Diethyl Blechnic Exhibits Anti-Inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells
Abstract
:1. Introduction
2. Results
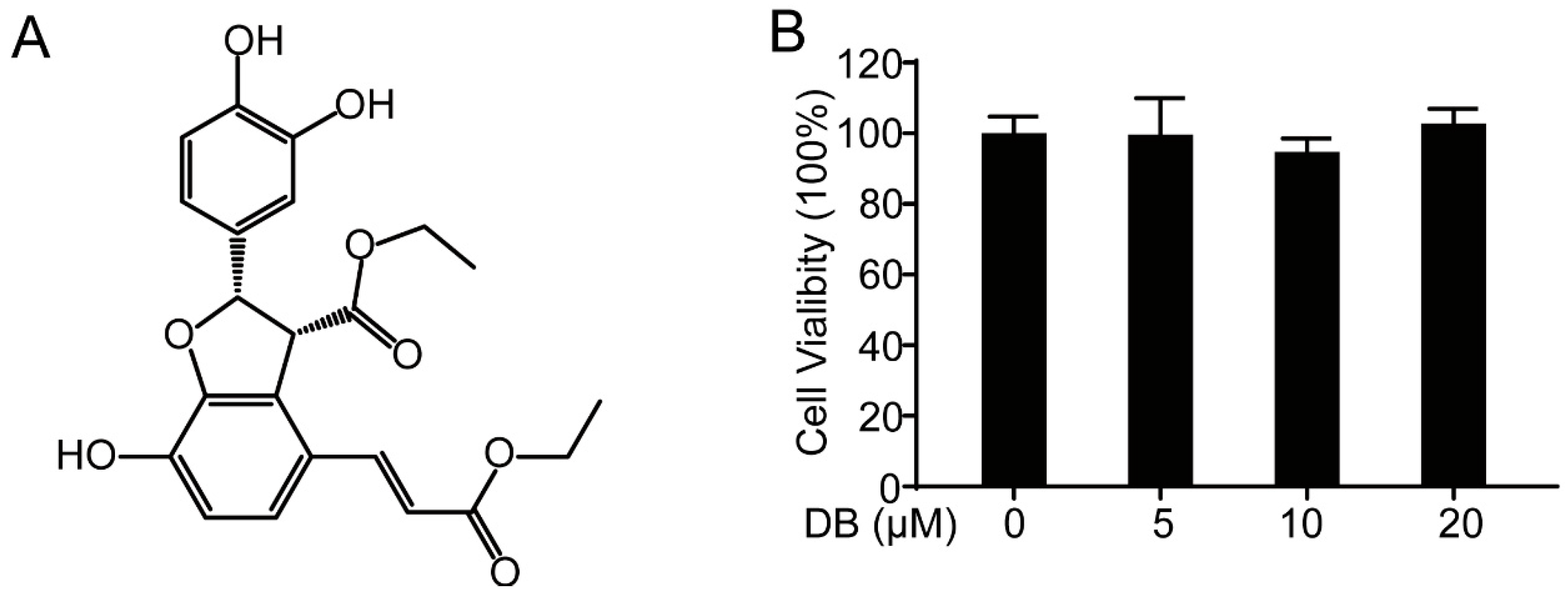
2.1. DB Suppresses LPS-Induced inflammatory Response in RAW264.7 Cells
2.2. DB Suppresses the Release of Pro-Inflammatory Cytokines in LPS-Stimulated RAW264.7 Cells
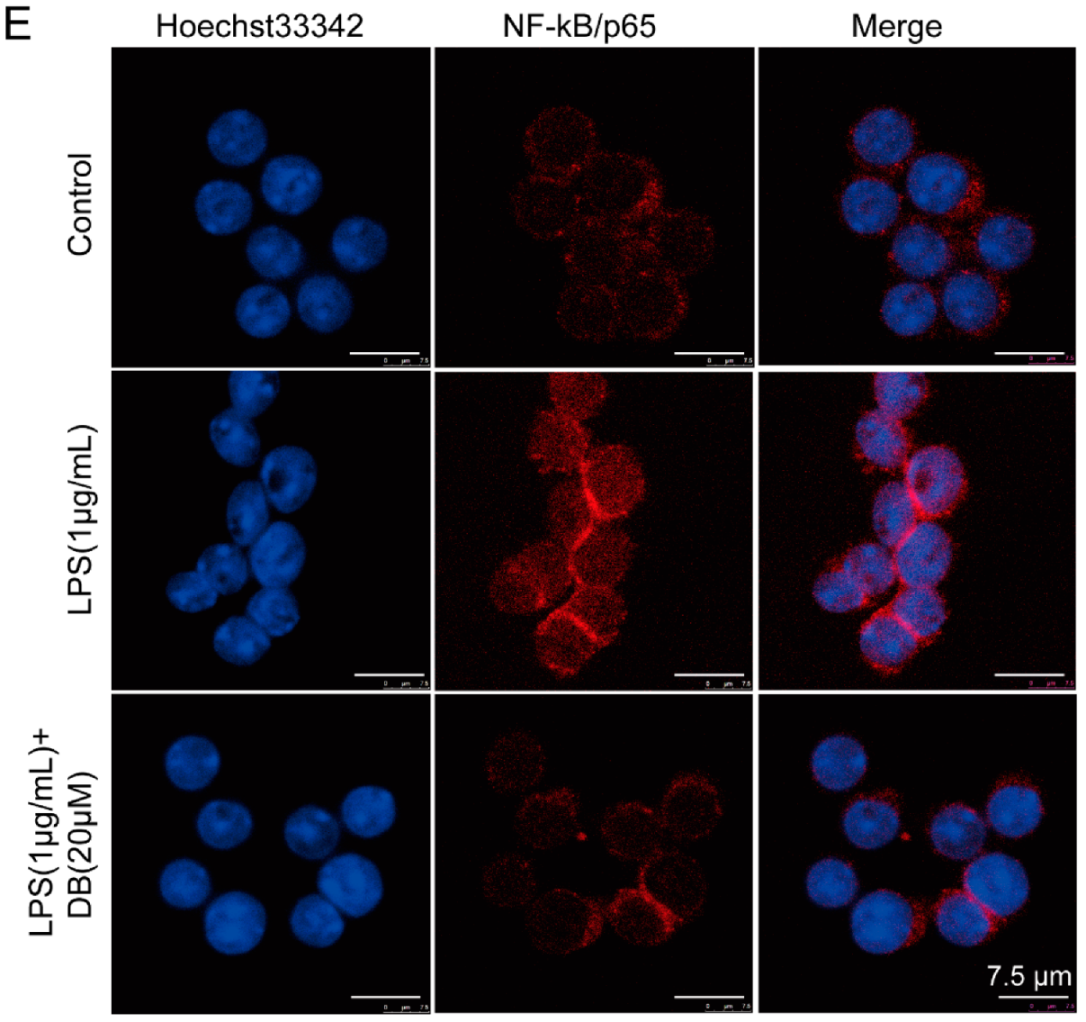
2.3. DB Suppresses LPS-Induced NF-κB Nuclear Translocation in RAW264.7 Cells
2.4. DB Suppresses the TLR4-MyD88 Pathway in LPS-Stimulated RAW264.7 Cells
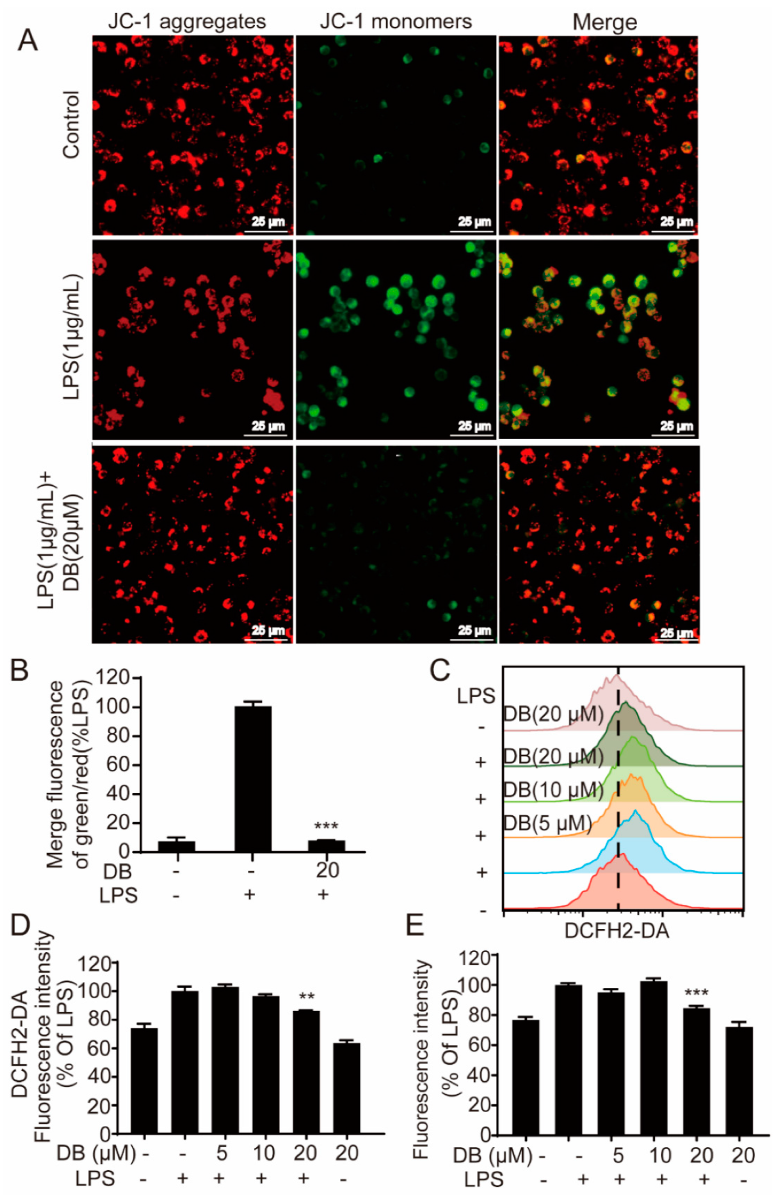
2.5. DB Suppresses the Loss of MMP and LPS-Induced ROS Generation
2.6. DB Suppresses the LPS-Stimulated Inflammatory Response Through the Nrf2 Pathway
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Qualtiy Control of DB
4.3. Cell Culture
4.4. MTT Assay
4.5. Griess Reagent Assay
4.6. Assessment of Cytokine Release
4.7. ROS Kit
4.8. Flow Cytometry Assay
4.9. Western Blotting Analysis
4.10. Quantitative Real-Time PCR (qRT-PCR) Assay
| IL-1β-F | GAAAGACGG CACACCCACCCT |
| IL-1β-R | GCTCTGCTTGTGAGGTGCTGATGTA |
| IL-6-F | TCCAGTTGCCTTC TTGGGAC |
| IL-6-R | GTGTAATTAAGCCTCCGACTTG |
| TNF-α-F | TTCTGTCTACTGAACTTCGGGGTGATCGGTCC |
| TNF-α-R | GTATGAGATAGCAAATCGGCTGACGGTGTGGG |
| NF-κB/p65-F | GCACGGATGACAGAGGCGTGTATAAGG |
| NF-κB/p65-R | GGCGGATGATCTCCTTCTCTCTGTCTG |
| IκB-α-F | TGCTGAGGCACTTCTGAG |
| IκB-α-R | CTGTATCCGGGTGCTTGG |
| HO-1-F | TCAGTCCCAAACCTCGCGGT |
| HO-1-R | GCTGTGCAGGTGTTAGCC |
| NRF2-F | AGCAGGACATGGAGCAAGTT |
| NRF2-R | TTCTTTTTCCAGCGAGGAGA |
| GAPDH-F | CATGACCACAGTCCATGCCATCAC |
| GAPDH-R | TGAGGTCCACCACCC TGTTGCTGT |
4.11. Immunofluorescence Assay
4.12. Fluorescence Assay
