Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of the Extraction Mode on the Kinetics of Extraction and on the Global Extraction Yields Using Raw Dry Plants
2.2. Influence of the Plant Grinding on the Extraction Kinetics and on the Global Extraction Yield
2.3. Quantification of Total Polyphenol, Flavonoid, and Proanthocyanidin Contents. Comparison with Commercialized Standardized Extracts and Antioxidant Activity
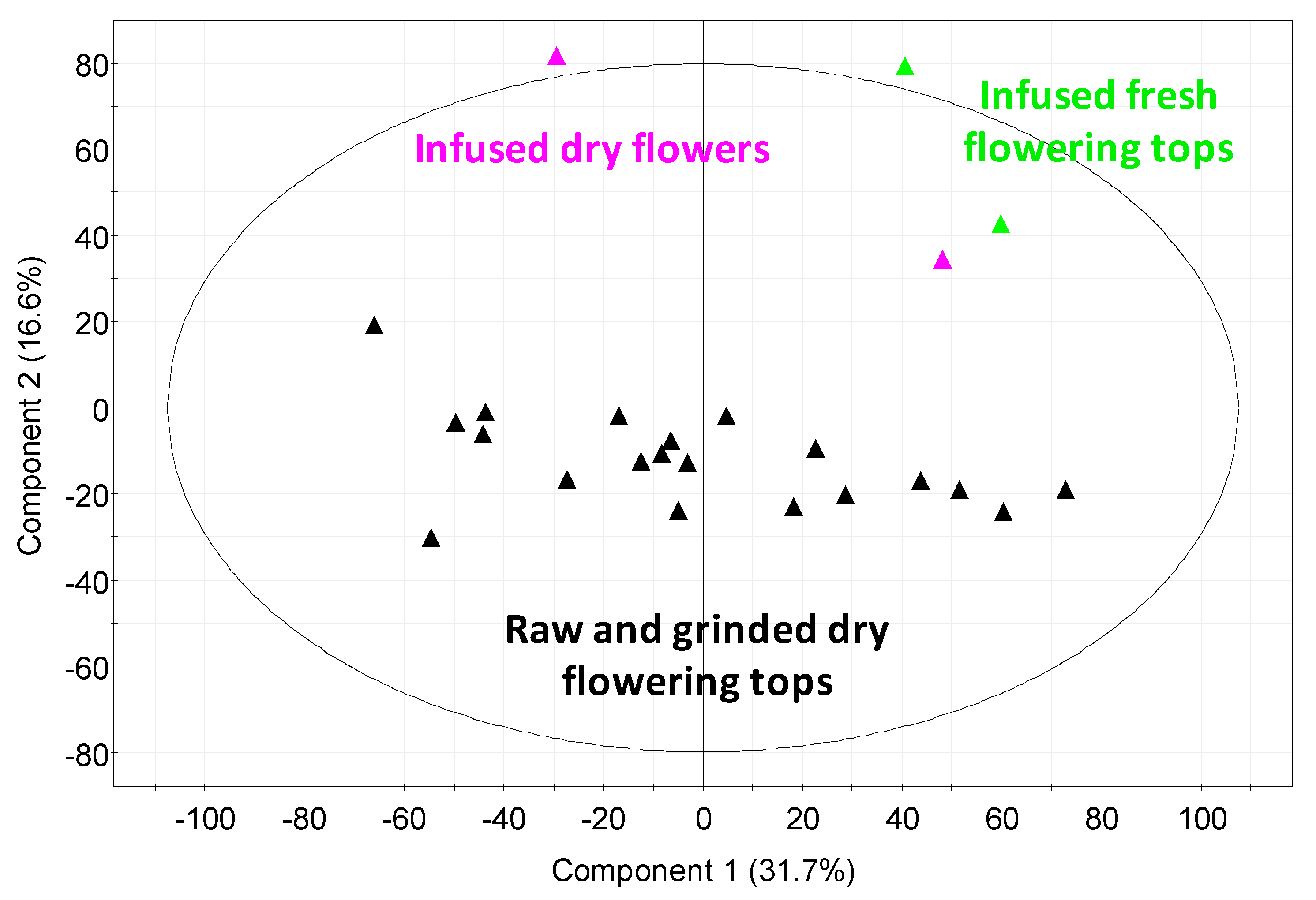
2.4. Quantification Influence of the Extraction Mode and the Nature/State of the Hawthorn on the Extracted UHPLC Profiles
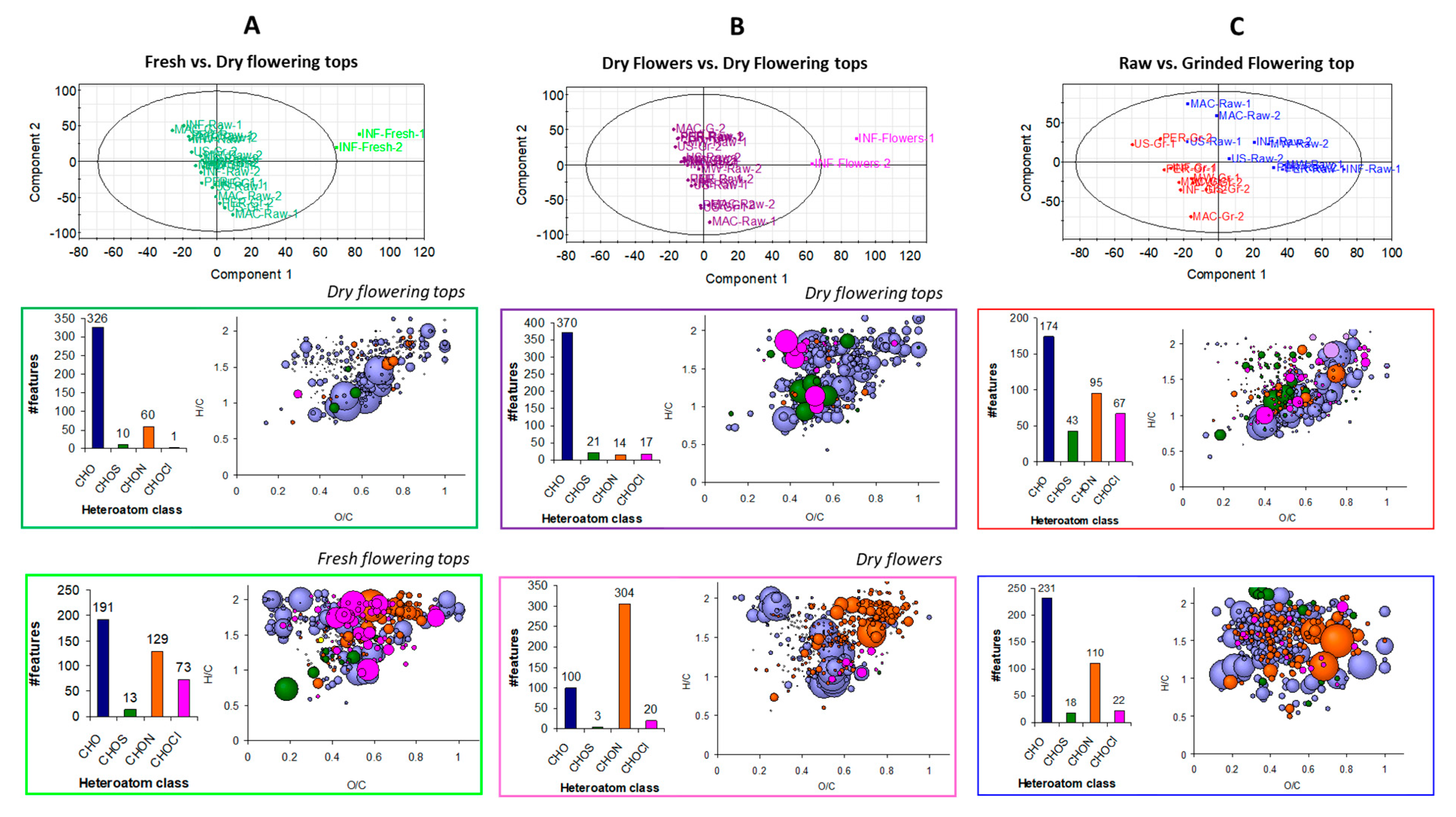
2.5. Influence of Extraction Mode and the Nature/State of the Hawthorn Studied by ESI FT-ICR-MS in Negative Mode
2.6. Optimization of the Homemade Infusion Protocol and Characterization of the Plant Granulometry
2.7. Variability between Hawthorn Lots
3. Materials and Methods
3.1. Chemicals
3.2. Ground Hawthorn, Density, and Granulometry
3.3. Infusion Extraction
3.4. Maceration Extraction
3.5. Ultrasound-Assisted Extraction
3.6. Microwave-Assisted Extraction
3.7. Percolation Extraction
3.8. Optimized Infusion Extraction
3.9. Kinetic Monitoring
3.10. Total Polyphenols Content (TPC)
3.11. Total Flavonoids Content (TFC)
3.12. Total Proanthocyanidin Oligomers Content (OPC)
3.13. UHPLC and UHPLC-ESI-MS Analysis
3.14. (−) ESI FT-ICR-MS Analysis
3.15. Anti-Oxidant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chikezie, P.C.; Ibegbulem, C.O.; Mbagwu, F.N. Bioactive principles from medicinal plants. Res. J. Phytochem. 2015, 9, 88–115. [Google Scholar] [CrossRef]
- Nirmal, S.A.; Pal, S.C.; Otimenyin, S.O.; Aye, T.; Elachouri, M.; Kundu, S.K.; Thandavarayan, R.A.; Mandal, S.C. Contribution of herbal products in global market. Pharma Rev. 2013, 95–104. [Google Scholar]
- Hariharan, P.; Subburaju, T. Medicinal Plants and Its Standardization—A global and industrial overview. Glob. J. Med. Plant Res. 2012, 1, 10–13. [Google Scholar]
- Islami, F.; Poustchi, H.; Pourshams, A.; Khoshnia, M.; Gharavi, A.; Kamangar, F.; Dawsey, S.M.; Abnet, C.C.; Brennan, P.; Sheikh, M.; et al. A prospective study of tea drinking temperature and risk of esophageal squamous cell carcinoma. Int. J. Cancer 2019. [Google Scholar] [CrossRef]
- Tai, W.P.; Nie, G.J.; Chen, M.J.; Yaz, T.Y.; Guli, A.; Wuxur, A.; Huang, Q.Q.; Lin, Z.G.; Wu, J. Hot food and beverage consumption and the risk of esophageal squamous cell carcinoma: A case-control study in a northwest area in China. Medicine 2017, 96, 50. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Habtemariam, S.; Ahmed, T.; Sureda, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.M. Polyphenolic composition of Crataegus monogyna jacq.: From chemistry to medical applications. Nutrients 2015, 7, 7708–7728. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Zuo, Z.; Harrison, F.; Sing, M.; Chow, S. Hawthorn. J. Clin. Pharmacol. 2002, 42, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Tan, D.; Liu, G. Hawthorn—A health food. Appl. Mech. Mater. 2012, 140, 350–354. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, V.; Bhat, Z.A.; Khan, N.A.; Prasad, D.N. The genus Crataegus: Chemical and pharmacological perspectives. Braz. J. Pharmacogn. 2012, 22, 1187–1200. [Google Scholar] [CrossRef]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Dordević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-inflammatory, gastroprotective, free-radical-scavenging, and antimicrobial activities of hawthorn berries ethanol extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlcek, J.; Balla, S.; Szekeres, L.; Adam, V.; Kizek, R. Polyphenolic profile and biological activity of Chinese hawthorn (Crataegus pinnatifida BUNGE) fruits. Molecules 2012, 17, 14490–14509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Peng, W.; Qin, R.; Zhou, H. Crataegus pinnatifida: Chemical constituents, pharmacology, and potential applications. Molecules 2014, 19, 1685–1712. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Dao, J.; Shao, Z.H. Hawthorn: Potential roles in cardiovascular disease. Am. J. Chin. Med. 2005, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holubarsch, C.J.F.; Colucci, W.S.; Eha, J. Benefit-risk assessment of Crataegus extract WS 1442: An evidence-based review. Am. J. Cardiovasc. Drugs 2018, 18, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Koch, E.; Malek, F.A. Standardized extracts from hawthorn leaves and flowers in the treatment of cardiovascular disorders preclinical and clinical studies. Planta Med. 2011, 77, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Degenring, F.H.; Suter, A.; Weber, M.; Saller, R. A randomised double blind placebo controlled clinical trial of a standardised extract of fresh Crataegus berries (Crataegisan®) in the treatment of patients with congestive heart failure NYHA II. Phytomedicine 2003, 10, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Tassell, M.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 2010, 4, 32–41. [Google Scholar]
