High Sensitive Immunoelectrochemical Measurement of Lung Cancer Tumor Marker ProGRP Based on TiO2-Au Nanocomposite
Abstract
1. Introduction
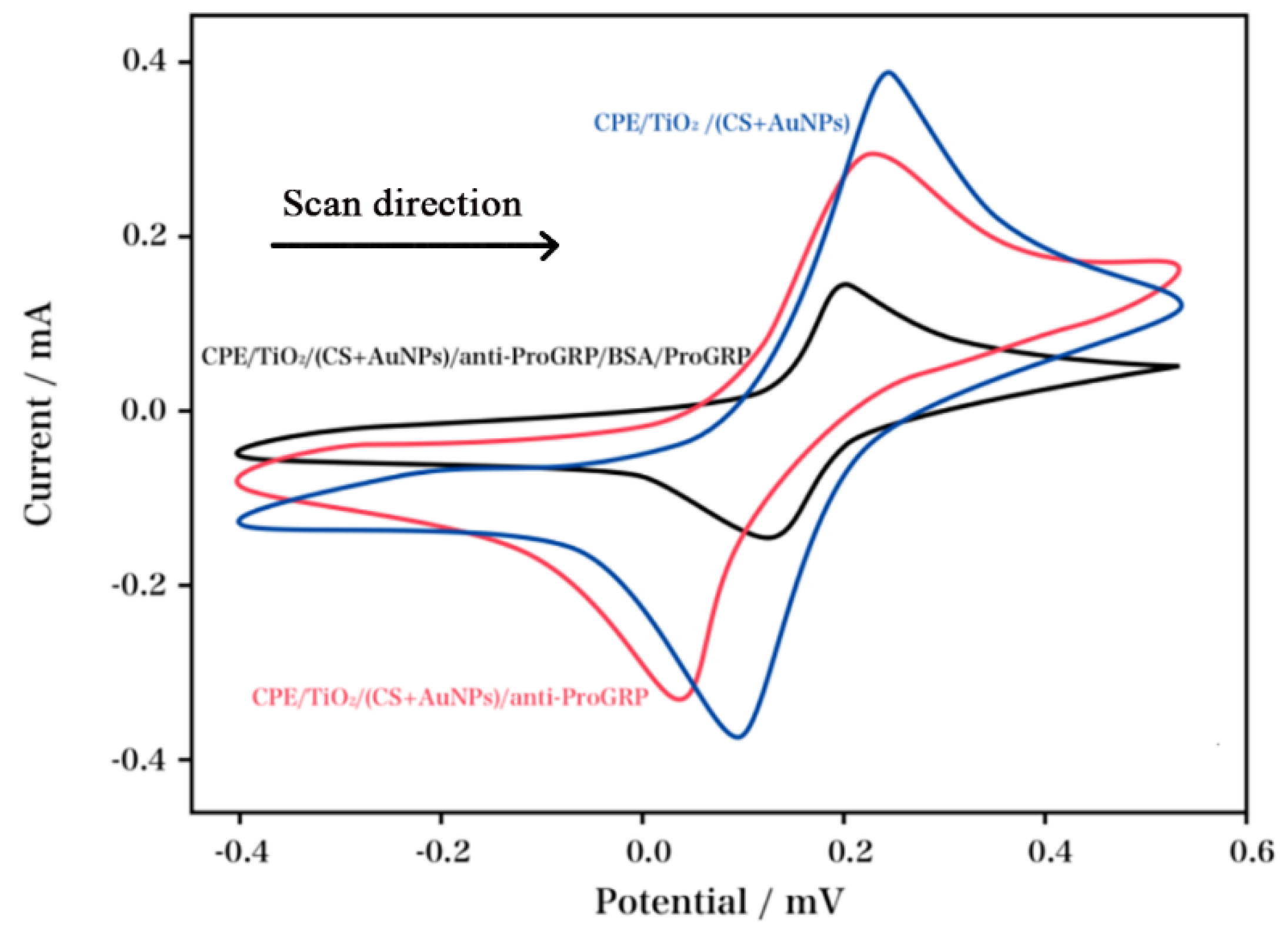
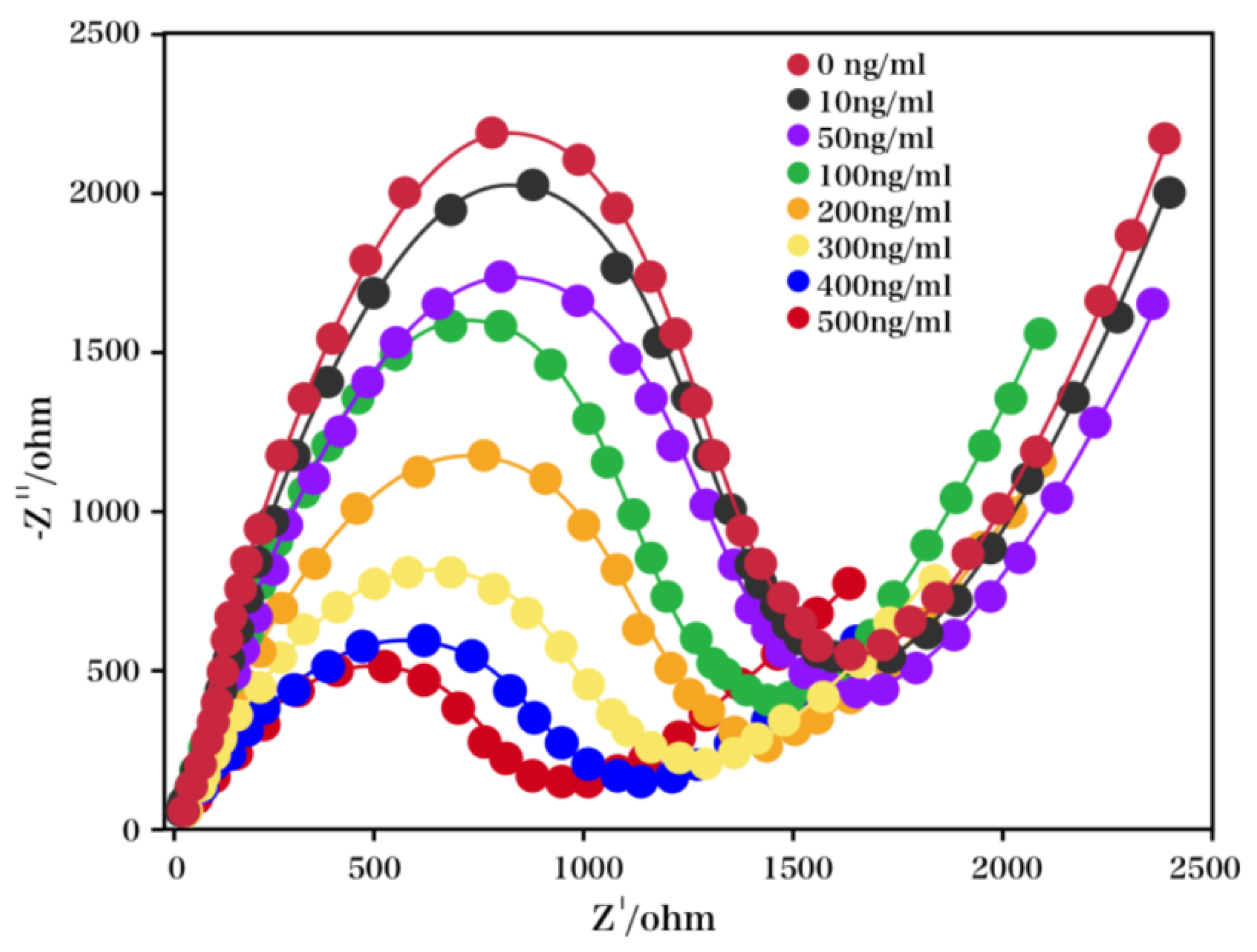
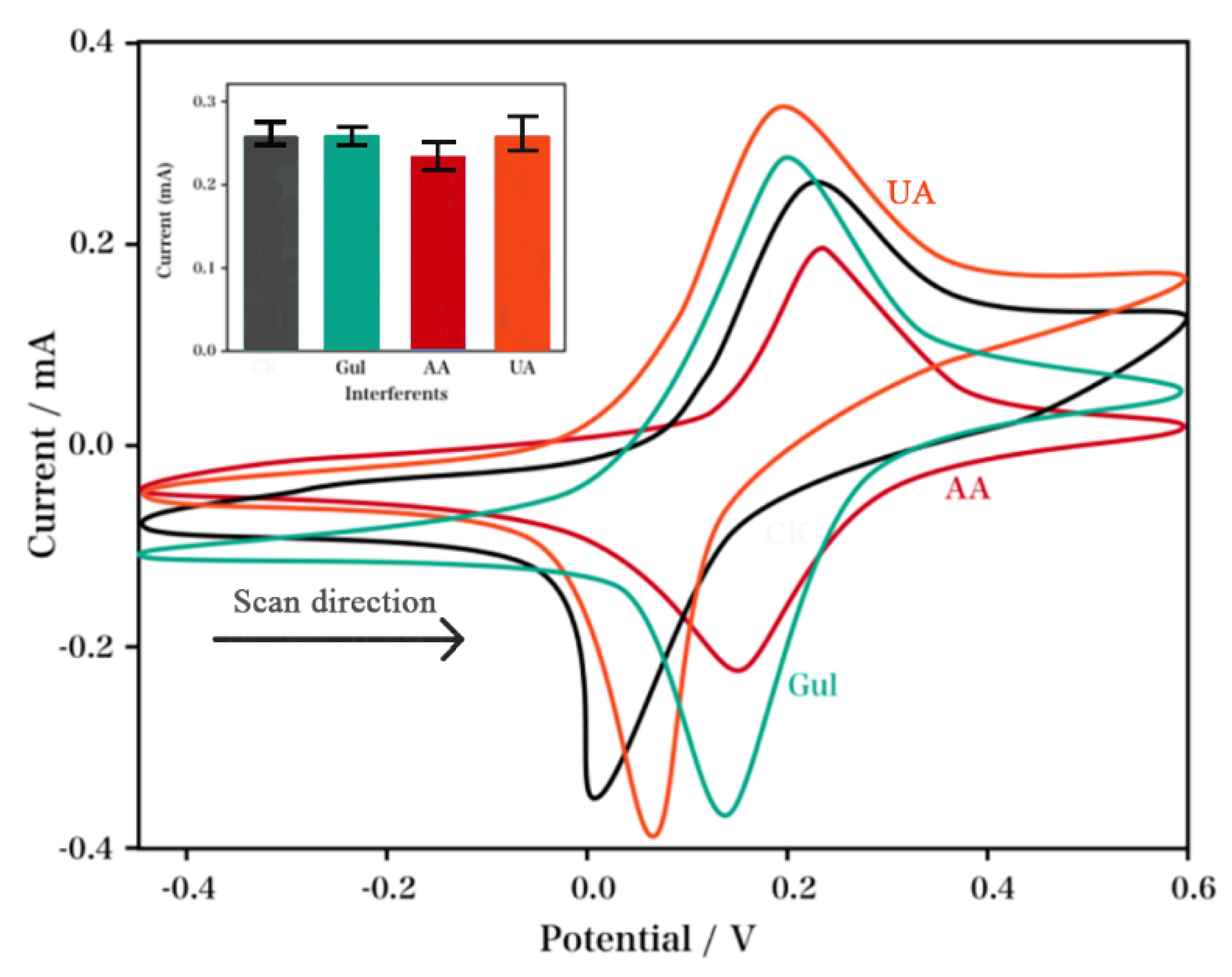
2. Results and Discussion
3. Experiments
3.1. Chemicals
3.2. Nanocomposite Preparation
3.3. Immunosensor Fabrication
3.4. Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cai, X.; Weng, S.; Guo, R.; Lin, L.; Chen, W.; Zheng, Z.; Huang, Z.; Lin, X. Ratiometric Electrochemical Immunoassay Based on Internal Reference Value for Reproducible and Sensitive Detection of Tumor Marker. Biosens. Bioelectron. 2016, 81, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Yu, J.; Ge, S.; Song, X. All-Graphene Composite Materials for Signal Amplification toward Ultrasensitive Electrochemical Immunosensing of Tumor Marker. Biosens. Bioelectron. 2015, 71, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Farzin, L.; Tabrizi, M.A.; Molaabasi, F. Highly Sensitive Label Free Electrochemical Detection of Vgef 165 Tumor Marker Based on “Signal Off” and “Signal on” Strategies Using an Anti-Vegf 165 Aptamer Immobilized Bsa-Gold Nanoclusters/Ionic Liquid/Glassy Carbon Electrode. Biosens. Bioelectron. 2015, 74, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Peng, N.; Qing, Y.; Shan, J.; Li, M.; Guan, W.; Dai, N.; Gu, X.; Wang, D. An Electrochemical Immunosensor for Simultaneous Multiplexed Detection of Neuron-Specific Enolase and Pro-Gastrin-Releasing Peptide Using Liposomes as Enhancer. Electrochim. Acta 2011, 56, 5624–5629. [Google Scholar] [CrossRef]