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, S.K.; Brown, L. Cholesterol versus Inflammation as Cause of Chronic Diseases. Nutrients 2019, 11, 2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.C.; Lin, H.L.; Wey, S.P.; Jan, T.R. Areca-nut extract modulates antigen-specific immunity and augments inflammation in ovalbumin-sensitized mice. Immunopharmacol. Immunotoxicol. 2011, 33, 315–322. [Google Scholar] [CrossRef] [PubMed]
- PJ, M.; TA, W. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar]
- Raetz, C.R.H.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi-Matsui, M.; Yano, S.; Matsumoto, N.; Futai, M. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem. Biophys. Res. Commun. 2012, 425, 144–149. [Google Scholar] [CrossRef]
- Doyle, S.L.; O’Neill, L.A. Toll-like receptors: From the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 2006, 72, 1102–1113. [Google Scholar] [CrossRef]
- Dinarello, C.A. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019, 15, 612–632. [Google Scholar] [CrossRef]
- Plociennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Ge, B.X. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 2008, 18, 745–755. [Google Scholar] [CrossRef]
- Shim, J.H.; Xiao, C.; Paschal, A.E.; Bailey, S.T.; Rao, P.; Hayden, M.S.; Lee, K.Y.; Bussey, C.; Steckel, M.; Tanaka, N.; et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005, 19, 2668–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, G.; Al-Sadoon, M.K.; El-Toni, A.M.; Daghestani, M. Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFκB and ERK signaling. Lipids Health Dis. 2012, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Zheng, J.; Fan, R.; Wu, H.; Yao, H.; Yan, Y.; Liu, J.; Ran, L.; Sun, Z.; Yi, L.; Dang, L.; et al. Directed self-assembly of herbal small molecules into sustained release hydrogels for treating neural inflammation. Nat. Commun. 2019, 10, 1604. [Google Scholar] [CrossRef]
