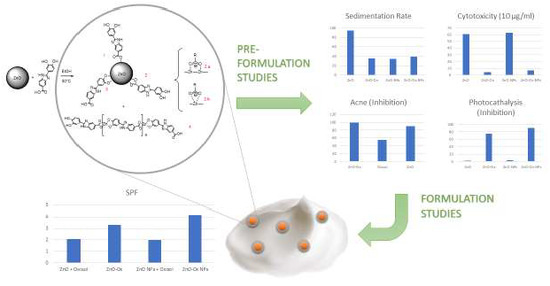
Synthesis and Characterization of New Multifunctional Self-Boosted Filters for UV Protection: ZnO Complex with Dihydroxyphenyl Benzimidazole Carboxylic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Adduct Characterization (FT-IR)
2.2. Thermogravimetric Analysis (TGA)
2.3. Colloidal Characterization (CSA, DLS–ELS, ζ)
2.4. Efficacy Tests
2.4.1. SPF
2.4.2. Photochemiluminescence (PCL)
2.5. Release Test
2.6. Safety Tests
2.6.1. Photocatalytic Activity
2.6.2. Cytotoxicity
2.7. Solubility Test
2.8. Anti-Acne Activity
3. Materials and Methods
3.1. Materials
3.2. Zinc Oxide (ZnO) Functionalization: ZnO–Ox
3.3. Synthesis of Zn2+–Oxisol Complex
3.4. Evaluation of Stability of Oxisol–ZnO Particles
3.5. Sedimentation Rate
3.6. Zn Release from the Zinc-Based Materials (ZnO–Ox and Zn@Oxisol)
3.7. Digestion Analysis (ICP–MS)
3.8. Photocatalysis
3.9. Formulation
3.10. Characterization
3.10.1. FT-IR Analysis
3.10.2. Thermo-Gravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
3.10.3. ζ Potential (DLS–ELS)
3.10.4. SEM
3.11. Cytotoxicity
3.12. Anti-Acne Activity: Determination of MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration)
3.13. Oxisol Release from the Emulsions
3.14. Photochemiluminescence (PCL)
3.15. In Vitro Evaluation of Filtering Parameters
3.16. Statistical Evaluations
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabros, S.; Zito, P.M. Sunscreens and Photoprotection; StatPearls Publishing: Tampa, FL, USA, 2019. [Google Scholar]
- Charles, N.P. The Influence of Sunlight in the Production of Cancer of the Skin; H.K. Lewis & Company, Limited: Chicago, IL, USA, 1918. [Google Scholar]
- Lehmann, P. Sun exposed skin disease. Clin. Dermatol. 2011, 29, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Cullen, A.P.; de Gruijl, F.R.; Longstreth, J.; Takizawa, Y.; Lucas, R.M.; Noonan, F.P.; van der Leun, J.C. The effects on human health from stratospheric ozone depletion and its interactions with climate change. Photochem. Photobiol. Sci. 2007, 6, 232–251. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Ziosi, P.; Manfredini, S.; Brazzo, F.; Vaccarelli, C.; Vertuani, S.; Reggio, S.; Bustacchini, S. SPF BOOSTER Nuovo approccio nello sviluppo di Prodotti Solari. Valutazione di efficacia in vitro ed in vivo. Cosmet. Technol. 2006, 9. [Google Scholar]
- Bino, A.; Baldisserotto, A.; scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 527–537. [Google Scholar] [CrossRef]
- Nakayama, N.; Hayashi, T. Preparation of TiO2 nanoparticles surface-modified by both carboxylic acid and amine: Dispersibility and stabilization in organic solvents. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 543–550. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc therapy in dermatology: A review. Dermatol. Res. Pract. 2014, 2014. [Google Scholar] [CrossRef]