- Verma, S.K.; Jain, V.; Khamesra, R. Crataegus Oxyacantha—A cardioprotective Herb. J. Herb. Med. Toxicol. 2007, 1, 65–71. [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). European Union Herbal Monograph on Crataegus Spp., Folium Cum Flore; Report No.: EMA/HMPC/159075/2014; European Medicines Agency: London, UK, 5 April 2016. [Google Scholar]
- Yang, B.; Liu, P.; Baoru Yang, P.L. Composition and health effects of phenolic compounds in hawthorn (Crataegus spp.) of different origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef]
- Schmidt, U.; Kuhn, U.; Ploch, M.; Hübner, W.D. Efficacy of the hawthorn (Crataegus) preparation LI 132 in 78 patients with chronic congestive heart failure defined as NYHA functional class II. Phytomedicine 1994, 1, 17–24. [Google Scholar] [CrossRef]
- Zick, S.M.; Gillespie, B.; Aaronson, K.D. The effect of Crataegus oxycantha special extract WS 1442 on clinical progression in patients with mild to moderate symptoms of heart failure. Eur. J. Heart Fail. 2008, 10, 587–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holubarsch, C.J.F.; Colucci, W.S.; Meinertz, T.; Gaus, W.; Tendera, M. Survival and prognosis: Investigation of Crataegus extract WS 1442 in congestive heart failure (SPICE)—Rationale, study design and study protocol. Eur. J. Heart Fail. 2000, 2, 431–437. [Google Scholar] [CrossRef]
- Bouaziz, A.; Khennouf, S.; Abdalla, S.; Djidel, S.; Abu Zarga, M.; Bentahar, A.; Dahamna, S.; Baghiani, A.; Amira, S. Phytochemical analysis, antioxidant activity and hypotensive effect of algerian azarole (Crataegus azarolus L.) leaves extracts. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 286–305. [Google Scholar]
- Bahorun, T.; Trotin, F.; Pommery, J.; Vasseur, J.; Pinkas, M. Antioxidant activities of Crataegus monogyna extracts. Planta Med. 1994, 60, 323–328. [Google Scholar] [CrossRef]
- Bahorun, T.; Aumjaud, E.; Ramphul, H.; Rycha, M.; Luximon-Ramma, A.; Trotin, F.; Aruoma, O.I. Phenolic constituents and antioxidant capacities of Crataegus monogyna (hawthorn) callus extracts. Food 2003, 47, 191–198. [Google Scholar] [CrossRef]
- Bahri-Sahloul, R.; Ammar, S.; Fredj, R.B.; Saguem, S.; Grec, S.; Trotin, F.; Skhiri, F.H. Polyphenol contents and antioxidant activities of extracts from flowers of two Crataegus azarolus L. varieties. Pak. J. Biol. Sci. 2009, 12, 660–668. [Google Scholar]
- Bernatoniene, J.; Masteikova, R.; Majiene, D.; Savickas, A.; Kevelaitis, E.; Bernatoniene, R.; Dvořáčkovâ, K.; Civinskiene, G.; Lekas, R.; Vitkevičius, K.; et al. Free radical-scavenging activities of Crataegus monogyna extracts. Medicina 2008, 44, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.L.; Chen, H.S.; Shen, Y.C.; Lai, G.H.; Lin, P.K.; Wang, C.M. Phytochemical composition, antioxidant activity and neuroprotective effect of Crataegus pinnatifida fruit. S. Afr. J. Bot. 2013, 88, 432–437. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, Y.; Gao, H.; Yuan, J.; Feng, F.; Cao, W.; Zheng, J. Protective effect of extract of Crataegus pinnatifida pollen on DNA damage response to oxidative stress. Food Chem. Toxicol. 2013, 59, 709–714. [Google Scholar] [CrossRef]
- Cui, T.; Nakamura, K.; Tian, S.; Kayahara, H.; Tian, Y.-L.L. Polyphenolic content and physiological activities of Chinese hawthorn extracts. Biosci. Biotechnol. Biochem. 2006, 70, 2948–2956. [Google Scholar] [CrossRef] [Green Version]
- Cui, T.; Li, J.Z.; Kayahara, H.; Ma, L.; Wu, L.X.; Nakamura, K. Quantification of the polyphenols and triterpene acids in Chinese hawthorn fruit by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 4574–4581. [Google Scholar] [CrossRef] [PubMed]
- Belšcak-Cvitanovic, A.; Durgo, K.; Bušić, A.; Franekić, J.; Komes, D. Phytochemical attributes of four conventionally extracted medicinal plants and cytotoxic evaluation of their extracts on human laryngeal carcinoma (HEp2) Cells. J. Med. Food 2014, 17, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Jia, Y.N.; Cui, T.Y.; Han, Z.; Qin, A.X.; Kang, X.H.; Pan, Y.L.; Cui, T. Quantification of ten polyphenols in the leaves of Chinese hawthorn (Crataegus pinnatifida bge. var. major N.E. BR.) by HPLC. Asian J. Chem. 2013, 25, 10344–10348. [Google Scholar] [CrossRef]