- Gao, H.; Huang, L.; Ding, F.; Yang, K.; Feng, Y.; Tang, H.; Xu, Q.M.; Feng, J.; Yang, S. Simultaneous purification of dihydrotanshinone, tanshinone I, cryptotanshinone, and tanshinone IIA from Salvia miltiorrhiza and their anti-inflammatory activities investigation. Sci. Rep. 2018, 8, 8460. [Google Scholar] [CrossRef]
- Gao, H.; Sun, W.; Zhao, J.; Wu, X.; Lu, J.J.; Chen, X.; Xu, Q.M.; Khan, I.A.; Yang, S. Tanshinones and diethyl blechnics with anti-inflammatory and anti-cancer activities from Salvia miltiorrhiza Bunge (Danshen). Sci. Rep. 2016, 6, 33720. [Google Scholar] [CrossRef] [Green Version]
- Armaka, M.; Ospelt, C.; Pasparakis, M.; Kollias, G. The p55TNFR-IKK2-Ripk3 axis orchestrates arthritis by regulating death and inflammatory pathways in synovial fibroblasts. Nat. Commun. 2018, 9, 618. [Google Scholar] [CrossRef]
- Donato, A.J.; Black, A.D.; Jablonski, K.L.; Gano, L.B.; Seals, D.R. Aging is associated with greater nuclear NF κ B, reduced I κ B α, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 2008, 7, 805–812. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.; Dhar, D.; El Zahraa Mohammed, F.; Habtesion, A.; Davies, N.A.; Jover-Cobos, M.; Macnaughtan, J.; Sharma, V.; Olde Damink, S.W.; Mookerjee, R.P.; et al. Prevention of acute kidney injury in a rodent model of cirrhosis following selective gut decontamination is associated with reduced renal TLR4 expression. J. Hepatol. 2012, 56, 1047–1053. [Google Scholar] [CrossRef]
- Fu, C.Y.; Chen, J.; Lu, X.Y.; Zheng, M.Z.; Wang, L.L.; Shen, Y.L.; Chen, Y.Y. Dimethyl fumarate attenuates lipopolysaccharide-induced mitochondrial injury by activating Nrf2 pathway in cardiomyocytes. Life Sci. 2019, 235, 116863. [Google Scholar] [CrossRef]