- Zhuo, Y.; Chai, Y.; Yuan, R.; Mao, L.; Yuan, Y.; Han, J. Glucose Oxidase and Ferrocene Labels Immobilized at Au/TiO2 Nanocomposites with High Load Amount and Activity for Sensitive Immunoelectrochemical Measurement of Progrp Biomarker. Biosens. Bioelectron. 2011, 26, 3838–3844. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, A.; Lai, G.; Lin, C.; Yu, J.; Yu, A.; Liu, Z.; Xie, K.; Su, W. A Glassy Carbon Electrode Modified with N-Doped Carbon Dots for Improved Detection of Hydrogen Peroxide and Paracetamol. Microchim. Acta 2018, 185, 87. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Liu, Z.; Liu, N.; Ma, Z. A Label-Free Immunosensor Based on Graphene Nanocomposites for Simultaneous Multiplexed Electrochemical Determination of Tumor Markers. Biosens. Bioelectron. 2014, 53, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Ho, C.; Cheng, A.; Yu, H. Immobilization of Redox-Labeled Hairpin DNA Aptamers on Gold: Electrochemical Quantitation of Epithelial Tumor Marker Mucin 1. Electrochim. Acta 2013, 110, 139–145. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, N. Design of Immunoprobes for Electrochemical Multiplexed Tumor Marker Detection. Expert Rev. Mol. Diagn. 2015, 15, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Burgio, S.; Menna, C.; Carretta, E.; Rossi, L.; Bianchi, E.; Masini, C.; Amadori, D.; Giorgi, U. Chromogranin a Is a Potential Prognostic Marker in Prostate Cancer Patients Treated with Enzalutamide. Prostate 2014, 74, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Dayyani, F.; Morgenstern, D.; Holdenrieder, S. In Response to “Serum Tumor Marker Use in Patients with Advanced Solid Tumors”. J. Oncol. Pract. 2016, 12, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yin, P.; Tan, Y.; Dong, L.; Hu, C.; Huang, Q.; Lu, X.; Wang, H.; Xu, G. Metabolomics Study of Hepatocellular Carcinoma: Discovery and Validation of Serum Potential Biomarkers by Using Capillary Electrophoresis–Mass Spectrometry. J. Proteome Res. 2014, 13, 3420–3431. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mei, Q.; Jia, S.; Koh, K.; Wang, K.; Liu, X. High Specific Detection of Osteopontin Using a Three-Dimensional Copolymer Layer Support Based on Electrochemical Impedance Spectroscopy. Analyst 2014, 139, 4476–4481. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Ye, X.; Wu, T.; Deng, H.; Wu, P.; Li, C. Sensing Platform for Neuron Specific Enolase Based on Molecularly Imprinted Polymerized Ionic Liquids in between Gold Nanoarrays. Biosens. Bioelectron. 2018, 99, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Gu, H.; Jeon, W.; Youn, H.; Her, J.; Kim, S.; Lee, J.; Shin, J.; Ban, C. Electrochemical Aptasensor of Cardiac Troponin I for the Early Diagnosis of Acute Myocardial Infarction. Anal. Chem. 2015, 87, 9869–9875. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Sung, H. Proteomic Approaches in Lung Cancer Biomarker Development. Expert Rev. Proteomic. 2009, 6, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Palmblad, M.; Tiss, A.; Cramer, R. Mass Spectrometry in Clinical Proteomics–from the Present to the Future. Proteom. Clin. Appl. 2009, 3, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhang, J.; Zhang, A.; Wang, Y.; Cai, X. Electrochemical Detecting Lung Cancer-Associated Antigen Based on Graphene-Gold Nanocomposite. Molecules 2017, 22, 392. [Google Scholar] [CrossRef] [PubMed]
- Anik, Ü.; Timur, S. Towards the Electrochemical Diagnosis of Cancer: Nanomaterial-Based Immunosensors and Cytosensors. RSC Adv. 2016, 6, 111831–111841. [Google Scholar] [CrossRef]