- Hamahameen, B.A.; Jamal, B. Determination of flavonoids in the leaves of hawthorn (Crataegus Azarolus) of Iraqi Kurdistan region by HPLC analysis. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 67–70. [Google Scholar]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, O.; Turkoglu, I. The investigation of some bioactive compounds and antioxidant properties of hawthorn (Crataegus monogyna subsp. monogyna jacq). J. Intercult. Ethnopharmacol. 2014, 3, 51. [Google Scholar] [CrossRef]
- Liu, T.; Cao, Y.; Zhao, M. Extraction optimization, purification and antioxidant activity of procyanidins from hawthorn (C. pinnatifida Bge. var. major) fruits. Food Chem. 2010, 119, 1656–1662. [Google Scholar] [CrossRef]
- Liu, P.; Yang, B.; Kallio, H. Characterization of phenolic compounds in Chinese hawthorn (Crataegus pinnatifida Bge. var. major) fruit by high performance liquid chromatography-electrospray ionization mass spectrometry. Food Chem. 2010, 121, 1188–1197. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Yang, B. Phenolic compounds in hawthorn (Crataegus grayana) fruits and leaves and changes during fruit ripening. J. Agric. Food Chem. 2011, 59, 11141–11149. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Lü, D.; Zhou, C.; Yang, B. Quantitative analysis of phenolic compounds in Chinese hawthorn (Crataegus spp.) fruits by high performance liquid chromatography-electrospray ionisation mass spectrometry. Food Chem. 2011, 127, 1370–1377. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Fan, Y.; Zhao, C.; Mao, X.; Chen, X.; Huang, H.; Gao, W. Effect of different solvents on the chemical composition, antioxidant activity and alpha-glucosidase inhibitory activity of hawthorn extracts. Int. J. Food Sci. Technol. 2016, 51, 1244–1251. [Google Scholar] [CrossRef]
- Orhan, I.; Özçelik, B.; Kartal, M.; Özdeveci, B.; Duman, H. HPLC quantification of vitexine-2″-O-rhamnoside and hyperoside in three Crataegus species and their antimicrobial and antiviral activities. Chromatographia 2007, 66, 153–157. [Google Scholar] [CrossRef]
- Badalica-Petrescu, M.; Dragan, S.; Ranga, F.; Fetea, F.; Socaciu, C. Comparative HPLC-DAD-ESI(+)MS fingerprint and quantification of phenolic and flavonoid composition of aqueous leaf extracts of Cornus mas and Crataegus monogyna, in relation to their cardiotonic potential. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Popovic-Milenkovic, M.T.; Tomovic, M.T.; Brankovic, S.R.; Ljujic, B.T.; Jankovic, S.M. Antioxidant and anxiolytic activities of Crataegus nigra wald. et kit. berries. Acta Pol. Pharm. Drug Res. 2014, 71, 279–285. [Google Scholar]
- Prinz, S.; Ring, A.; Huefner, A.; Pemp, E.; Kopp, B. 4‴-Acetylvitexin-2″-O-rhamnoside, isoorientin, orientin, and 8-methoxykaempferol-3-O-glucoside as markers for the differentiation of Crataegus monogyna and Crataegus pentagyna from Crataegus laevigata (Rosaceae). Chem. Biodivers. 2007, 4, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Quettier-Deleu, C.; Voiselle, G.; Fruchart, J.C.; Duriez, P.; Teissier, E.; Bailleul, F.; Vasseur, J.; Trotin, F. Hawthorn extracts inhibit LDL oxidation. Pharmazie 2003, 58, 577–581. [Google Scholar]
- Rezaei-Golmisheh, A.; Malekinejad, H.; Asri-Rezaei, S.; Farshid, A.A.; Akbari, P. Hawthorn ethanolic extracts with triterpenoids and flavonoids exert hepatoprotective effects and suppress the hypercholesterolemia-induced oxidative stress in rats. Iran. J. Basic Med. Sci. 2015, 18, 691–699. [Google Scholar]
- Rohr, G.E.; Meier, B.; Sticher, O. Quantitative reversed-phase high-performance liquid chromatography of procyanidins in Crataegus leaves and flowers. J. Chromatogr. A 1999, 835, 59–65. [Google Scholar] [CrossRef]
- Salmanian, S.; Sadeghi Mahoonak, A.R.; Alami, M.; Ghorbani, M. Phenolic content, antiradical, antioxidant, and antibacterial properties of hawthorn (Crataegus elbursensis) seed and pulp extract. J. Agric. Sci. Technol. 2014, 16, 343–354. [Google Scholar]
- Simirgiotis, M.J. Antioxidant capacity and HPLC-DAD-MS profiling of chilean peumo (Cryptocarya alba) fruits and comparison with german peumo (Crataegus monogyna) from Southern Chile. Molecules 2013, 18, 2061–2080. [Google Scholar] [CrossRef] [Green Version]