- Lenz, A.; Selegård, L.; Söderlind, F.; Larsson, A.; Holtz, P.O.; Uvdal, K.; Ojamäe, L.; Käll, P. ZnO nanoparticles functionalized with organic acids: An experimental and quantum-chemical study. J. Phys. Chem. C 2009, 113, 17332–17341. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Tetrahedral vs Octahedral Zinc Complexes with Ligands of Biological Interest: A DFT/CDM Study. J. Am. Chem. Soc. 2000, 122, 11146–11153. [Google Scholar] [CrossRef]
- Jiang, C.; Aiken, G.R.; Hsu-Kim, H. Effects of Natural Organic Matter Properties on the Dissolution Kinetics of Zinc Oxide Nanoparticles. Environ. Sci. Technol. 2015, 49, 11476–11484. [Google Scholar] [CrossRef] [PubMed]
- Mohd Omar, F.; Abdul Aziz, H.; Stoll, S. Aggregation and disaggregation of ZnO nanoparticles: Influence of pH and adsorption of Suwannee River humic acid. Sci. Total Environ. 2013, 468–469, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO Nanoparticles in Solution. Influence of pH, Dissolution, Aggregation and Disaggregation Effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Schmidt-Ott, A.; van den Berg, K.J.; Dik, J.; Kooyman, P.J.; van Driel, B.A. A quick assessment of the photocatalytic activity of TiO2 pigments—From lab to conservation studio! Microchem. J. 2015, 126, 162–171. [Google Scholar]
- Bukallah, S.B.; Rauf, M.A.; Ashraf, S.S. Photocatalytic decoloration of Coomassie Brilliant Blue with titanium oxide. Dye. Pigment. 2007, 72, 353–356. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, L.; Li, S. Photocatalytic degradation of Reactive Brilliant Blue KN-R by TiO2/UV process. Desalination 2010, 258, 48–53. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Mädler, L.; et al. Use of a rapid cytotoxicity screening approach to engineer a safer zinc oxide nanoparticle through iron doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- Çopur, M. Optimization of dissolution of Zn and Cd metals from Waelz sintering waste by in aqueous (NH4)2SO4 solution. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2010, 25, 17–19. [Google Scholar]
- Huang, Z.; Zheng, X.; Yan, D.; Yin, G.; Liao, X.; Kang, Y.; Yao, Y.; Huang, D.; Hao, B. Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 2008, 24, 4140–4144. [Google Scholar] [CrossRef]
- Singh, B.; Srivastava, R.; Narang, K.K. Synthesis and spectral studies of zinc chloride complexes with some acetophenone and 4-hydroxy-acetophenone acylhydrazones. Synth. React. Inorg. Met. Chem. 2000, 30, 1175–1192. [Google Scholar] [CrossRef]
- Detloff, T.; Sobisch, T.; Lerche, D. Particle size distribution by space or time dependent extinction profiles obtained by analytical centrifugation (concentrated systems). Powder Technol. 2007, 174, 50–55. [Google Scholar] [CrossRef]
- Wang, D.; Lin, z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Badetti, E.; Calgaro, L.; Falchi, L.; Bonetto, A.; Bettiol, C.; Leonetti, B.; Ambrosi, E.; Zendri, E.; Marcomini, A. Interaction between Copper Oxide Nanoparticles and Amino Acids: Influence on the Antibacterial Activity. Nanomaterials 2019, 9, 792. [Google Scholar] [CrossRef] [Green Version]