- Fallah, M.; Mohammadi, H.; Shaki, F.; Hosseini-Khah, Z.; Moloudizargari, M.; Dashti, A.; Ziar, A.; Mohammadpour, A.; Mirshafa, A.; Modanloo, M.; et al. Doxorubicin and liposomal doxorubicin induce senescence by enhancing nuclear factor κ B and mitochondrial membrane potential. Life Sci. 2019, 232, 116677. [Google Scholar] [CrossRef]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2013, 65, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ohta, T.; Maruyama, A.; Hosoya, T.; Nishikawa, K.; Maher, J.A.; Shibahara, S.; Itoh, K.; Yamamoto, M. BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress. Mol. Cell. Biol. 2006, 26, 7942–7952. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Dong, H.; Song, E.; Xu, X.; Liu, L.; Song, Y. Nrf2/ARE pathway activation, HO-1 and NQ01 induction by polychlorinated biphenyl quinone is associated with reactive oxygen. species and PI3K/AKT signaling. Chem.-Biol. Interact. 2014, 209, 56–67. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Yao, Y.D.; Shen, X.Y.; Machado, J.; Luo, J.F.; Dai, Y.; Lio, C.K.; Yu, Y.; Xie, Y.; Luo, P.; Liu, J.X.; et al. Nardochinoid B Inhibited the Activation of RAW264.7 Macrophages Stimulated by Lipopolysaccharide through Activating the Nrf2/HO-1 Pathway. Molecules 2019, 24, 2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surh, Y.J.; Chun, K.S.; Cha, H.H.; Han, S.S.; Keum, Y.S.; Park, K.K.; Lee, S.S. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-κ B activation. Mutat. Res. 2001, 480, 243–268. [Google Scholar] [CrossRef]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelova, H.; Hosek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef] [Green Version]
- Matias, M.L.; Gomes, V.J.; Romao-Veiga, M.; Ribeiro, V.R.; Nunes, P.R.; Romagnoli, G.G.; Peracoli, J.C.; Peracoli, M.T.S. Silibinin Downregulates the NF-κB Pathway and NLRP1/NLRP3 Inflammasomes in Monocytes from Pregnant Women with Preeclampsia. Molecules 2019, 24, 1548. [Google Scholar] [CrossRef] [Green Version]
