Blotting Paper-Derived Activated Porous Carbon/Reduced Graphene Oxide Composite Electrodes for Supercapacitor Applications
Abstract
:1. Introduction
2. Results and Discussion
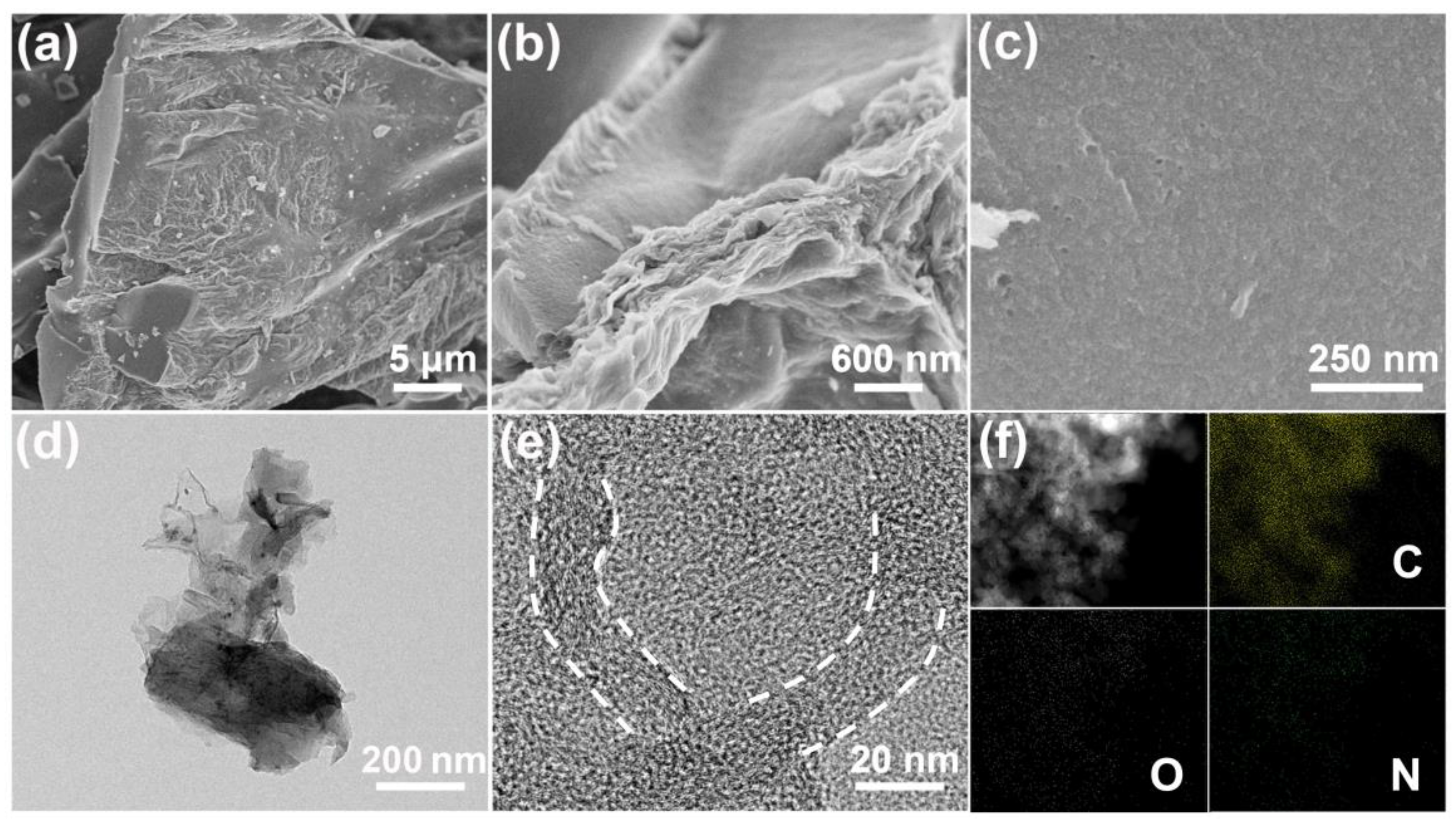
2.1. Characterizations of GBPCs
2.2. Electrochemical Performance in 6 M KOH Electrolyte
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of Graphene Oxide
3.3. Synthesis of GBPCs
3.4. Material Characterization
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. J. Mater. Chem. A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, N.; Ma, Y.; Wang, S.; Liu, W.; Luo, C.; Zhang, H.; Cheng, F.; Rao, J.; Hu, X.; et al. Highly Self-Healable 3D Microsupercapacitor with MXene-Graphene Composite Aerogel. Acs Nano 2018, 12, 4224–4232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Huang, H.; Xiao, C.; Zheng, S.; Shi, X.; Qin, J.; Fu, Q.; Bao, X.; Feng, X.; Mullen, K.; et al. Electrochemically Scalable Production of Fluorine-Modified Graphene for Flexible and High-Energy Ionogel-Based Microsupercapacitors. J. Am. Chem Soc. 2018, 140, 8198–8205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, K.; Li, S.; Li, M.; Li, J.; Ren, K. Synthesis of garlic skin-derived 3D hierarchical porous carbon for high-performance supercapacitors. Nanoscale 2018, 10, 2427–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Li, Z.; Xing, W.; Shen, H.; Bi, X.; Zhu, T.; Qiu, Z.; Zhuo, S. A New Approach to Tuning Carbon Ultramicropore Size at Sub-Angstrom Level for Maximizing Specific Capacitance and CO2 uptake. Adv. Funct. Mater. 2016, 26, 7955–7964. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, H.; Liu, J.; Xu, L.; Wu, H.; Zou, M.; Wang, Q.; He, X.; Li, Y.; Cao, A. Controlled Air-Etching Synthesis of Porous-Carbon Nanotube Aerogels with Ultrafast Charging at 1000 A g−1. Small 2018, 14, 1802394. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, Y.; Fan, H.; Liu, M.; Zhuo, O.; Wang, X.; Wu, Q.; Yang, L.; Ma, Y.; Hu, Z. Porous 3D Few-Layer Graphene-like Carbon for Ultrahigh-Power Supercapacitors with Well-Defined Structure-Performance Relationship. Adv Mater. 2017, 29, 1604569. [Google Scholar] [CrossRef]
