Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications
Abstract
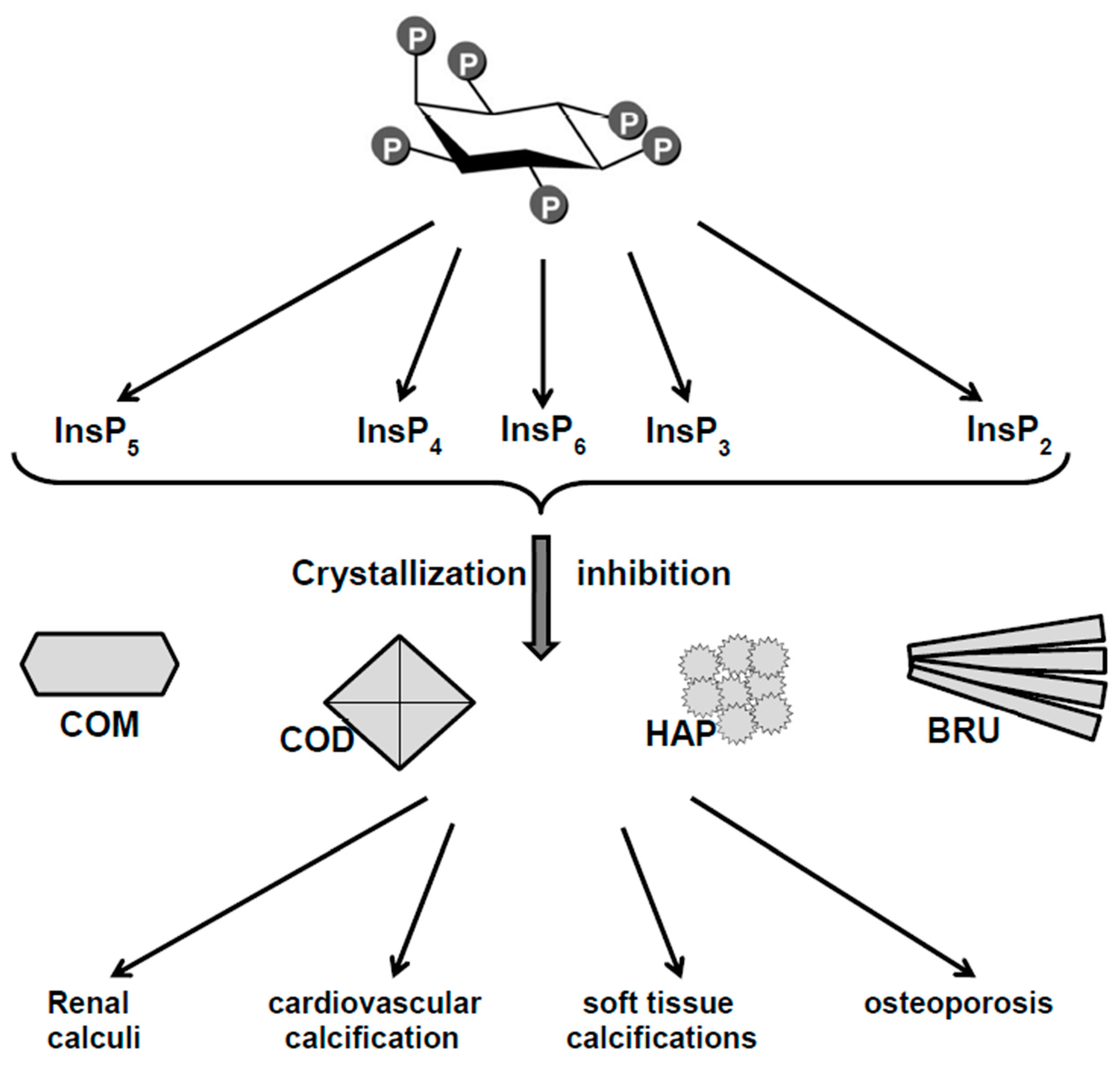
1. Introduction
2. Phytate (InsP6) and Inositol Phosphates (InsPs) as Crystallization Inhibitors of Calcium Salts: In Vitro Studies
3. Relationship Between Oral or Topical Intake of Phytate and Pathological Calcifications
3.1. Animals
3.2. Humans
4. Relationship Between Intake of InsP6 and Excretion of InsPs
4.1. Animals
4.2. Humans
5. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- O’Dell, B.L.; de Boland, A.R.; Koirtyohann, S.R. Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J. Agric. Food Chem. 1972, 20, 718–723. [Google Scholar] [CrossRef]
- Harland, B.F.; Oberleas, D. Phytate in foods. World Rev. Nutr. Diet. 1987, 52, 235–259. [Google Scholar] [CrossRef]
- Harland, B.F. CRC Handbook of Dietary Fibre in Human Nutrition; Spiller, G.A., Ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 674. [Google Scholar]
- Harland, B.F.; Smikle-Williams, S.; Oberleas, D. High performance liquid chromatography analysis of phytate (IP6) in selected foods. J. Food Compos. Anal. 2004, 17, 227–233. [Google Scholar] [CrossRef]
- Dorsch, J.A.; Cook, A.; Young, K.A.; Anderson, J.M.; Bauman, A.T.; Volkmann, C.J.; Murthy, P.P.; Raboy, V. Seed phosphorus and inositol phosphate phenotype of barley low phytic acid genotypes. Phytochemistry 2003, 62, 691–706. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frolich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Hartig, T. Über das Klebermehl. Bot. Ztg. 1855, 13, 881–882. [Google Scholar]
- Hartig, T. Weitere Mitteilungen, das Klebermehl (Aleuron) betreffend. Bot. Ztg. 1856, 14, 257–269. [Google Scholar]
- Anderson, R.J. A contribution to the chemistry of phytin. J. Biol. Chem. 1914, 17, 171–190. [Google Scholar]
- McCance, R.A.; Widdowson, E.M. Mineral metabolism of healthy adults on white and brown bread dietaries. J. Physiol. 1942, 101, 44–85. [Google Scholar] [CrossRef]
- McCance, R.A.; Walsham, C.M. The digestibility and absorption of the calories, proteins, purines, fat and calcium in wholemeal wheaten bread. Br. J. Nutr. 1948, 2, 26–41. [Google Scholar] [CrossRef]
- Halsted, J.A.; Ronaghy, H.A.; Abadi, P.; Haghshenass, M.; Amirhakemi, G.H.; Barakat, R.M.; Reinhold, J.G. Zinc deficiency in man. Am. J. Med. 1972, 53, 277–284. [Google Scholar] [CrossRef]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int. J. Food Sci. Technol. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Perello, J.; Costa-Bauza, A.; Prieto, R.M. Effect of phytate on element bioavailability in the second generation of rats. J. Trace Elem. Med. Biol. 2004, 17, 229–234. [Google Scholar] [CrossRef]
- Graf, E.; Empson, K.L.; Eaton, J.W. Phytic acid. A natural antioxidant. J. Biol. Chem. 1987, 262, 11647–11650. [Google Scholar] [PubMed]
- Shamsuddin, A.M. Inositol phosphates have novel anticancer function. J. Nutr. 1995, 125, 725S–732S. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Shamsuddin, A.M. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A. Phytate (IP6) is a powerful agent for preventing calcifications in biological fluids: Usefulness in renal lithiasis treatment. Anticancer Res. 1999, 19, 3717–3722. [Google Scholar]
