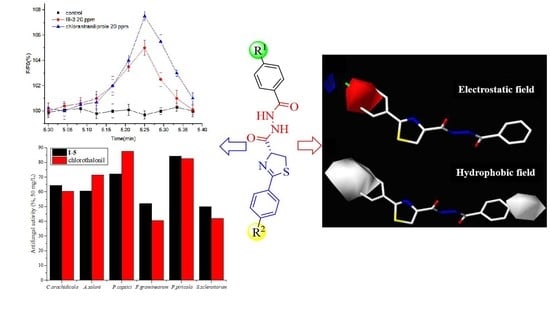
(R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs
Abstract
Share and Cite
Li, F.-Y.; Liu, J.-B.; Gong, J.-N.; Li, G. (R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs. Molecules 2019, 24, 4440. https://doi.org/10.3390/molecules24244440
Li F-Y, Liu J-B, Gong J-N, Li G. (R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs. Molecules. 2019; 24(24):4440. https://doi.org/10.3390/molecules24244440
Chicago/Turabian StyleLi, Feng-Yun, Jing-Bo Liu, Jia-Ning Gong, and Gen Li. 2019. "(R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs" Molecules 24, no. 24: 4440. https://doi.org/10.3390/molecules24244440
APA StyleLi, F.-Y., Liu, J.-B., Gong, J.-N., & Li, G. (2019). (R)-2-Phenyl-4,5-Dihydrothiazole-4-Carboxamide Derivatives Containing a Diacylhydrazine Group: Synthesis, Biological Evaluation, and SARs. Molecules, 24(24), 4440. https://doi.org/10.3390/molecules24244440