- Yang, J.; Wu, H.; Zhu, M.; Ren, W.; Lin, Y.; Chen, H.; Pan, F. Optimized mesopores enabling enhanced rate performance in novel ultrahigh surface area meso-/microporous carbon for supercapacitors. Nano Energy 2017, 33, 453–461. [Google Scholar] [CrossRef]
- Xie, Q.; Huang, X.; Zhang, Y.; Wu, S.; Zhao, P. High performance aqueous symmetric supercapacitors based on advanced carbon electrodes and hydrophilic poly(vinylidene fluoride) porous separator. Appl. Surf. Sci. 2018, 443, 412–420. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Zhou, Z.; Yu, J.; Xing, W.; Han, G.; Si, W.; Zhuo, S. Nitrogen-doped hierarchical porous carbon materials prepared from meta-aminophenol formaldehyde resin for supercapacitor with high rate performance. Electrochim. Acta 2015, 153, 68–75. [Google Scholar] [CrossRef]
- Jiang, Q.; Pang, X.; Geng, S.; Zhao, Y.; Wang, X.; Qin, H.; Liu, B.; Zhou, J.; Zhou, T. Simultaneous cross-linking and pore-forming electrospun carbon nanofibers towards high capacitive performance. Appl. Surf. Sci. 2019, 479, 128–136. [Google Scholar] [CrossRef]
- Xue, G.; Zhong, J.; Cheng, Y.; Wang, B. Facile fabrication of cross-linked carbon nanofiber via directly carbonizing electrospun polyacrylonitrile nanofiber as high performance scaffold for supercapacitors. Electrochim. Acta 2016, 215, 29–35. [Google Scholar] [CrossRef]
- Su, X.L.; Chen, J.R.; Zheng, G.P.; Yang, J.-H.; Guan, X.X.; Liu, P.; Zheng, X.C. Three-dimensional porous activated carbon derived from loofah sponge biomass for supercapacitor applications. Appl. Surf. Sci. 2018, 436, 327–336. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Wei, T.; Fan, Z.; Yan, P. Nitrogen and sulfur co-doped porous carbon nanosheets derived from willow catkin for supercapacitors. Nano Energy 2016, 19, 165–175. [Google Scholar] [CrossRef]
- Lin, T.; Chen, W.; Liu, F.; Yang, C.; Bi, H.; Xu, F.; Huang, F. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 2015, 350, 1508. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Du, M.; Zhang, M.; Zou, M.; Yang, T.; Fu, Y.; Yao, J. The design and construction of 3D rose-petal-shaped MoS2 hierarchical nanostructures with structure-sensitive properties. J. Mater. Chem. A 2014, 2, 7680. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Zhu, Q.; Sun, N.; Anasori, B.; Hu, L.; Wang, F.; Guan, Y.; Gogotsi, Y. Reduced graphene oxide as a multi-functional conductive binder for supercapacitor electrodes. Energy Storage Mater. 