- Lee, S.H.; Park, H.J.; Chun, H.K.; Cho, S.Y.; Cho, S.M.; Lillehoj, H.S. Dietary phytic acid lowers the blood glucose level in diabetic KK mice. Nutr. Res. 2006, 26, 474–479. [Google Scholar] [CrossRef]
- Jariwalla, R.J.; Sabin, R.; Lawson, S.; Herman, Z.S. Lowering of serum cholesterol and triglycerides and modulation of divalent cations by dietary phytate. J. Appl. Nutr. 1990, 42, 18–28. [Google Scholar]
- Lee, S.H.; Park, H.J.; Chun, H.K.; Cho, S.Y.; Jung, H.J.; Cho, S.M.; Kim, D.Y.; Kang, M.S.; Lillehoj, H.S. Dietary phytic acid improves serum and hepatic lipid levels in aged ICR mice fed a high-cholesterol diet. Nutr. Res. 2007, 27, 505–510. [Google Scholar] [CrossRef]
- Thomas, W.C.; Tilden, M.T. Inhibition of mineralization by hydrolysates of phytic acid. Johns Hopkins Med. J. 1972, 131, 133–142. [Google Scholar] [PubMed]
- Van den Berg, C.J.; Hill, L.F.; Stanbury, S.W. Inositol phosphates and phytic acid as inhibitors of biological calcification in the rat. Clin. Sci. 1972, 43, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Garcia-Ferragut, L.; Costa-Bauza, A. Study of the early stages of renal stone formation: Experimental model using urothelium of pig urinary bladder. Urol. Res. 1996, 24, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Garcia-Ferragut, L.; Costa-Bauza, A. Development of calcium oxalate crystals on urothelium: Effect of free radicals. Nephron 1998, 78, 296–301. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A.; March, J.G. Artificial simulation of the early stages of renal stone formation. Br. J. Urol. 1994, 74, 298–301. [Google Scholar] [CrossRef]
- Grases, F.; Kroupa, M.; Costa-Bauza, A. Studies on calcium oxalate monohydrate crystallization: Influence of inhibitors. Urol. Res. 1994, 22, 39–43. [Google Scholar] [CrossRef]
- Grases, F.; Rodriguez, A.; Costa-Bauza, A. Efficacy of mixtures of magnesium, citrate and phytate as calcium oxalate crystallization inhibitors in urine. J. Urol. 2015, 194, 812–819. [Google Scholar] [CrossRef]
- Saw, N.K.; Chow, K.; Rao, P.N.; Kavanagh, J.P. Effects of inositol hexaphosphate (phytate) on calcium binding, calcium oxalate crystallization and in vitro stone growth. J. Urol. 2007, 177, 2366–2370. [Google Scholar] [CrossRef]
- Fakier, S.; Rodgers, A.; Jackson, G. Potential thermodynamic and kinetic roles of phytate as an inhibitor of kidney stone formation: Theoretical modelling and crystallization experiments. Urolithiasis 2019, 47, 493–502. [Google Scholar] [CrossRef]
- Fleisch, H.; Russell, R.G.; Bisaz, S.; Casey, P.A.; Mühlbauer, R.C. The influence of pyrophosphate analogues (diphosphonates) on the precipitation and dissolution. Calcif. Tissue Res. 1968, 2, 10. [Google Scholar] [CrossRef]
- Francis, M.D.; Russell, R.G.; Fleisch, H. Diphosphonates Inhibit Formation of Calcium Phosphate Crystals in vitro and Pathological Calcification in vivo. Science 1969, 165, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H. Development of bisphosphonates. Breast Cancer Res. 2002, 4, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Garcia-Gonzalez, R.; Torres, J.J.; Llobera, A. Effects of phytic acid on renal stone formation in rats. Scand. J. Urol. Nephrol. 1998, 32, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Prieto, R.M.; Simonet, B.M.; March, J.G. Phytate prevents tissue calcifications in female rats. Biofactors 2000, 11, 171–177. [Google Scholar] [CrossRef]