- Song, S.J.; Li, L.Z.; Gao, P.Y.; Yuan, Y.Q.; Wang, R.P.; Liu, K.C.; Peng, Y. Isolation of antithrombotic phenolic compounds from the leaves of Crataegus pinnatifida. Planta Med. 2012, 78, 1967–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierling, W.; Brand, N.; Gaedcke, F.; Sensch, K.H.; Schneider, E.; Scholz, M. Investigation of the pharmaceutical and pharmacological equivalence of different hawthorn extracts. Phytomedicine 2003, 10, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Guo, X.; Liu, R.H.; You, L.; Abbasi, A.M.; Fu, X. Phenolic contents and cellular antioxidant activity of Chinese hawthorn “Crataegus pinnatifida”. Food Chem. 2015, 186, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Guo, R.; You, L.; Abbasi, A.M.; Li, T.; Fu, X.; Liu, R.H.; You, L.; Fu, X. Major triterpenoids in Chinese hawthorn “Crataegus pinnatifida” and their effects on cell proliferation and apoptosis induction in MDA-MB-231 cancer cells. Food Chem. Toxicol. 2017, 100, 149–160. [Google Scholar] [CrossRef]
- Ying, X.; Wang, R.; Xu, J.; Zhang, W.; Li, H.; Zhang, C.; Li, F. HPLC determination of eight polyphenols in the leaves of Crataegus pinnatifida Bge. var. major. J. Chromatogr. Sci. 2009, 47, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chang, Q.; Zhu, M.; Huang, Y.; Ho, W.K.K.; Chen, Z.Y. Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, R.; Wang, L.; Yan, R.; Hou, R.; Gao, S.; Yang, B. Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major N.E.Br. fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2013, 148, 563–569. [Google Scholar] [CrossRef]
- Arslan, R.; Bor, Z.; Bektas, N.; Meriçli, A.H.; Ozturk, Y. Antithrombotic effects of ethanol extract of Crataegus orientalis in the carrageenan-induced mice tail thrombosis model. Thromb. Res. 2011, 127, 210–213. [Google Scholar] [CrossRef]
- Martino, E.; Collina, S.; Rossi, D.; Bazzoni, D.; Gaggeri, R.; Bracco, F.; Azzolina, O. Influence of the extraction mode on the yield of hyperoside, vitexin and vitexin-2-O-rhamnoside from Crataegus monogyna Jacq. (hawthorn). Phytochem. Anal. 2008, 19, 534–540. [Google Scholar] [CrossRef]
- Öztürk, N.; Tunçel, M. Assessment of phenolic acid content and in vitro antiradical characteristics of hawthorn. J. Med. Food 2011, 14, 664–669. [Google Scholar] [CrossRef]
- Chu, C.Y.; Lee, M.J.; Liao, C.L.; Lin, W.L.; Yin, Y.F.; Tseng, T.H. Inhibitory effect of hot-water extract from dried fruit of Crataegus pinnatifida on low-density lipoprotein (LDL) oxidation in cell and cell-free systems. J. Agric. Food Chem. 2003, 51, 7583–7588. [Google Scholar] [CrossRef] [PubMed]
- Predescu, N.C.; Papuc, C.; Nicorescu, V.; Gajaila, I.; Goran, G.V.; Petcu, C.D.; Stefan, G. The influence of solid-to-solvent ratio and extraction method on total phenolic content, flavonoid content and antioxidant properties of some ethanolic plant extracts. Rev. Chim. 2016, 67, 1922–1927. [Google Scholar]
- Sözer, U.; Dönmez, A.A.; Meriçli, A.H. Constituents from the leaves of Crataegus davisii Browicz. Sci. Pharm. 2006, 74, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.P.; Wang, X.T.; Chen, L.L.; Qi, J.; Xu, T.; Yu, B.Y. Quality and antioxidant activity detection of Crataegus leaves using on-line high-performance liquid chromatography with diode array detector coupled to chemiluminescence detection. Food Chem. 2010, 120, 929–933. [Google Scholar] [CrossRef]
- Rehwald, A.; Meier, B.; Sticher, O. Qualitative and quantitative reversed-phase high-performance liquid chromatography of flavonoids in Crataegus leaves and flowers. J. Chromatogr. A 1994, 677, 25–33. [Google Scholar] [CrossRef]
- Yin, J.; Qu, J.; Zhang, W.; Lu, D.; Gao, Y.; Ying, X.; Kang, T. Tissue distribution comparison between healthy and fatty liver rats after oral administration of hawthorn leaf extract. Biomed. Chromatogr. 2014, 28, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Liu, Y.; Kim, H.S. Hawthorn fruit extract elevates expression of Nrf2/HO-1 and improves lipid profiles in ovariectomized rats. Nutrients 2016, 8, 283. [Google Scholar] [CrossRef] [Green Version]
- Abuashwashi, M.A.; Palomino, O.M.; Gómez-Serranillos, M.P. Geographic origin influences the phenolic composition and antioxidant potential of wild Crataegus monogyna from Spain. Pharm. Biol. 2016, 54, 2708–2713. [Google Scholar] [CrossRef] [Green Version]
- Amanzadeh, Y.; Khanavi, M.; Khatamsaz, M.; Rajabi, A.; Ebrahimi, S.E.S. High-performance thin-Layer chromatographic fingerprints of flavonoids and phenol carboxylic acids for standardization of Iranian species of the genus Crataegus L. J. Pharm. Sci. 2007, 3, 143–152. [Google Scholar]
- Long, S.R.; Carey, R.A.; Crofoot, K.M.; Proteau, P.J.; Filtz, T.M. Effect of hawthorn (Crataegus oxycantha) crude extract and chromatographic fractions on multiple activities in a cultured cardiomyocyte assay. Phytomedicine 2006, 13, 643–650. [Google Scholar] [CrossRef]