2018, 12, 128–136. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, H.; Sun, H.; Hua, W.; Wang, H.; Liu, X.; Wei, B. One-pot synthesis of nitrogen-doped ordered mesoporous carbon spheres for high-rate and long-cycle life supercapacitors. Carbon 2018, 127, 85–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Pan, Z.; Sun, J.; Zhao, J.; Zhang, J.; Zhang, C.; Tang, L.; Luo, J.; Song, B.; et al. Wrapping Aligned Carbon Nanotube Composite Sheets around Vanadium Nitride Nanowire Arrays for Asymmetric Coaxial Fiber-Shaped Supercapacitors with Ultrahigh Energy Density. Nano Lett. 2017, 17, 2719–2726. [Google Scholar] [CrossRef]
- Shao, J.; Song, M.; Wu, G.; Zhou, Y.; Wan, J.; Ren, X.; Ma, F. 3D carbon nanocage networks with multiscale pores for high-rate supercapacitors by flower-like template and in-situ coating. Energy Storage Mater. 2018, 13, 57–65. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, Y.; Wang, L.; Liu, L.; Liu, Y.; Leng, J. Conductive Shape Memory Microfiber Membranes with Core-Shell Structures and Electroactive Performance. Acs Appl. Mater. Interfaces 2018, 10, 35526–35532. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, B.; Ding, Y.; Liang, H.; Li, C.; Yu, Z.; Wu, Z.; Chen, W.; Yu, S. Wood-Derived Ultrathin Carbon Nanofiber Aerogels. Angew. Chem. Int. Ed. 2018, 57, 1–7. [Google Scholar]
- Herou, S.; Ribadeneyra, M.C.; Madhu, R.; Araullo-Peters, V.; Jensen, A.; Schlee, P.; Titirici, M. Ordered mesoporous carbons from lignin: A new class of biobased electrodes for supercapacitors. Green Chem. 2019, 21, 550–559. [Google Scholar] [CrossRef]
- Heo, Y.J.; Lee, H.I.; Lee, J.W.; Park, M.; Rhee, K.Y.; Park, S.J. Optimization of the pore structure of PAN-based carbon fibers for enhanced supercapacitor performances via electrospinning. Compos. Part. B Eng. 2019, 161, 10–17. [Google Scholar] [CrossRef]