- Fengjin, Q.; Hou, X.; Ma, X. Research Progress in the Sensor Application of Ferrocene and Its Derivatives. Chin. J. Appl. Chem. 2013, 30, 1393–1398. [Google Scholar]
- Kiani, M.; Tehrani, A.; Sayahi, H. Reusable and Robust High Sensitive Non-Enzymatic Glucose Sensor Based on Ni (Oh) 2 Nanoparticles. Anal. Chim. Acta 2014, 839, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Stefan-van Staden, R.I.; Comnea, I.R.; van Staden, J.F.; Gavan, C.S. Stochastic Microsensors as Screening Tools for Neuron Specific Enolase. RSC Adv. 2014, 4, 26383–26388. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, R.; Chai, Y.; Zhuo, Y.; Su, H.; Zhang, Y. Horseradish Peroxidase-Loaded Nanospheres Attached to Hollow Gold Nanoparticles as Signal Enhancers in an Ultrasensitive Immunoassay for Alpha-Fetoprotein. Microchim. Acta 2014, 181, 679–685. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, S.; Ye, C.; Liu, X.; Gao, J.; Cui, N.; Guo, P.; Lai, G.; Wei, Q.; Yang, M.; et al. Sensitivity Enhancement of Potassium Ion (K+) Detection Based on Graphene Field-Effect Transistors with Surface Plasma Pretreatment. Sens. Actuators B Chem. 2019, 285, 333–340. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhang, H.; Zhuang, W.; Zhang, H.; Wang, A.; Su, W.; Yu, Y.; Lin, C. Enhanced Electrochemical Voltammetric Fingerprints for Plant Taxonomic Sensing. Biosens. Bioelectron. 2018, 120, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xiao, X.; Wang, A. Reduced Graphene Oxide Coupled with G-C3n4 Nanodots as 2d/0d Nanocomposites for Enhanced Photocatalytic Activity. J. Phys. Chem. Solids 2018, 122, 104–108. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, Y.; Ye, C.; Sun, H.; Dai, D.; Wei, Q.; Lai, G.; Wu, T.; Yu, A.; Fu, L.; et al. Highly Stable and Regenerative Graphene–Diamond Hybrid Electrochemical Biosensor for Fouling Target Dopamine Detection. Biosens. Bioelectron. 2018, 111, 117–123. [Google Scholar] [CrossRef]