- Refaat, A.T.; Shahat, A.A.; Ehsan, N.A.; Yassin, N.; Hammouda, F.; Tabl, E.A.; Ismail, S.I. Phytochemical and biological activities of Crataegus sinaica growing in Egypt. Asian Pac. J. Trop. Med. 2010, 3, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Belkhir, M.; Rebai, O.; Dhaouadi, K.; Congiu, F.; Tuberoso, C.I.G.; Amri, M.; Fattouch, S. Comparative analysis of Tunisian wild Crataegus azarolus (yellow azarole) and Crataegus monogyna (red azarole) leaf, fruit, and traditionally derived syrup: Phenolic profiles and antioxidant and antimicrobial activities of the aqueous-acetone extracts. J. Agric. Food Chem. 2013, 61, 9594–9601. [Google Scholar] [CrossRef] [PubMed]
- Froehlicher, T.; Hennebelle, T.; Martin-Nizard, F.; Cleenewerck, P.; Hilbert, J.L.; Trotin, F.; Grec, S. Phenolic profiles and antioxidative effects of hawthorn cell suspensions, fresh fruits, and medicinal dried parts. Food Chem. 2009, 115, 897–903. [Google Scholar] [CrossRef]
- Kwok, C.Y.; Li, C.; Cheng, H.L.; Ng, Y.F.; Chan, T.Y.; Kwan, Y.W.; Leung, G.P.H.; Lee, S.M.Y.; Mok, D.K.W.; Yu, P.H.F.; et al. Cholesterol lowering and vascular protective effects of ethanolic extract of dried fruit of Crataegus pinnatifida, hawthorn (Shan Zha), in diet-induced hypercholesterolaemic rat model. J. Funct. Foods 2013, 5, 1326–1335. [Google Scholar] [CrossRef]
- Shatoor, A.S. Cardio-tonic effect of the aqueous extract of whole plant of Crataegus aronia syn: Azarolus (L) on isolated rabbit’s heart. Afr. J. Pharm. Pharmacol. 2012, 6, 1901–1909. [Google Scholar]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef]
- Urbonavičiute, A.; Jakštas, V.; Kornyšova, O.; Janulis, V.; Maruška, A. Capillary electrophoretic analysis of flavonoids in single-styled hawthorn (Crataegus monogyna Jacq.) ethanolic extracts. J. Chromatogr. A 2006, 1112, 339–344. [Google Scholar] [CrossRef]
- Liu, P.; Kallio, H.; Lü, D.; Zhou, C.; Ou, S.; Yang, B. Acids, sugars, and sugar alcohols in Chinese hawthorn (Crataegus spp.) fruits. J. Agric. Food Chem. 2010, 58, 1012–1019. [Google Scholar] [CrossRef]
- Rabiei, K.; Bekhradnia, S.; Nabavi, S.M.; Nabavi, S.F.; Ebrahimzadeh, M.A. Antioxidant activity of polyphenol and ultrasonic extracts from fruits of Crataegus pentagyna subsp. elburensis. Nat. Prod. Res. 2012, 26, 2353–2357. [Google Scholar] [CrossRef]
- Pan, G.; Yu, G.; Zhu, C.; Qiao, J. Optimization of ultrasound-assisted extraction (UAE) of flavonoids compounds (FC) from hawthorn seed (HS). Ultrason. Sonochemistry 2012, 19, 486–490. [Google Scholar] [CrossRef]
- Svedström, U.; Vuorela, H.; Kostiainen, R.; Huovinen, K.; Laakso, I.; Hiltunen, R. HPLC determination of oligomeric procyanidins from dimers up to the hexamer in hawthorn. J. Chromatogr. A 2002, 968, 53–60. [Google Scholar] [CrossRef]
- Svedström, U.; Vuorela, H.; Kostiainen, R.; Tuominen, J.; Kokkonen, J.; Rauha, J.P.; Laakso, I.; Hiltunen, R. Isolation and identification of oligomeric procyanidins from Crataegus leaves and flowers. Phytochemistry 2002, 60, 821–825. [Google Scholar] [CrossRef]
- Keating, L.; Hayes, J.; Moane, S.; Lehane, M.; O’Doherty, S.; Kingston, R.; Furey, A. The effect of simulated gastro-intestinal conditions on the antioxidant activity of herbal preparations made from native Irish hawthorn. J. Herb. Med. 2014, 4, 127–133. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Portnaya, I.; Cogan, U.; Azaizeh, H.; Bomzon, A. Antioxidant activity of Crataegus aronia aqueous extract used in traditional Arab medicine in Israel. J. Ethnopharmacol. 2005, 101, 153–161. [Google Scholar] [CrossRef]
- Cheng, S.; Qiu, F.; Huang, J.; He, J. Simulataneous determination of vitexin-2″-O-glucoside, vitexin-2″-O-rhamnoside, rutin, and hyperoside in the extract of hawthorn (Crataegus pinnatifida Bge.) leaves by RP-HPLC with ultraviolet photodiode array detection. J. Sep. Sci. 2007, 30, 717–721. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Bahramian, F. Antioxidant activity of Crataegus pentaegyna subsp. elburensis fruits extracts used in traditional medicine in Iran. Pak. J. Biol. Sci. 2009, 12, 413–419. [Google Scholar] [CrossRef]
- Hamburger, M.; Baumann, D.; Adler, S. Supercritical carbon dioxide extraction of selected medicinal plants—Effects of high pressure and added ethanol on yield of extracted substances. Phytochem. Anal. 2004, 15, 46–54. [Google Scholar] [CrossRef]
- Shortle, E.; Kerry, J.; Furey, A.; Gilroy, D. Optimisation of process variables for antioxidant components from Crataegus monogyna by supercritical fluid extraction (CO2) using Box-Behnken experimental design. J. Supercrit. Fluids 2013, 81, 112–118. [Google Scholar] [CrossRef]
- Mudge, E.M.; Liu, Y.; Lund, J.A.; Brown, P.N. Single-laboratory validation for the determination of flavonoids in hawthorn leaves and finished products by LC-UV. Planta Med. 2016, 82, 1487–1492. [Google Scholar] [CrossRef]