- Lv, T.; Liu, M.; Zhu, D.; Gan, L.; Chen, T. Nanocarbon-Based Materials for Flexible All-Solid-State Supercapacitors. Adv Mater. 2018, 30, e1705489. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Liu, X.; Ma, C.; Li, J.; Zielinska, B.; Kalenczuk, R.J.; Chen, X.; Chu, P.K.; Tang, T.; Mijowska, E. Biomass-derived robust three-dimensional porous carbon for high volumetric performance supercapacitors. J. Power Sources 2019, 412, 1–9. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Fan, Z. Biomass-derived carbon materials with structural diversities and their applications in energy storage. Sci. China Mater. 2017, 61, 133–158. [Google Scholar] [CrossRef] [Green Version]
- Meng, C.Z.; Liu, C.H.; Chen, L.Z.; Hu, C.H.; Fan, S. Highly Flexible and All-Solid-State Paperlike Polymer Supercapacitors. Nano Lett. 2010, 10, 4025–4031. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Bi, X.; Zhou, T.; Zhou, J.; Zhao, J.; Miao, Z.; Yi, W.; Fu, P.; Zhuo, S. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar] [CrossRef]
- Sun, K.; Yu, S.; Hu, Z.; Li, Z.; Lei, G.; Xiao, Q.; Ding, Y. Oxygen-containing hierarchically porous carbon materials derived from wild jujube pit for high-performance supercapacitor. Electrochim. Acta 2017, 231, 417–428. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, P.; Zhou, F.; Zeng, W.; Su, H.; Li, G.; Gao, J.; Sun, R.; Wong, C.P. Flexible Asymmetrical Solid-State Supercapacitors Based on Laboratory Filter Paper. Acs Nano 2016, 10, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Wahid, M.; Puthusseri, D.; Phase, D.; Ogale, S. Enhanced Capacitance Retention in a Supercapacitor Made of Carbon from Sugarcane Bagasse by Hydrothermal Pretreatment. Energy Fuels 2014, 28, 4233–4240. [Google Scholar] [CrossRef]
- Liu, N.; Ma, W.; Tao, J.; Zhang, X.; Su, J.; Li, L.; Yang, C.; Gao, Y.; Golberg, D.; Bando, Y. Cable-type supercapacitors of three-dimensional cotton thread based multi-grade nanostructures for wearable energy storage. Adv. Mater. 2013, 25, 4925–4931. [Google Scholar] [CrossRef]
- He, X.; Ling, P.; Yu, M.; Wang, X.; Zhang, X.; Zheng, M. Rice husk-derived porous carbons with high capacitance by ZnCl2 activation for supercapacitors. Electrochim. Acta 2013, 105, 635–641. [Google Scholar] [CrossRef]
- Gund, G.S.; Park, J.H.; Harpalsinh, R.; Kota, M.; Gogotsi, Y.; Park, H.S. MXene/Polymer Hybrid Materials for Flexible AC-Filtering Electrochemical Capacitors. Joule 2018, 3, 164–176. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.X.; Lin, Z.Y.; Huang, X.Q.; Wang, Y.; Huang, Y.; Duan, X.F. Functionalized Graphene Hydrogel-Based High-Performance Supercapacitors. Adv. Mater. 2013, 25, 5779–5784. [Google Scholar] [CrossRef]