- Bak, M.-J.; Hong, S.-G.; Lee, J.-W.; Jeong, W.-S. Red Ginseng Marc Oil Inhibits iNOS and COX-2 via NF κ B and p38 Pathways in LPS-Stimulated RAW 264.7 Macrophages. Molecules 2012, 17, 13769–13786. [Google Scholar] [CrossRef] [Green Version]
- Yuan, R.; Huang, L.; Du, L.J.; Feng, J.F.; Li, J.; Luo, Y.Y.; Xu, Q.M.; Yang, S.L.; Gao, H.; Feng, Y.L. Dihydrotanshinone exhibits an anti-inflammatory effect in vitro and in vivo through blocking TLR4 dimerization. Pharmacol. Res. 2019, 142, 102–114. [Google Scholar] [CrossRef]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. MITOCHONDRIAL REACTIVE OXYGEN SPECIES (ROS) AND ROS-INDUCED ROS RELEASE. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Li, L.; Zhang, C.; Cheng, X.; Zhang, Y.; Guo, Y.; Long, M.; Yang, S.; He, J. Palmitic Acid and β-Hydroxybutyrate Induce Inflammatory Responses in Bovine Endometrial Cells by Activating Oxidative Stress-Mediated NF-κB Signaling. Molecules 2019, 24, 2421. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Tian, K.; Zhang, T.; Fan, C.H.; Zhou, P.; Zeng, D.; Zhao, S.; Li, L.S.; Smith, H.S.; Li, J.; et al. Cyanate Induces Oxidative Stress Injury and Abnormal Lipid Metabolism in Liver through Nrf2/HO-1. Molecules 2019, 24, 3231. [Google Scholar] [CrossRef] [Green Version]
- Francis, N.; Rao, S.; Blanchard, C.; Santhakumar, A. Black Sorghum Phenolic Extract Regulates Expression of Genes Associated with Oxidative Stress and Inflammation in Human Endothelial Cells. Molecules 2019, 24, 3321. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.-L.; Lin, S.-W.; Lee, C.-C.; Lin, K.-Y.; Liao, C.-H.; Yang, T.-Y.; Wang, H.-M.; Huang, H.-C.; Wu, C.-R.; Hseu, Y.-C. Induction of Nrf2-mediated genes by Antrodia salmonea inhibits ROS generation and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Funct. 2015, 6, 230–241. [Google Scholar] [CrossRef]