- Hellenbrand, N.; Sendker, J.; Lechtenberg, M.; Petereit, F.; Hensel, A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of hawthorn (Crataegus spp.). Fitoterapia 2015, 104, 14–22. [Google Scholar] [CrossRef]
- Littleton, R.M.; Miller, M.; Hove, J.R. Whole plant based treatment of hypercholesterolemia with Crataegus laevigata in a zebrafish model. BMC Complement. Altern. Med. 2012, 12, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Chen, G.; Cui, T. Determination of flavones in Crataegus pinnatifida by capillary zone electrophoresis. J. Chromatogr. Sci. 2003, 41, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Comparing the composition and bioactivity of Crataegus monogyna flowers and fruits used in folk medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Garjani, A.; Nazemiyeh, H.; Maleki, N.; Valizadeh, H. Effects of extracts from flowering tops of Crataegus meyeri A. Pojark. on ischaemic arrhythmias in anaesthetized rats. Phyther. Res. 2000, 14, 428–431. [Google Scholar] [CrossRef]
- Włoch, A.; Kapusta, I.; Bielecki, K.; Oszmiański, J.; Kleszczyńska, H. Activity of hawthorn leaf and bark extracts in relation to biological membrane. J. Membr. Biol. 2013, 246, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhang, P.; Zhao, C.; Zhang, Y.; Liu, H.; Hu, L.; Gao, X.; Zhang, D. Prevention effect in selenite-induced cataract in vivo and antioxidative effects in vitro of Crataegus pinnatifida leaves. Biol. Trace Elem. Res. 2011, 142, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Roullier-Gall, C.; Witting, M.; Gougeon, R.D.; Schmitt-Kopplin, P. High precision mass measurements for wine metabolomics. Front. Chem. 2014, 2, 102. [Google Scholar] [CrossRef] [Green Version]
- Roullier-Gall, C.; Boutegrabet, L.; Gougeon, R.D.; Schmitt-Kopplin, P. A grape and winechemodiversity comparison of different appellations in Burgundy: Vintage vs terroir effects. Food Chem. 2014, 152, 100–107. [Google Scholar] [CrossRef]
- Condoret, J.S. Teaching transport phenomena around a cup of coffee. Chem. Eng. Educ. 2007, 41, 137–143. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–153. [Google Scholar]
- Lamaison, J.L.; Carnat, A. Teneurs en Principaux Flavonoides des fleurs et des feuilles de Crataegus monogyna Jacq et de Crataegus laevigata (Poiret) DC. (Rosaceae). Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Kanawati, B.; Bader, T.M.; Wanczek, K.-P.; Li, Y.; Schmitt-Kopplin, P. Fourier transform (FT)-artifacts and power-function resolution filter in Fourier transform mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Tziotis, D.; Hertkorn, N.; Schmitt-Kopplin, P. Kendrick-analogous network visualisation of ion cyclotron resonance Fourier transform mass spectra: Improved options for the assignment of elemental compositions and the classification of organic molecular complexity. Eur. J. Mass Spectrom. 2011, 17, 415–421. [Google Scholar] [CrossRef]
- Liu, P. Composition of Hawthorn (Crataegus spp.) Fruits and Leaves and Emblic Leaf Flower (Phyllanthus Emblica) Fruits. Ph.D. Thesis, University of Turku, Turku, Finland, 2012. [Google Scholar]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. An easy and fast test to compare total free radical scavenger capacity of foodstuffs. Phytochem. Anal. 2000, 11, 330–338. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour. Technol. 2007, 98, 2963–2967. [Google Scholar] [CrossRef]
- Tilburt, J.C.; Kaptchuk, T.J. Herbal medicine research and global health: An ethical analysis. Bull. World Health Organ. 2008, 86, 594–599. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2013, 4, 177. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of all plant extracts are available from the authors. |







| Extraction Mode | |||||
|---|---|---|---|---|---|
| Parameter of Extraction | Infusion | Maceration | Ultrasonic | Microwave | Percolation |
| Temperature (°C) | Decreasing from 90 upon time | 20, 40, 60, 80 | 20, 40, 60 | 96 (300 W) | 100 |
| Stirring speed (rpm) | 250, 500, 750, 1000 (Magnetic stirring) | 500 (magnetic stirring) | 250 (mechanical stirring) | No | No |
| Extraction time (min) | 5, 10, 30 | 5, 10, 30 | 5, 10, 30 | 5, 10, 30 | 5, 10 |
| Plant state | Fresh, fresh after 1 year, dry | fresh, dry | dry | dry | dry |
| Plant granulometry | Raw, ground (1 mm, 2 mm, coarse, fine, ultrafine 10″ and 30″) | raw, ground 1 mm | raw, ground 1 mm | raw, ground 1 mm | raw, ground 1 mm |
| Plant Nature & Granulometry | Extraction Mode | Experimental Conditions | t70% (min) | A(t) at 30 min | 10 min Extraction Time | 30 min Extraction Time | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extraction Yield (%) b | TPC c | TFC d | OPC e | Vitexin O-rhamnoside f | Extraction Yield (%) b | TPC c | TFC d | OPC e | |||||