- Zhou, T.; Jiang, Q.; Wang, L.; Qiu, Z.; Liu, Y.; Zhou, J.; Liu, B. Facile preparation of nitrogen-enriched hierarchical porous carbon nanofibers by Mg(OAc) 2 -assisted electrospinning for flexible supercapacitors. Appl. Surf. Sci. 2018, 456, 827–834. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, T.; Hou, G.; Kou, T.; Yue, L.; Guan, R.; Li, Y. Hierarchically porous carbon foams for electric double layer capacitors. Nano Res. 2016, 9, 2875–2888. [Google Scholar] [CrossRef]
- You, B.; Yang, J.; Sun, Y.; Su, Q. Easy synthesis of hollow core, bimodal mesoporous shell carbon nanospheres and their application in supercapacitor. Chem. Commun. 2011, 47, 12364–12366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, Y.; Kopec, M.; Lee, J.; Wang, Z.; Liu, S.; Yan, J.; Yuan, R.; Kowalewski, T.; Bockstaller, M.R.; et al. Facile Aqueous Route to Nitrogen-Doped Mesoporous Carbons. J. Am. Chem. Soc. 2017, 139, 12931–12934. [Google Scholar] [CrossRef] [PubMed]
- Vishnuprakash, P.; Nithya, C.; Premalatha, M. Exploration of V2O5 nanorod@rGO heterostructure as potential cathode material for potassium-ion batteries. Electrochim. Acta 2019, 309, 234–241. [Google Scholar] [CrossRef]
- Chang, B.; Zhang, S.; Sun, L.; Yin, H.; Yang, B. 2D graphene-like hierarchically porous carbon nanosheets from a nano-MgO template and ZnCl2 activation: Morphology, porosity and supercapacitance performance. Rsc Adv. 2016, 6, 71360–71369. [Google Scholar] [CrossRef]
- Foo, C.Y.; Sumboja, A.; Tan, D.J.H.; Wang, J.; Lee, P.S. Flexible and Highly Scalable V2O5-rGO Electrodes in an Organic Electrolyte for Supercapacitor Devices. Adv. Energy Mater. 2014, 4, 1400236. [Google Scholar] [CrossRef]
- Fan, Y.M.; Liu, Y.; Liu, X.; Liu, Y.; Fan, L.Z. Hierarchical porous NiCo2S4-rGO composites for high-performance supercapacitors. Electrochim. Acta 2017, 249, 1–8. [Google Scholar] [CrossRef]
- Huang, J.L.; Wang, J.Y.; Wang, C.W.; Zhang, H.N.; Lu, C.X.; Wang, J.Z. Hierarchical Porous Graphene Carbon-Based Supercapacitors. Chem. Mater. 2015, 27, 2107–2113. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhang, H.T.; Sun, X.Z.; Zhang, D.C.; Ma, Y.W. High-performance supercapacitors based on a graphene-activated carbon composite prepared by chemical activation. Rsc Adv. 2012, 2, 7747–7753. [Google Scholar] [CrossRef]
