 | - | - | >500 | >500 | >500 | 50 | - |
| Reticuline 19 | 250 | >500 | >500 | 100 | 100 | 250 | - | - | 100 | 100 | >500 | >500 | - |
| Annona squamosal [90] | |||||||||||||
| (−)-(R)-anonaine 8 [90] | - | - | - | - | - | - | - | - | - | - | - | - | 30–39 * |
| Cleistopholine 1 | - | - | - | 250 | 250 | 250 | - | - | - | - | - | 250 | - |
| Chloramphenicol | 50 | 25 | 25 | 50 | 50 | 50 | 50 | 850 | 12.5 | 12.5 | 12.5 | 12.5 | - |
| Alkaloid | Plants | Part of Plant | Country | Anticancer Activity | Ref(s) |
|---|---|---|---|---|---|
| (−)-Anonaine 8 | Nelumbo nucifera Gaertn (Nelumbonaceae) | Leaves | Taiwan | Anti-proliferative effects with IC50 > 500 µM against AGS and 150.1 ± 0.3 µM against DU-145 | [99] |
| Michelia alba D.C. (Magnoliaceae) | Leaves | Taiwan | Inhibited viability of HeLa cancer cells (23 ± 1%) more effectively than non-cancer cells (Vero and MDCK cells, 75 ± 3% and 95 ± 4%, respectively) at concentration of 100 µM. | [100] | |
| Annomontine 53 | Acanthostrongylophora ingens (Petrosiidae) | Sponges | Indonesia | Pronounced cytotoxicity against L5178Y cell line with ED50 7.8 µg/mL compared to the positive control kahalalide F (ED50 6.3 μg/mL) | [97] |
| Acanthostrongylophora ingens (Petrosiidae) | Sponges | Indonesia | Pronounced cytotoxicity against L5178Y cell line with EC50 0.49 µg/mL | [101] | |
| Artabonatine B 23 | Artabotrys hexapetalus (L.f.) Bhandari (Annonaceae) | Roots, stems, and leaves | Taiwan | Active against both Hep G2 and 2,2,15 cell lines with IC50 9.1 and 11.0 µg/mL, respectively | [102] |
| (−)-Asimilobine 9 | Nelumbo nucifera Gaertn (Nelumbonaceae) | Leaves | Taiwan | Anti-proliferative effects against AGS and DU-145 cell lines with IC50 > 500 µM | [99] |
| Cleistopholine 1 | Cananga odorata (Lam.) Hook.f. & Thomson (Annonaceae) | Fruits | Taiwan | Displayed potent cytotoxicity against Hep G2 (human hepatoma cell) and Hep 2,2,15 (Hep G2 cell line transfected with hepatitis B virus) cell lines with IC50 value of 0.22 µg/mL and 0.54 µg/mL, respectively | [103] |
| Disepalum pulchrum (King) J.Sinclair (Enicosanthellum pulchrum, Annonaceae) | Roots | Malaysia | Active against CAOV-3 and SKOV-3 with IC50 value of 61.4 μM and 67.3 μM, respectively. This was comparable with that of the positive control cisplatin (62.8 μM and 67.1 μM) at 24 h of treatment. Cleistopholine (1) at >200 μM showed less cytotoxic effect against normal ovarian cells (SV40). | [104] | |
| Saprosma hainanense Merr. (Rubiaceae) | Stems | China | Inactive against against BEL-7402, SGC-7901, and K-562 cell lines | [105] | |
| (−)-Corydine 29 | Dicranostigma leptopodum (Maxim.) Fedde (Papaveraceae) | Whole plant | China | Showed its cytotoxicity against H1299, MCF-7, and SMCC-7721 with IC50 > 100 µM | [106] |
| Stephania dinklagei (Engl.) Diels (Menispermaceae) | Aerial parts | Ghana | Exhibited cytotoxic activity against KB cell line with IC50 733 μM | [107] | |
| (−)-Corydine 29 | Stephania dinklagei (Engl.) Diels (Menispermaceae) | Stem | Ghana | (−)-Corydine 29 showed DNA-damaging activity in the yeast bioassay (IC50 values YCp50 gal, pRAD52 GAL, Prad52 GLU were27.5, >73.9, and 22.5 μg/mL, respectively | [108] |
| Stephania kwangsiensis H.S. Lo. (Menispermaceae) | Root | India | Three different concentrations (20, 10, 5 µg/mL) could all significantly increase the apoptosis rate (8.77%, 9.12%, and 12.38%, respectively) of NCI-H446 cells after 48 h of treatment compared to the control group (1.02%). (−)-Corydine 29 can inhibit the proliferation of lung cancer NCI-H446 cells and induce their apoptosis | [109] | |