| Flowering tops (Raw) | Infusion | 250 rpm | 9 | 1.41 | - | - | - | - | - | 14.29 ± 0.56 | - | - | - |
| 500 rpm | 8 | 1.51 | 15.64 ± 0.83 | 18.90 ± 1.72 | 2.33 ± 0.19 | 1.24 ± 0.10 | 33.56 ± 1.30 | 16.14 ± 0.45 | 18.78 ± 0.68 | 2.47 ± 0.07 | 1.70 ± 0.16 | ||
| 750 rpm | 6 | 1.47 | - | - | - | - | - | 15.03 ± 0.71 | - | - | - | ||
| 1000 rpm | 4 | 1.58 | - | - | - | - | - | 16.02 ± 0.89 | - | - | - | ||
| Maceration (at 500 rpm) | 20 °C | 12 | 0.66 | - | - | - | - | - | 9.75 ± 0.56 | - | - | - | |
| 40 °C | 12 | 0.80 | - | - | - | - | - | 11.87 ± 0.75 | - | - | - | ||
| 60 °C | 13 | 1.17 | 12.02 ± 0.81 | 12.57 ± 0.74 | 1.47 ± 0.15 | 1.06 ± 0.02 | 30.91 ± 2.13 | 14.18 ± 0.67 | 14.60 ± 1.67 | 1.86 ±0.18 | 1.10 ± 0.07 | ||
| 80 °C | 13 | 1.76 | - | - | - | - | - | 16.19 ± 1.07 | - | - | - | ||
| US (at 250 rpm) | 20 °C | 10 | 1.38 | - | - | - | - | - | 13.65 ± 1.22 | - | - | - | |
| 40 °C | 8 | 1.63 | - | - | - | - | - | 14.48 ± 1.19 | - | - | - | ||
| 60 °C | 8 | 2.14 a | 17.21 ± 0.53 | 21.17 ± 2.57 | 2.46 ± 0.03 | 2.37 ± 0.20 | 34.86 ± 0.78 | 20.78 ± 1.09 | 24.17 ± 0.57 | 2.75 ± 0.05 | 2.46 ± 0.06 | ||
| MW | 300 W | - | - | 17.30 ± 1.67 | 22.69 ± 1.30 | 2.80 ± 0.06 | 2.35 ± 0.20 | 38.50 ± 0.89 | 21.57 ± 0.20 | 30.14 ± 0.44 | 3.21 ± 0.17 | 3.82 ± 0.08 | |
| Percolation | - | - | - | 18.16 ± 1.34 | 23.87 ± 2.14 | 2.89 ± 0.12 | 3.01 ± 0.22 | 34.07 ± 2.70 | - | - | - | - | |
| Flowering tops (Ground 1 mm) | Infusion | 250 rpm | <1.5 | 2.27 a | - | - | - | - | - | - | - | - | - |
| 500 rpm | <1.5 | 2.41 a | 22.20 ± 0.59 | 32.79 ± 0.67 | 3.45 ± 0.20 | 3.93 ± 0.09 | 34.35 ± 0.48 | 23.19 ± 0.66 | 34.67 ± 0.93 | 3.56 ± 0.06 | 4.63 ± 0.39 | ||
| 750 rpm | <1.5 | 2.43 a | - | - | - | - | - | - | - | - | - | ||
| 1000 rpm | <1.5 | 2.52 a | - | - | - | - | - | - | - | - | - | ||
| Maceration (at 500 rpm) | 20 °C | 12 | 1.76 | - | - | - | - | - | 19.11 ± 0.35 | - | - | - | |
| 40 °C | 4.5 | 2.07 a | - | - | - | - | - | 19.20 ± 0.48 | - | - | - | ||
| 60 °C | <1.5 | 2.30 a | 20.59 ± 0.29 | 28.45 ± 0.26 | 3.12 ± 0.07 | 3.52 ± 0.14 | 33.85 ± 0.80 | 21.18 ± 1.29 | 30.28 ± 0.37 | 3.20 ± 0.07 | 3.78 ± 0.08 | ||
| 80 °C | <1.5 | 2.58 a | - | - | - | - | - | 23.30 ± 0.78 | - | - | - | ||
| US (at 250 rpm) | 20 °C | - | - | - | - | - | - | - | 14.28 ± 1.67 | - | - | - | |
| 40 °C | - | - | - | - | - | - | - | 17.50 ± 1.72 | - | - | - | ||
| 60 °C | <1.5 | 3.05 a | 24.24 ± 0.98 | 33.58 ± 1.23 | 3.64 ± 0.47 | 4.29 ± 0.10 | 32.67 ± 1.08 | 25.10 ± 1.86 | 33.26 ± 1.72 | 3.74 ± 0.17 | 4.32 ± 0.19 | ||
| MW | 300 W | - | - | 23.28 ± 0.42 | 34.73 ± 1.57 | 3.93 ± 0.17 | 4.04 ± 0.08 | 34.40 ± 0.49 | 23.81 ± 1.37 | 37.31 ± 0.87 | 3.85 ± 0.05 | 4.73 ± 0.21 | |
| Percolation | - | - | - | 18.98 ± 0.64 | 27.15 ± 1.78 | 2.95 ± 0.20 | 3.62 ± 0.13 | 34.59 ± 1.90 | - | - | - | - | |
| Flowering tops (Fresh) | Infusion | 500 rpm | 16.5 | 0.72 | 10.07 ± 1.02 | 8.28 ± 0.66 | 1.19 ± 0.05 | 0.41 ± 0.04 | 51.07 ± 4.09 | 12.27 ± 1.22 | 10.41 ± 0.66 | 1.60 ± 0.19 | 0.86 ± 0.08 |
| Maceration | 60 °C, 500 rpm | 13 | 0.58 | 6.69 ± 1.84 | 2.94 ± 1.38 | 0.63 ± 0.06 | 0.14 ± 0.04 | - | 11.09 ± 0.78 | 9.80 ± 0.89 | 1.35 ± 0.09 | 0.65 ± 0.05 | |
| Flowers only (Raw) | Infusion | 500 rpm | - | - | 10.18 ± 0.87 | 11.50 ± 0.32 | 1.66 ± 0.01 | 0.66 ± 0.05 | 21.55 ± 0.34 | - | - | - | - |
| Standardized Extracts | Uptake Quantity | Dry Extract Content | Dry Plant: Dry Extract Ratio | Plant Organ | Excipients | Extraction Solvent | TPC | TFC | OPC | Price (€) | Treatment Cost/Day (cents) | Cost/30 Days (€) a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPS Phyto-prevent® | 5–10 mL | 900 mg/5 mL | - | Leaves & flowers | Glycerol | - | - | 7.5–12.5 mg eq. Q/5 mL (0.8–1.4 %) | - | 19.89/150 mL | 66.3/5 mL | 19.9 |
| WS1442® crataegutt novo 450 (or Cardiplant® 450 or Cardio Max WS 1442®) | 1–2 tablets | 450 mg/1 tablet | 4.0–6.6:1 | Leaves & flowers | Glucose, SiO2, Fe2O3, TiO2, sucrose, gelatine, macrogol, citric acid, … | EtOH (45 %) | - | - | 78–90.6 mg eq. epiCAT/1 tablet (17.3–20.1 %) | 16.13/50 tablets | 64.5/2 tablets | 19.4 |
| Crataegisan ® Bioforce | 30–90 drops (0.75−2.25 mL) | 690 mg/90 drops | 3.2:1 | Fruits | EtOH (46−54%) | EtOH (50 %) | 12.7 mg eq. GA/90 drops (1.84 %) | - | 6.4 mg/90 drops (0.93%) | 10.50/50 mL | 52.5/90 drops | 15.8 |
| Faros 300® LI 132 | 3 Tablets | 300 mg/1 tablet | 4.0–7.0:1 | Leaves, flowers & fruits | Glucose, SiO2, lactose, TiO2, sucrose, gelatine, macrogol, … | MeOH (70 %) | - | 6.6 mg eq. HY/1 tablet (2.2 %) | - | 23.25/100 tablets | 69.7/3 tablets | 20.9 |