- Brunelli, A.; Badetti, E.; Basei, G.; Izzo, F.C.; Hristozov, D.; MArcomini, A. Effects of organic modifiers on the colloidal stability of TiO2 nanoparticles. A methodological approach for NPs categorization by multivariate statistical analysis. NanoImpact 2018, 9, 114–123. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Buso, P.G.; Radice, M.; Dissette, V.; Lampronti, I.; Gambari, R.; Manfredini, S.; Vertuani, S. Moringa oleifera leaf extracts as multifunctional ingredients for “natural and organic” sunscreens and photoprotective preparations. Molecules 2018, 23, 664. [Google Scholar] [CrossRef] [Green Version]
- Lewin, G.; Popov, I. Oxidants and antioxidants part B—Antioxidative homeostasis: Characterization by means of chemiluminescent technique. Methods Enzymol. 1999, 300, 437–456. [Google Scholar]
- Popov, I.; Lewin, G. Photochemiluminescent detection of antiradical activity III: A simple assay of ascorbate in blood plasma. J. Biochem. Biophys. Methods 1994, 28, 277–282. [Google Scholar]
- Dimitrovska Cvetkovska, A.; Manfredini, S.; Ziosi, P.; Molesini, S.; Dissette, V.; Magri, I.; Scapoli, C.; Carrieri, A.; Durini, E.; Vertuani, S. Factors affecting SPF in vitro measurement and correlation with in vivo results. Int. J. Cosmet. Sci. 2017, 39, 310–319. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |











| Weight Loss Referred to Organic Fraction (%) | |
|---|---|
| Nanometric ZnO–Ox | 21.08 ± 0.8 |
| Non-nanometric ZnO–Ox | 42.26 ± 1.1 |
| Zn complex | 78.67 ± 0.1 |
| Organic Fraction (%) | |
|---|---|
| Nanometric ZnO–Ox | 26.44 ± 1.3 |
| Non-nanometric ZnO–Ox | 38.16 ± 2.3 |
| Zn complex | 77.69 ± 1.4 |
| DLS (nm) ± SD | CSA (nm) ± SD | Sedimentation Rate (μm/s) ± SD | |
|---|---|---|---|
| Non nanometric ZnO | 1612 ± 290 411.4 ± 59 10.7 ± 1.5 | 247.27 ± 15.2 | 94.22 ± 0.5 |
| Non-nanometric ZnO–Ox | 162 ± 36 | 135.37 ± 1.9 | 35.90 ± 1.5 |
| Nanometric ZnO | 167 ± 34 | 162.63 ± 1.2 | 33.91 ± 0.3 |
| Nanometric ZnO–Ox | 46 ± 39 | 164.63 ± 3.2 | 38.67 ± 0.8 |
| A. Pre-Irradiation | SPF | UVA-PF | UVA/UVB | ʎc |
| Emulsion base | 0.99 ± 0.04 | 0.98 ± 0.04 | 0.99 ± 0.04 | 290 ± 1.00 |
| Emulsion with Oxisol | 1.31 ± 0.12 | 1.17 ± 0.11 | 1.00 ± 0.1 | 370 ± 1.00 |
| Emulsion with non-nanometric ZnO | 1.62 ± 0.15 | 1.60 ± 0.15 | 1.00 ± 0.12 | 377 ± 1.00 |
| Emulsion mixture (Non nanometric ZnO+Oxisol) | 2.08 ± 0.15 | 2.10 ± 0.17 | 1.03 ± 0.15 | 381 ± 1.00 |
| Emulsion with non-nanometric ZnO–Ox | 3.32 ± 0.28 | 2.50 ± 0.24 | 0.93 ± 0.15 | 379 ± 1.00 |
| Emulsion with nanometric ZnO | 1.58 ± 0.15 | 1.55 ± 0.15 | 1.00 ± 0.12 | 374 ± 1.00 |
| Emulsion with nanometric ZnO–Ox | 2.01 ± 0.18 | 2.08 ± 0.19 | 1.04 ± 0.13 | 381 ± 1.00 |
| Emulsion mixture (Nanometric ZnO+Oxisol) | 4.17 ± 0.34 | 2.65 ± 0.27 | 0.86 ± 0.15 | 375 ± 1.00 |