- Mo, M.; Chen, C.; Gao, H.; Chen, M.; Li, D. Wet-spinning assembly of cellulose nanofibers reinforced graphene/polypyrrole microfibers for high performance fiber-shaped supercapacitors. Electrochim. Acta 2018, 269, 11–20. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X.; Han, J.; Yu, L.; Chen, J.; Wu, Q.; Jiang, J. Effects of nanocellulose on sodium alginate/polyacrylamide hydrogel: Mechanical properties and adsorption-desorption capacities. Carbohydr. Polym. 2019, 206, 289–301. [Google Scholar] [CrossRef]
- Song, K.; Ni, H.; Fan, L.Z. Flexible graphene-based composite fifilms for supercapacitors with tunable areal capacitance. Electrochim. Acta 2017, 235, 233–241. [Google Scholar]
- Chang, Y.; Zhou, L.; Xiao, Z.; Liang, J.; Kong, D.; Li, Z.; Zhang, X.; Li, X.; Zhi, L. Embedding reduced graphene oxide in bacterial cellulose-derived carbon nanofifibril networks for supercapacitors. ChemElectroChem 2017, 4, 2448–2452. [Google Scholar] [CrossRef]
- Zou, B.X.; Wang, Y.; Huang, X.; Lu, Y. Hierarchical N- and O-doped porous carbon composites for high-performance supercapacitors. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Tran, C.D.; Ho, H.C.; Keum, J.K.; Chen, J.; Gallego, N.C.; Naskar, A.K. Sustainable energy-storage materials from lignin-graphene nanocomposite-derived porous carbon fifilm. Energy Technol. 2017, 5, 1927e1935. [Google Scholar]
- Tang, Z.J.; Pei, Z.X.; Wang, Z.F.; Li, H.F.; Zeng, J.; Ruan, Z.H.; Huang, Y.; Zhu, M.; Xue, Q.; Yu, J.; et al. Highly anisotropic, multichannel wood carbon with optimized heteroatom doping for supercapacitor and oxygen reduction reaction. Carbon 2018, 130, 532–543. [Google Scholar] [CrossRef]
- Hu, P.; Meng, D.; Ren, G.; Yan, R.; Peng, X. Nitrogen-doped mesoporous carbon thin fifilm for binder-free supercapacitor. Appl. Mater. Today 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Purkait, T.; Singh, G.; Singh, M.; Kumar, D.; Dey, R.S. Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci. Rep. 2017, 7, 15239. [Google Scholar]