| Infusion (lot no. 20335) | 1–2 infusions | 555 mg/10 min infusion (from 2.5 g ground dry plant) | 4.3–4.6:1 | Leaves & flowers | Water | Water | 82 mg eq. GA/1 infusion | 8.6 mg eq. Q/1 infusion | 9.8 mg eq. CY/1 infusion | 29.25/1 kg | 7.3/1 infusion | 2.2 |
| Peak | Retention Time (min) | λmax (nm) | [M + H]+ | Other Ions in the Spectrum | Identified Compound | Standard Used for Identification | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 2.71 | 204, 218, 260 | 288 | Cyanidin | R | [40] | |
| 2 | 3.73 | 218, 236, 324 | 355 | 377, 711 | 5-O-Caffeoylquinic acid | R1 | [40] |
| 3 | 7.49 | 219, 238, 325 | 355 | 377, 711 | Chlorogenic acid (3-O-caffeoyquinic acid) | R1 | [40] |
| 4 | 9.1 | 227, 280 | 579 | 427, 289 | Procyanidin B2 | R | [40] |
| 5 | 9.45 | 224 279 | 291 | 147, 139, 123 | Epicatechin | R | [40] |
| 6 | 12.24 | 280 | 867 | 579 | Procyanidin C1 | R | [40] |
| 7 | 13.37 | 219, 280 | 1155 | 287, 413, 575 | Cinnamtannin A2 | R | [40] |
| 8 | 15.59 | 216, 269, 338 | 579 | 433, 313 | Vitexin 2-O-rhamnoside | R1 | [40] |
| 9 | 16.13 | 220, 256, 353 | 465 | 303 | Hyperoside | R1 | [40] |
| 10 | 16.35 | 219, 280 | 577 | 289 | Procyanidin A2 | R | [40] |
| 11 | 16.52 | 202, 257, 353 | 303 | 621 | Isoquercetin | R | [40] |
| 12 | 19.85 | 268, 337 | 433 | 621 | Apigenin-C-hexoside | R1 | [40] |
| Celia® Bag | Stirring | Lot Number | Plant Organs | Plant Material | Container | TPC mg eq. GA 🟀 | TFC mg eq. Q 🟀 | OPC mg eq. CY 🟀 | Extraction Yield (%) | Vitexin 2-O-rhamnoside (mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | 55849 | Flowering tops | Ground 1 mm | Cup | 26.6 ± 1.6 | 2.88 ± 0.17 | 2.24 ± 0.18 | 19.8 ± 0.9 | - |
| Mug | 33.5 ± 1.5 | 3.71 ± 0.19 | 3.40 ± 0.16 | 21.7 ± 2.0 | - | |||||
| Bowl | 35.9 ± 0.9 | 4.27 ± 0.19 | 3.77 ± 0.23 | 23.1 ± 0.2 | - | |||||
| Ground 2 mm | Cup | 26.9 ± 0.7 | 2.57 ± 0.10 | 2.23 ± 0.09 | 21.5 ± 0.1 | - | ||||
| Mug | 32.2 ± 0.4 | 3.42 ± 0.23 | 3.07 ± 0.02 | 24.1 ± 0.2 | - | |||||
| Bowl | 35.3 ± 1.2 | 3.92 ± 0.21 | 3.56 ± 0.22 | 24.2 ± 0.3 | - | |||||
| Yes | Yes | 55849 | Flowering tops | Ground 1 mm | Mug | 24.9 ± 0.2 | 3.01 ± 0.22 | 2.29 ± 0.05 | 16.9 ± 1.0 | - |
| Yes | Yes | CB58120 | Flowering tops | Raw | Mug | 13.6 ± 0.3 | 1.71 ± 0.02 | 0.70 ± 0.06 | 16.8 ± 0.1 | - |
| Fine | 20.6 ± 1.6 | 3.02 ± 0.11 | 1.72 ± 0.08 | 22.3 ± 0.5 | - | |||||
| Ultrafine 10″ | 10.2 ± 0.4 | 1.45 ± 0.11 | 0.73 ± 0.05 | 11.7 ± 0.3 | - | |||||
| No | Yes | CB58120 | Flowering tops | Fine | Bodum® | 21.6 ± 0.4 | 3.13 ± 0.10 | 1.81 ± 0.09 | 22.5 ± 0.3 | - |
| No | No * | CB58120 | Flowering tops | Fine | Bodum® | 20.1 ± 0.4 | 2.86 ± 0.02 | 1.81 ± 0.05 | 21.7 ± 0.1 | 25.9 ± 0.3 |
| Coarse | 21.8 ±0.1 | 2.46 ±0.15 | 1.64 ±0.07 | 21.1 ±0.4 | - | |||||
| Ultrafine 10″ | 21.3 ± 0.4 | 2.98 ± 0.16 | 1.85 ± 0.06 | 21.7 ± 0.3 | - | |||||
| H18001534 | Fine | 34.2 ± 1.8 | 3.67 ± 0.21 | 1.76 ± 0.05 | 22.4 ± 0.5 | 14.7 ± 0.3 | ||||
| 1221478 | Fine | 28.0 ± 1.3 | 3.66 ± 0.19 | 1.64 ± 0.06 | 22.0 ± 0.3 | 10.7 ± 0.9 | ||||
| R78925 | Fine | 23.8 ± 0.9 | 2.98 ± 0.15 | 1.21 ± 0.06 | 22.4 ± 0.1 | 25.0 ± 1.5 | ||||
| No | No * | 20334 | Flowers | Fine | Bodum® | 37.2 ± 0.7 | 3.46 ± 0.09 | 1.96 ± 0.05 | 21.7 ± 0.6 | 15.5 ± 0.5 |
| No | No * | - | Fresh (after 1 year) | Fine | Bodum® | 44.6 ± 1.3 | 4.06 ± 0.14 | 4.24 ± 0.17 | 27.8 ± 0.5 | 25.6 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngoc, P.C.; Leclercq, L.; Rossi, J.-C.; Desvignes, I.; Hertzog, J.; Fabiano-Tixier, A.-S.; Chemat, F.; Schmitt-Kopplin, P.; Cottet, H. Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations. Molecules 2019, 24, 4420. https://doi.org/10.3390/molecules24234420
Ngoc PC, Leclercq L, Rossi J-C, Desvignes I, Hertzog J, Fabiano-Tixier A-S, Chemat F, Schmitt-Kopplin P, Cottet H. Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations. Molecules. 2019; 24(23):4420. https://doi.org/10.3390/molecules24234420
Chicago/Turabian StyleNgoc, Phu Cao, Laurent Leclercq, Jean-Christophe Rossi, Isabelle Desvignes, Jasmine Hertzog, Anne-Sylvie Fabiano-Tixier, Farid Chemat, Philippe Schmitt-Kopplin, and Hervé Cottet. 2019. "Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations" Molecules 24, no. 23: 4420. https://doi.org/10.3390/molecules24234420
APA StyleNgoc, P. C., Leclercq, L., Rossi, J.-C., Desvignes, I., Hertzog, J., Fabiano-Tixier, A.-S., Chemat, F., Schmitt-Kopplin, P., & Cottet, H. (2019). Optimizing Water-Based Extraction of Bioactive Principles of Hawthorn: From Experimental Laboratory Research to Homemade Preparations. Molecules, 24(23), 4420. https://doi.org/10.3390/molecules24234420