| Corytuberine 16 | Dicranostigma leptopodum (Maxim.) Fedde (Papaveraceae) | Whole plant | China | Cytotoxicity against H1299, MCF-7, and SMCC-7721 with IC50 value of 53.58 ± 5.47 µM, 72.30 ± 1.72 µM, and 73.22 ± 2.35 µM, respectively | [106] |
| Demethylsonodione 79 | Hernandia nymphaefolia (Presi) Kubitzk (Hernandiaceae) | Trunk bark | Taiwan | Exhibited cytotoxic activity against P-388, KB16, A549 (human lung adenocarcinoma), and HT-29 (human colon carcinoma cell lines with ED50 value of 0.766, 0.507, 0.223, and 0.772 µg/mL | [110] |
| Dielsiquinone 3 | Goniothalamus tamirensis Pierre ex Finet & Gagnep. (Annonaceae) | Stem bark | Thailand | Showed cytotoxic activity against A549, HT029, MCF7, RPMI and U251 with ED50 value of 0.11, 1.12, 0.11, 0.11 and 0.37 µM, respectively | [111] |
| Glaucine 68 | Cassytha filiformis L. (Lauraceae) | Whole plant | Benin | Active compound against HeLa cell line with IC50 value of 8.2 µM | [112] |
| Codiaeum variegatum (L.) Rumph. ex A.Juss. (Euphorbiaceae) | Leaves | Egypt | Showed cytotoxic activity against HepG2, MCF7, HCT116, and A549 cell lines with % of inhibition of cell viability of 38.4%, 46.3%, 66.8%, and 17.3%, respectively (at concentration of 100 µg/mL) | [113] | |
| Corydalis turtschaninovii Bess. (Papaveraceae) | Tuber | Korea | Showed cytotoxic activity against A549, SK-OV-3, SK-MEL-2 and HCT-15 cell lines with IC50 value of 26.76 ± 3.82, 21.57 ± 1.01, 20.39 ± 1.45 and 18.63 ± 4.15 µM, respectively | [114] | |
| Isocoreximine 39 | Guatteria blepharophylla Mart (Annonaceae) | Bark | Brazil | Showed anti-proliferative activity against UACC-62, MCF-7, NCI-H460, OVCAR-03, PC-3, HT-29, alagnd 786-0 with TGI value of >764.52 µM, and NCI-ADR/RES (TGI 131.50 µM). This compound showed selective activity for ovarian expressing phenotype for multiple drug resistance (NCI-ADR/RES) with a TGI value of 131.50 µM, but was less active than doxorubicin (TGI value of 14.80 µM) | [98] |
| (+)-Isocorydine 74 | Cassytha filiformis L. (Lauraceae) | Whole plant | Benin | Inactive against HeLa cell with IC50 > 80 µM | [112] |
| Papaver rhoeas L. Papaver rhopalothece Stapf, Papaver macrostomum Boiss. & A.Huet (Papaveraceae) | Aerial parts | Turkey | Nontoxic against normal Vero cell with IC50 value of >300 μg/mL | [115] | |
| Lanuginosine 10 | Magnolia grandiflora L. (Magnoliaceae) | Leaves | Egypt | Exhibited cytotoxicity against U251 and HEPG2 with IC50 value of 4 μg/mL and 2.5 μg/mL, respectively. Lanuginosine 10 was found to be inactive against the HeLa cancer cell. | [116] |
| Liriodenine 11 | Anomianthus dulcis (Dunal) J. Sinclair (Annonaceae) | Stem bark | Thailand | Exhibit the growth of NCIH187, BC, and KB cell lines with IC50 values at 1.02, 13.45 and 14.57 µg/mL, respectively | [117] |
| Broussonetia papyrifera (L.) L′Hér. ex Vent. (Moraceae) | Fruits | China | Exhibit strong cytotoxic effect against A375, BEL-7402, and HeLa cell lines with IC50 value of 5.38 ± 0.27, 6.61 ± 0.57, and 5.97 ± 0.39 µg/mL, respectively | [118] | |
| Cananga odorata (Lam.) Hook.f. & Thomson (Annonaceae) | Stem bark | Bangladesh | Show cytotoxic activity based on brine shrimp method with LC50 value of 4.89 μg/mL | [119] | |
| Cyathostemma argenteum Wild & R.B.Drumm (Vitaceae) | Roots | Malaysia | Found to be similarly and moderately cytotoxic against MCF-7 ADR MDA-MB435 and MT-1 cells lines with IC50 values of 15.6, 16.7, 6.4 and 18.2 µM, respectively | [120] | |
| Disepalum plagioneurum (Diels) D.M.Johnson (Syn Polyalthia plagioneura Diels, Annonaceae) | Stem | China | Cytotoxic activity against GSC-7901, K562, and SPCA-1 cell lines with IC50 value of of 3.87, 37.61, and 6.19 µM, respectively | [121] | |