- Takahashi, S.; Anzai, J. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials 2013, 6, 5742–5762. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Zhang, S.; Wang, W.; Chen, Z. A Highly Sensitive Nonenzymatic Glucose Sensor Based on Cuo Nanoparticles Decorated Carbon Spheres. Sens. Actuators B Chem. 2015, 211, 385–391. [Google Scholar] [CrossRef]
- Saravanan, R.; Khan, M.; Gupta, V.; Mosquera, E.; Gracia, F.; Narayanan, V.; Stephen, A. Zno/Ag/Mn2O3 Nanocomposite for Visible Light-Induced Industrial Textile Effluent Degradation, Uric Acid and Ascorbic Acid Sensing and Antimicrobial Activity. RSC Adv. 2015, 5, 34645–34651. [Google Scholar] [CrossRef]
- Mathur, S.; Erdem, A.; Cavelius, C.; Barth, S.; Altmayer, J. Amplified Electrochemical DNA-Sensing of Nanostructured Metal Oxide Films Deposited on Disposable Graphite Electrodes Functionalized by Chemical Vapor Deposition. Sens. Actuators B Chem. 2009, 136, 432–437. [Google Scholar] [CrossRef]
- Lucarelli, L.; Nadtochenko, V.; Kiwi, J. Environmental Photochemistry: Quantitative Adsorption and Ftir Studies During the Tio2-Photocatalyzed Degradation of Orange Ii. Langmuir 2000, 16, 1102–1108. [Google Scholar] [CrossRef]
- Kavan, L.; Rathouský, J.; Grätzel, M.; Shklover, V.; Zukal, A. Surfactant-Templated Tio2 (Anatase): Characteristic Features of Lithium Insertion Electrochemistry in Organized Nanostructures. J. Phys. Chem. B 2000, 104, 12012–12020. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Zhang, P.; Zhu, J.; Zhang, H.; Zhang, L.; Su, W. Embedding Leaf Tissue in Graphene Ink to Improve Signals in Electrochemistry-Based Chemotaxonomy. Electrochem. Commun. 