- Nguyen, T.D.; Shopsowitz, K.E.; MacLachlan, M.J. Mesoporous nitrogen-doped carbon from nanocrystalline chitin assemblies. J. Mater. Chem 2014, 2, 5915. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Zhang, J.; Jin, L.; Zhao, X.; Xu, T. A top-down approach for fabricating free-standing bio-carbon supercapacitor electrodes with a hierarchical structure. Sci. Rep. 2015, 5, 14155. [Google Scholar] [CrossRef] [Green Version]
- Kostoglou, N.; Koczwara, C.; Prehal, C.; Terziyska, V.; Babic, B.; Matovic, B.; Constantinides, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; et al. Nanoporous activated carbon cloth as a versatile material for hydrogen adsorption, selective gas separation and electrochemical energy storage. Nano Energy 2017, 40, 49–64. [Google Scholar] [CrossRef]
Sample Availability: Samples of the GBPC-x reported in this article are available from the authors. |
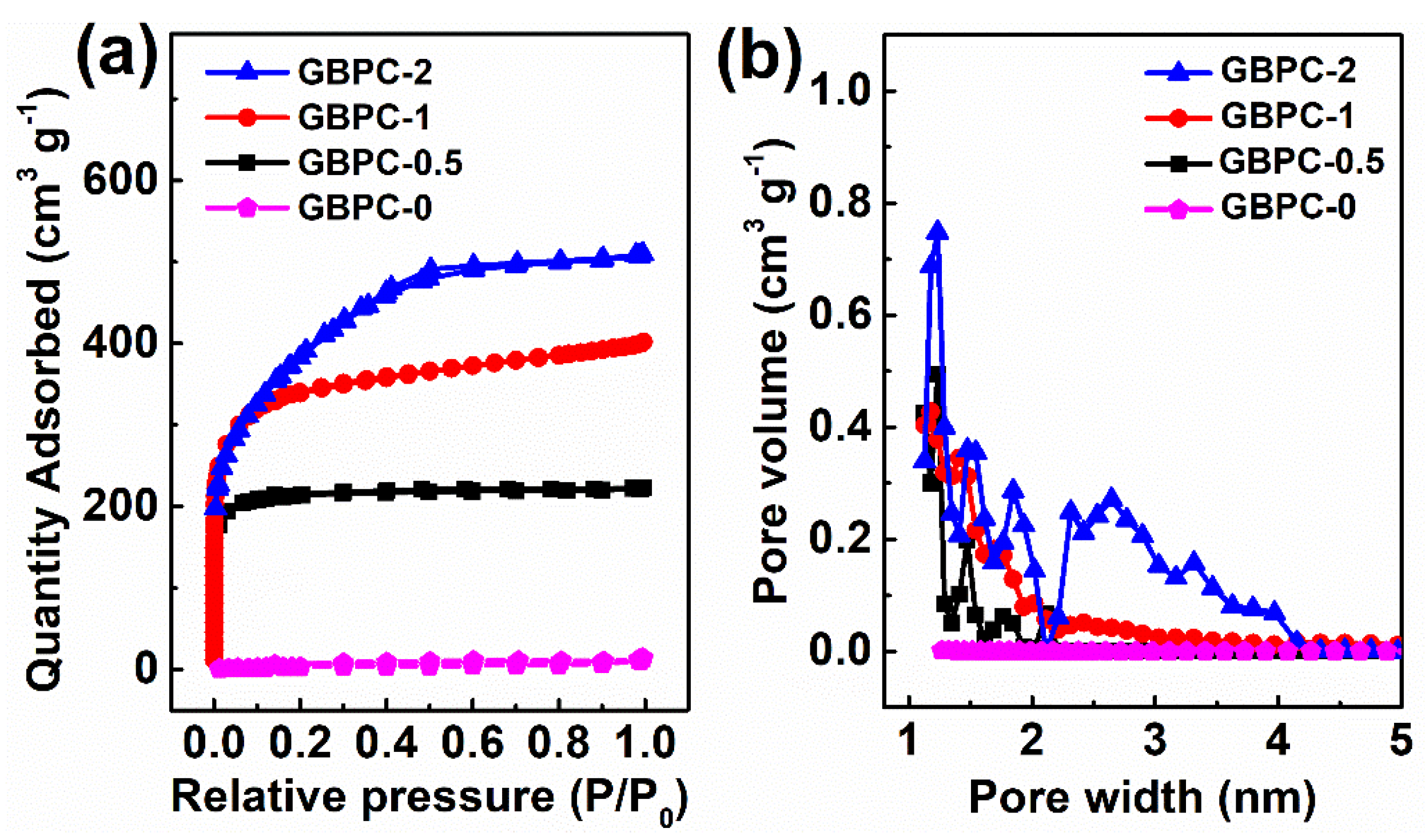





| Sample | SBET /m2 g−1 | Smicro /m2 g−1 | VT /cm3 g−1 | Vmicro /cm3 g−1 | Vmacro /cm3 g−1 | D /nm |
|---|---|---|---|---|---|---|
| GBPC-0 | 13.4 | 3.73 | 0.02 | 0.001 | 0.0019 | 5.97 |
| GBPC-0.5 | 715.7 | 416.87 | 0.34 | 0.32 | 0.02 | 1.93 |
| GBPC-1 | 1066.9 | 613.59 | 0.62 | 0.33 | 0.29 | 2.33 |
| GBPC-2 | 1388.1 | 541.96 | 0.79 | 0.36 | 0.43 | 2.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Liu, D.; Liu, B.; Zhou, T.; Zhou, J. Blotting Paper-Derived Activated Porous Carbon/Reduced Graphene Oxide Composite Electrodes for Supercapacitor Applications. Molecules 2019, 24, 4625. https://doi.org/10.3390/molecules24244625
Jiang Q, Liu D, Liu B, Zhou T, Zhou J. Blotting Paper-Derived Activated Porous Carbon/Reduced Graphene Oxide Composite Electrodes for Supercapacitor Applications. Molecules. 2019; 24(24):4625. https://doi.org/10.3390/molecules24244625
Chicago/Turabian StyleJiang, Qinting, Dandan Liu, Bo Liu, Tong Zhou, and Jin Zhou. 2019. "Blotting Paper-Derived Activated Porous Carbon/Reduced Graphene Oxide Composite Electrodes for Supercapacitor Applications" Molecules 24, no. 24: 4625. https://doi.org/10.3390/molecules24244625
APA StyleJiang, Q., Liu, D., Liu, B., Zhou, T., & Zhou, J. (2019). Blotting Paper-Derived Activated Porous Carbon/Reduced Graphene Oxide Composite Electrodes for Supercapacitor Applications. Molecules, 24(24), 4625. https://doi.org/10.3390/molecules24244625