| Disepalum pulchrum (King) J.Sinclair (syn Enicosanthellum pulchrum (King) Heusden, Annonaceae) | Root | Malaysia | Inhibited CAOV-3 cell growth with IC50 value of.3 ± 1.06 µM after 24 h of exposure. Exhibited less activity against SKOV-3 cells, with IC50 values of 68.0 ± 1.56 µM. | [122] | |
| Goniothalamus gitingensis Elmer (Annonaceae) | Leaves | Philippines | Effective antiproliferative effects against HUVEC and K-562 cell lines with GI50 value of 8.2 ± 0.3 and 6.1 ± 0.8, respectively. | [123] | |
| Liriodenine 11 | Guatteria aberrans Erkens & Maas (Syn Guatteria f riesiana (W.A. Rodrigues) Erkens & Maas, Annonaceae) | Stem bark | Brazil | Anticancer potent against B16-F10 (mouse melanoma), HepG2 (human hepatocellular carcinoma), HL-60 (human promyelocytic leukemia), and K562 (human chronic myelocytic leukemia) tumor cell lines with IC50 values of >10, 8.3, 5.5, and 5.0 μM for the respectively | [124] |
| Guatteria blepharophylla Mart. (Annonaceae) | Bark | Brazil | Showed anti-proliferative activity against UACC-62, MCF-7, NCI-H460, OVCAR-03, PC-3, HT-29, 786-0 and NCI-ADR/RES with TGI value of 63.02, 37.67, 87.41, 372.18, >909.09, >909.09, >909.09 and >909.09 µM, respectively. This compound undermined positive control doxorubicin against MCF-7 with TGI value of 46.04 µM. | [98] | |
| Magnolia duperreana Pierre (Syn Kmeria duperreana (Pierre) Dandy, Magnoliaceae) | Stem bark | Thailand | Found to be active against KB and P388 cell lines with ED50 value of of 1.7 and 0.8 µg/mL, respectively | [125] | |
| Magnolia floribunda (Finet & Gagnep.) Figlar (Syn Michelia floribunda Finet & Gagnep., Magnoliaceae) | Stem bark | Thailand | Indicated cytotoxic activity against KB and P388 cell lines with with ED50 value of <2.5 µg/mL | [126] | |
| Michelia compressa var. formosana (Magnoliaceae) | Heartwood | Taiwan | Exhibited powerful inhibitory activity against TW01, H226, Jurkat, A498, A549, and HT1080 carcinoma cell lines with IC50 value of were 8.99, 14.71, 15.7, 4.52, 8.82 and 9.75 μM, respectively | [127] | |
| Michelia compressa var. lanyuensis (Magnoliaceae) | Roots | Taiwan | Possessed cytotoxicity against B16F10 cells after 24 h treatment at high concentration (100 μM) with 80% of cell viability. | [128] | |
| Microcos paniculata L. (Malvaceae) | Branche | Vitenam | Showed low activity against HT-29 cancer cell line with IC50 values greater than 10 μM. | [129] | |
| Miliusa sinensis Finet & Gagnep. (Annonaceae) | Leaves and branches | Vietnam | Indicated a good activity against MCF-7, KB, LU and Hep-G2 cancer cell lines with IC50 value of 2.89, 2.30, 6.66 and 5.23 μg/mL, respectively | [130] | |
| Nelumbo nucifera Gaertn (Nelumbonaceae) | Leaves | Taiwan | Showed anti-proliferative effects against AGS and DU-145 cell lines with IC50 value of >500 and 95.4 ± 0.4 µM, respectively | [99] | |
| Polyalthia longifolia var. pendula (Annonaceae) | Bark | Taiwan | Showed activity against MCF-7 (breast cancer) and MDA-MB-231 cell line with IC50 value of 4.46 and 10.28 µg/mL, respectively | [131] | |
| Liriodenine 11 | Pseuduvaria setosa (King) J. Sinclair (Annonaceae) | Aerial part | Thailand | Strongly cytotoxic to KB and BC cell lines with IC50 2.4 µg/mL and 2.3 µg/mL, respectively | [132] |
| Saprosma hainanense Merr. (Rubiaceae) | Stems | China | Exhibit cytotoxic activities against BEL-7402, SGC-7901, and K-562 cell lines with IC50 value of 71.7, 33.7, and 197.7 µM, respectively | [105] | |
| Stephania dinklagei (Engl.) Diels (Menispermaceae) | Aerial parts | Ghana | Exhibit cytotoxic activity against KB cell line with IC50 value of 26.9 ± 2.4 μM | [107] | |