| B. Post-irradiation | SPF | UVA-PF | UVA/UVB | ʎc |
| Emulsion base | 0.98 ± 0.04 | 0.97 ± 0.05 | 0.99 ± 0.04 | 290 ± 1.00 |
| Emulsion with Oxisol | 1.21 ± 0.13 | 1.10 ± 0.15 | 0.91 ± 0.1 | 369 ± 1.00 |
| Emulsion with non-nanometric ZnO | 1.51 ± 0.16 | 1.50 ± 0.17 | 1.00 ± 0.12 | 376 ± 1.00 |
| Emulsion mixture (Non nanometric ZnO+Oxisol) | 1.91 ± 0.15 | 1.95 ± 0.17 | 1.02 ± 0.15 | 383 ± 1.00 |
| Emulsion with non-nanometric ZnO–Ox | 3.02 ± 0.31 | 2.33 ± 0.35 | 0.77 ± 0.15 | 375 ± 1.00 |
| Emulsion with nanometric ZnO | 1.47 ± 0.13 | 1.46 ± 0.15 | 0.99 ± 0.12 | 375 ± 1.00 |
| Emulsion with nanometric ZnO–Ox | 1.87 ± 0.20 | 1.93 ± 0.21 | 1.03 ± 0.13 | 382 ± 1.00 |
| Emulsion mixture (Nanometric ZnO+Oxisol) | 3.88 ± 0.32 | 2.49 ± 0.33 | 0.64 ± 0.15 | 372 ± 1.00 |
| Formulation | μmoli TE/Gram |
|---|---|
| Emulsion with Oxisol 0.5% | 41.96 ± 2.07 |
| Emulsion mixture (Non nanometric ZnO+Oxisol) | 1.31 ± 0.06 |
| Emulsion mixture (Naometric ZnO+Oxisol) | 1.81 ± 0.07 |
| Emulsion with non-nanometric ZnO–Ox | 1.98 ± 0.05 |
| Emulsion with nanometric ZnO–Ox | 1.65 ± 0.04 |
| Substrate | Solvent | pH | Time (h) | Oxisol Desorption (%) |
|---|---|---|---|---|
| 12.0 | <1.0 | |||
| CH3CH2OH/H2O | 6.1 | <1.0 | ||
| 2.7 | <1.0 | |||
| Nanometric | CH3CH2OH | - | 4.0 ± 0.5 | <1.0 |
| ZnO | 12.0 | 23.49 ± 1.55 | ||
| H2O | 6.1 | 9.01 ± 1.80 | ||
| 2.7 | <1.0 | |||
| 12.0 | <1.0 | |||
| CH3CH2OH/H2O | 6.1 | <1.0 | ||
| Non- | 2.7 | <1.0 | ||
| nanometric | CH3CH2OH | - | 4.0 ± 0.5 | <1.0 |
| ZnO | 12.0 | 20.76 ± 1.21 | ||
| H2O | 6.1 | 6.72 ± 1.34 | ||
| 2.7 | <1.0 |
| Substrate | Issue of Release of the Emulsion Adduct | Time (h) | Oxisol Released in the Emulsion Test (%) |
|---|---|---|---|
| Nanometric ZnO–Ox | H2O 0.90% NaCl | 4.0 ± 0.5 | 8.73 ± 0.34 |
| Non nanometric ZnO–Ox | H2O 0.90% NaCl | 4.0 ± 0.5 | 7.20 ± 0.31 |
| Concentration (μM) | ||
| Only Acid Blue 9 solution (dark) | 109.99 ± 23.59 | |
| Only Acid Blue 9 solution (UV) | 86.50 ± 18.86 | |
| Dye Concentration (μM) (nanometric ZnO) | Dye Concentration (μM) (non-nanometric ZnO) | |
| ZnO–Ox (dark) | 93.68 ± 20.35 | 73.07 ± 16.13 |
| ZnO–Ox (UV) | 90.91 ± 19.73 | 74.77 ± 16.50 |
| ZnO (dark) | 91.22 ± 19.86 | 86.20 ± 18.86 |
| ZnO (UV) | 2.50 ± 2.08 | 2.11 ± 1.95 |
| Sample | Concentration (μg/mL) | % Inhibition ± Standard Deviation |
|---|---|---|
| Control | 0 | 0.00 ± 0.00 |
| Nanometric ZnO | 1 | 0.15 ± 6.56 |
| 10 | 61.11 ± 12.30 | |
| 100 | 87.89 ± 0.81 | |
| Nanometric ZnO–Ox | 1 | −9.33 ± 3.80 |
| 10 | 4.20 ± 4.56 | |
| 100 | 88.02 ± 0.80 | |
| Non-nanometric ZnO | 1 | −3.67 ± 5.53 |
| 10 | 53.25 ± 16.93 | |
| 100 | 87.86 ± 0.85 | |
| Non-nanometric ZnO–Ox | 1 | −11.23 ± 6.95 |
| 10 | 6.58 ± 3.23 | |
| 100 | 87.82 ± 0.96 |
| Zn2+ (%) | ||||
|---|---|---|---|---|
| Nanometric ZnO | Non Nanometric ZnO | Nanometric ZnO–Ox | Non-Nanometric ZnO–Ox | |
| 85.05 ± 6.0 | 77.67 ± 6.2 | 58.61 ± 2.9 | 39.51 ± 2.4 | |