- Grases, F.; Isern, B.; Sanchis, P.; Perello, J.; Torres, J.J.; Costa-Bauza, A. Phytate acts as an inhibitor information of renal calculi. Front. Biosci. 2007, 12, 2580–2587. [Google Scholar] [CrossRef]
- Grases, F.; Sanchis, P.; Perello, J.; Isern, B.; Prieto, R.M.; Fernandez-Palomeque, C.; Fiol, M.; Bonnin, O.; Torres, J.J. Phytate (myo-inositol hexakisphosphate) inhibits cardiovascular calcifications in rats. Front. Biosci. 2006, 11, 136–142. [Google Scholar] [CrossRef]
- Grases, F.; Sanchis, P.; Perello, J.; Isern, B.; Prieto, R.M.; Fernandez-Palomeque, C.; Saus, C. Phytate reduces age-related cardiovascular calcification. Front. Biosci. 2008, 13, 7115–7122. [Google Scholar] [CrossRef]
- Grases, F.; Perello, J.; Prieto, R.M.; Simonet, B.M.; Torres, J.J. Dietary myo-inositol hexaphosphate prevents dystrophic calcifications in soft tissues: A pilot study in Wistar rats. Life Sci. 2004, 75, 11–19. [Google Scholar] [CrossRef]
- Grases, F.; Prieto, R.M.; Sanchis, P.; Saus, C.; De Francisco, T. Role of phytate and osteopontin in the mechanism of soft tissue calcification. J. Nephrol. 2008, 21, 768–775. [Google Scholar]
- Grases, F.; Perello, J.; Isern, B.; Prieto, R.M. Study of a myo-inositol hexaphosphate-based cream to prevent dystrophic calcinosis cutis. Br. J. Dermatol. 2005, 152, 1022–1025. [Google Scholar] [CrossRef]
- Grases, F.; Sanchis, P.; Prieto, R.M.; Perello, J.; Lopez-Gonzalez, A.A. Effect of tetracalcium dimagnesium phytate on bone characteristics in ovariectomized rats. J. Med. Food 2010, 13, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Arriero, M.M.; Ramis, J.M.; Perello, J.; Monjo, M. Inositol hexakisphosphate inhibits osteoclastogenesis on RAW 264.7 cells and human primary osteoclasts. PLoS ONE 2012, 7, e43187. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willet, W.C.; Knight, E.L.; Stampfer, M.J. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch. Intern. Med. 2004, 164, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Piza, P.; Garcia-Raja, A.; Grases, F.; Costa-Bauza, A.; Prieto, R.M. Urinary lithogen risk test: Usefulness in the evaluation of renal lithiasis treatment using crystallization inhibitors (citrate and phytate). Arch. Esp. Urol. 1999, 52, 305–310. [Google Scholar] [PubMed]
- Sanchis, P.; Buades, J.M.; Berga, F.; Gelabert, M.M.; Molina, M.; Iñigo, M.V.; Garcia, S.; Gonzalez, J.; Bernabeu, M.R.; Costa-Bauza, A.; et al. Protective effect of myo-inositol hexaphosphate (phytate) on abdominal aortic calcification in patients with chronic kidney disease. J. Ren. Nutr. 2016, 26, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gonzalez, A.A.; Grases, F.; Roca, P.; Mari, B.; Vicente-Herrero, M.T.; Costa-Bauza, A. Phytate (myo-inositol hexaphosphate) and risk factors for osteoporosis. J. Med. Food 2008, 11, 747–752. [Google Scholar] [CrossRef]
- Grases, F.; Perello, J.; Isern, B.; Prieto, R.M. Determination of myo-inositol hexakisphosphate (phytate) in urine by inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2004, 510, 41–43. [Google Scholar] [CrossRef]