| Stephania dinklagei (Engl.) Diels (Menispermaceae) | Stem | Ghana | Showed DNA-damaging activity in the yeast bioassay against YCp50 gal, pRAD52 GAL, Prad52 GLU with IC50 value of 0.6, 1.5, and 0.5 μg/mL, respectively. | [108] | |
| Unonopsis guatterioides (A.DC.) R.E.Fr.(Sin Unonopsis buchtienii R. E.Fries, Annonaceae) | Stem | Bolivia | Possessed cytotoxic bioactivity against Vero cell line with IC50 value of 1 μg/mL | [133] | |
| Zanthoxylum nitidum (Roxb.) DC. (Rutaceae) | Stem bark | China | Exhibit cytotoxicity against three human cancer cell lines HT29, A549 and MDA-MB-231 with IC50 values of 9.12, 6.05, and 11.35 μM, respectively | [134] | |
| Zanthoxylum nitidum (Roxb.) DC. (Rutaceae) | Stem bark | Taiwan | Exhibit moderate cytotoxicity against MCF-7, NCI-H460, and SF-268 cancer cell lines with IC50 values of 3.19, 2.38, and 2.19, respectively. Liriodenine (11) was the most cytotoxic isolate in Zanthoxylum nitidum | [135] | |
| (+)-Nornuciferine 73 | Guatteria blepharophylla Mart (Annonaceae) | Stem bark | Brazil | Anti-proliferative activity against MCF-7 NCI-H460 PC-3 HT-29786-0 K562 and NCI-ADR/RES with TGI value of 215.58, 201.99, 542.38, 191.38, 615.23, 153.88 and 255.37 µM, respectively. | [98] |
| Nelumbo nucifera Gaertn (Nelumbonaceae) | Leaves | Taiwan | Anti-proliferative effects against AGS and DU-145 cell lines with IC50 value of >500 µM | [99] | |
| Phoebe grandis (Nees) Merr. (Lauraceae) | Leaves | Malaysia | Cytotoxic activity against NIH/3T3, HeLa and HL-60 with CD50 value of 17, 15 and 37 µg/mL, respectively. | [136] | |
| (+)-Reticuline 19 | Argemone Mexicana L. (Papaveraceae) | Whole plant | Taiwan | Cytotoxic effects against HONE-1 (96% of control) and NUGC (90% of control) at concentration of 150 µM | [137] |
| Dehaasia longipedicellata (Ridl.) Kosterm. (Lauraceae) | Stem bark | Malaysia | Cytotoxicity activities against A549 (IC50 > 200 µg/mL), A375 (IC50 97.600 µg/mL), and BxPC-3 (IC50 82.570 µg/mL) | [138] | |
| Hernandia nymphaefolia (Presi) Kubitzk (Hernandiaceae) | Trunk bark | Taiwan | Anticancer activity against P-388, KB16, A549 (human lung adenocarcinoma), and HT-29 (human colon carcinoma cell lines with ED50 > 50 µg/mL | [139] | |
| Roemerine 49 | Nelumbo nucifera Gaertn (Nelumbonaceae) | Leaves | Taiwan | Showed anti-proliferative effects against AGS and DU-145with IC50 value of >500 and 95.4 ± 0.4 µM, respectively | [99] |
| (−)-Stepholidine 20 | Polyalthia longifolia (Sonn.) Thwaites (Annonaceae) | Bark | Taiwan | Activity against MCF-7 (breast cancer) cell line with IC50 value of 16.56 µg/mL | [131] |
| Squamolone 82 | Artabotrys hexapetalus (L.f.) Bhandari (Syn Artabotrys uncinatus (Lam) Merr., Annonaceae) | Roots, stems, and leaves | Taiwan | Showed significant activity against Hep G2 and 2,2,15 cell lines with IC50 value of 2.8 and 1.6 µg/mL, respectively | [102] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities. Molecules 2019, 24, 4419. https://doi.org/10.3390/molecules24234419
Nugraha AS, Damayanti YD, Wangchuk P, Keller PA. Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities. Molecules. 2019; 24(23):4419. https://doi.org/10.3390/molecules24234419
Chicago/Turabian StyleNugraha, Ari Satia, Yuvita Dian Damayanti, Phurpa Wangchuk, and Paul A. Keller. 2019. "Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities" Molecules 24, no. 23: 4419. https://doi.org/10.3390/molecules24234419
APA StyleNugraha, A. S., Damayanti, Y. D., Wangchuk, P., & Keller, P. A. (2019). Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities. Molecules, 24(23), 4419. https://doi.org/10.3390/molecules24234419