- Fan, L.; Fan, Y.; Liu, L.; Tao, W.; Shan, X.; Dong, Y.; Li, L.; Zhang, S.; Wang, H. Chelerythrine Attenuates the Inflammation of Lipopolysaccharide-Induced Acute Lung Inflammation Through NF-κB Signaling Pathway Mediated by Nrf2. Front. Pharmacol. 2018, 9, 1047. [Google Scholar] [CrossRef]
- Bao, Y.; Meng, X.; Liu, F.; Wang, F.; Yang, J.; Wang, H.; Xie, G. Protective effects of osthole against inflammation induced by lipopolysaccharide in BV2 cells. Mol. Med. Rep. 2018, 17, 4561–4566. [Google Scholar] [CrossRef] [Green Version]
- Sawle, P.; Foresti, R.; Mann, B.E.; Johnson, T.R.; Green, C.J.; Motterlini, R. Carbon monoxide-releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br. J. Pharmacol. 2005, 145, 800–810. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Han, S.; Li, X.-X.; Wang, Q.-Q.; Cui, Y.; Chen, Y.; Gao, H.; Huang, L.; Yang, S. Diethyl Blechnic Exhibits Anti-Inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells. Molecules 2019, 24, 4502. https://doi.org/10.3390/molecules24244502
He J, Han S, Li X-X, Wang Q-Q, Cui Y, Chen Y, Gao H, Huang L, Yang S. Diethyl Blechnic Exhibits Anti-Inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells. Molecules. 2019; 24(24):4502. https://doi.org/10.3390/molecules24244502
Chicago/Turabian StyleHe, Jia, Shan Han, Xin-Xing Li, Qin-Qin Wang, Yushun Cui, Yangling Chen, Hongwei Gao, Liting Huang, and Shilin Yang. 2019. "Diethyl Blechnic Exhibits Anti-Inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells" Molecules 24, no. 24: 4502. https://doi.org/10.3390/molecules24244502
APA StyleHe, J., Han, S., Li, X.-X., Wang, Q.-Q., Cui, Y., Chen, Y., Gao, H., Huang, L., & Yang, S. (2019). Diethyl Blechnic Exhibits Anti-Inflammatory and Antioxidative Activity via the TLR4/MyD88 Signaling Pathway in LPS-Stimulated RAW264.7 Cells. Molecules, 24(24), 4502. https://doi.org/10.3390/molecules24244502