| Analyzed condition | Zn2+ in Solution (%) ** | |||
| pH 3 | 94 ± 6 | 100 ± 3 | 102 ± 2 | 103 ± 2 |
| pH 5 | 14 ± 1 | 15 ± 1 | 36 ± 2 | 60 ± 3 |
| pH 7 | 18 ± 1 | 12 ± 1 | 34 ± 1 | 57 ± 2 |
| pH 9 | 27 ± 1 | 31 ± 1 | 42 ± 1 | 52 ± 2 |
| pH 12 | 0 ± 0 | 1 ± 0 | 7 ± 1 | 11 ± 1 |
| DMEM * (pH 7.4) | 75 ± 4 | 81 ± 3 | 81 ± 3 | 55 ± 1 |
| Microorganism Cutibacterium Acnes (1 μg/mL) | Diameter | Inhibition (Halo Interpretation *) | Antimicrobial Activity Result * | |
|---|---|---|---|---|
| ZnO–Ox | 6.00 × 106 | 4.0 | +++ | S |
| Oxisol | 6.00 × 106 | 1.9 | + | L |
| ZnO | 6.00 × 106 | 2.4 | ++ | M |
| Microorganism Cutibacterium Acnes (1 μg/mL) | Result after Contact Time T1 | Reduction | Antimicrobial Activity Result * | |
|---|---|---|---|---|
| ZnO–Ox | 6.00 × 106 | 6.40 × 104 | 98.97% | M |
| Oxisol® | 6.00 × 106 | 2.70 × 106 | 55.00% | L |
| ZnO | 6.00 × 106 | 5.80 × 105 | 90.33% | M |
| Formulation | pH | Viscosity (ŋ) cP |
|---|---|---|
| Only ZnO | 6.53 | 11,230 |
| ZnO + Oxisol (mixture) | 6.67 | 29,520 |
| Functionalized ZnO (ZnO–Ox) | 6.50 | 39,650 |
| Only ZnO nanosized | 6.68 | 14,160 |
| ZnO nanosized + Oxisol (mixture) | 6.50 | 16,420 |
| Functionalized ZnO nanosized (nanometric ZnO–Ox) | 6.00 | 17,860 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battistin, M.; Durini, E.; Dissette, V.; Bonetto, A.; Marcomini, A.; Casagrande, E.; Brunetta, A.; Ziosi, P.; Molesini, S.; Gavioli, R.; et al. Synthesis and Characterization of New Multifunctional Self-Boosted Filters for UV Protection: ZnO Complex with Dihydroxyphenyl Benzimidazole Carboxylic Acid. Molecules 2019, 24, 4546. https://doi.org/10.3390/molecules24244546
Battistin M, Durini E, Dissette V, Bonetto A, Marcomini A, Casagrande E, Brunetta A, Ziosi P, Molesini S, Gavioli R, et al. Synthesis and Characterization of New Multifunctional Self-Boosted Filters for UV Protection: ZnO Complex with Dihydroxyphenyl Benzimidazole Carboxylic Acid. Molecules. 2019; 24(24):4546. https://doi.org/10.3390/molecules24244546
Chicago/Turabian StyleBattistin, Mattia, Elisa Durini, Valeria Dissette, Alessandro Bonetto, Antonio Marcomini, Elisa Casagrande, Andrea Brunetta, Paola Ziosi, Sonia Molesini, Riccardo Gavioli, and et al. 2019. "Synthesis and Characterization of New Multifunctional Self-Boosted Filters for UV Protection: ZnO Complex with Dihydroxyphenyl Benzimidazole Carboxylic Acid" Molecules 24, no. 24: 4546. https://doi.org/10.3390/molecules24244546
APA StyleBattistin, M., Durini, E., Dissette, V., Bonetto, A., Marcomini, A., Casagrande, E., Brunetta, A., Ziosi, P., Molesini, S., Gavioli, R., Nicoli, F., Manfredini, S., Vertuani, S., & Baldisserotto, A. (2019). Synthesis and Characterization of New Multifunctional Self-Boosted Filters for UV Protection: ZnO Complex with Dihydroxyphenyl Benzimidazole Carboxylic Acid. Molecules, 24(24), 4546. https://doi.org/10.3390/molecules24244546