- Costa-Bauza, A.; Grases, F.; Gomila, I.; Rodriguez, A.; Prieto, R.M.; Tur, F. A simple and rapid colorimetric method for determination of phytate in urine. Urol. Res. 2012, 40, 663–669. [Google Scholar] [CrossRef]
- Costa-Bauza, A.; Grases, F.; Fakier, S.; Rodriguez, A. A novel metal-dye system for urinary phytate detection at micro-molar levels in rats. Anal. Methods 2013, 5, 3016–3022. [Google Scholar] [CrossRef]
- Muñoz, J.A.; Valiente, M. Determination of phytic acid in urine by inductively coupled plasma mass spectrometry. Anal. Chem. 2003, 75, 6374–6378. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A.; Berga, F.; Rodriguez, A.; Gomila, R.M.; Martorell, G.; Martinez-Cignoni, M.R. Evaluation of inositol phosphates in urine after topical administration of myo-inositol hexaphosphate of female Wistar rats. Life Sci. 2018, 192, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tur, F.; Tur, E.; Lentheric, I.; Mendoza, P.; Encabo, M.; Isern, B.; Grases, F.; Maraschiello, C.; Perello, J. Validation of an LC-MS bioanalytical method for quantification of phytate levels in rat, dog and human plasma. J. Chromatogr. B 2013, 928, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Duong, Q.H.; Clark, K.D.; Lapsley, K.G.; Pegg, R.B. Quantification of inositol phosphate in almond meal and almond brown skins by HPLC/ESI/MS. Food Chem. 2017, 229, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Simonet, B.M.; March, J.G.; Prieto, R.M. Inositol hexakisphosphate in urine: The relationship between oral intake and urinary excretion. BJU Int. 2000, 85, 138–142. [Google Scholar] [CrossRef]
- Grases, F.; Costa-Bauza, A.; Berga, F.; Gomila, R.M.; Martorell, G.; Martinez-Cignoni, M.R. Intake of myo-inositol hexaphosphate and urinary excretion of inositol phosphates in Wistar rats: Gavage vs. oral administration with sugar. PLoS ONE 2019, 14, e0223959. [Google Scholar] [CrossRef]
- Grases, F.; Simonet, B.M.; Vucenik, I.; Prieto, R.M.; Costa-Bauza, A.; March, J.G.; Shamsuddin, A.M. Absorption and excretion of orally administered inositol hexaphosphate (IP6 or phytate) in humans. Biofactors 2001, 15, 53–61. [Google Scholar] [CrossRef]
- Prieto, R.M.; Fiol, M.; Perello, J.; Estruch, R.; Ros, E.; Sanchis, P.; Grases, F. Effects of Mediterranean diets with low and high proportions of phytate-rich foods on the urinary phytate excretion. Eur. J. Nutr. 2010, 49, 321–326. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grases, F.; Costa-Bauza, A. Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules 2019, 24, 4434. https://doi.org/10.3390/molecules24244434
Grases F, Costa-Bauza A. Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules. 2019; 24(24):4434. https://doi.org/10.3390/molecules24244434
Chicago/Turabian StyleGrases, Felix, and Antonia Costa-Bauza. 2019. "Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications" Molecules 24, no. 24: 4434. https://doi.org/10.3390/molecules24244434
APA StyleGrases, F., & Costa-Bauza, A. (2019). Key Aspects of Myo-Inositol Hexaphosphate (Phytate) and Pathological Calcifications. Molecules, 24(24), 4434. https://doi.org/10.3390/molecules24244434