2018, 92, 39–42. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lyu, F.; Lai, G.; Yu, J.; Lin, C.; Liu, Z.; Yu, A.; Su, W. A Solid-State Electrochemical Sensing Platform Based on a Supramolecular Hydrogel. Sens. Actuators B Chem. 2018, 262, 326–333. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, M.; Zhou, N.; Liu, L.; Song, X. Serum Human Epididymis Protein 4 (He4) May Be a Better Tumor Marker in Early Lung Cancer. Clin. Chim. Acta 2016, 455, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, D.; Zhang, F.; Ying, Y.; Song, L. Pro-Gastrin-Releasing Peptide and Neuron-Specific Enolase: Useful Predictors of Response to Chemotherapy and Survival in Patients with Small Cell Lung Cancer. Clin. Transl. Oncol. 2016, 18, 1019–1025. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lai, G.; Su, W.; Malherbe, F.; Yu, J.; Lin, C.; Yu, A. Defects Regulating of Graphene Ink for Electrochemical Determination of Ascorbic Acid, Dopamine and Uric Acid. Talanta 2018, 180, 248–253. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Sample | Addition | ELISA | Proposed Sensor |
|---|---|---|---|
| 1 | 1 ng/mL | 0.97 ng/mL | 0.98 ng/mL |
| 2 | 2 ng/mL | 1.72 ng/mL | 1.94 ng/mL |
| 3 | 5 ng/mL | Out of detection range | 4.88 ng/mL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Cai, X.; Zhang, J.; Fan, J.; Xu, J.; Xu, L. High Sensitive Immunoelectrochemical Measurement of Lung Cancer Tumor Marker ProGRP Based on TiO2-Au Nanocomposite. Molecules 2019, 24, 656. https://doi.org/10.3390/molecules24040656
Wei Z, Cai X, Zhang J, Fan J, Xu J, Xu L. High Sensitive Immunoelectrochemical Measurement of Lung Cancer Tumor Marker ProGRP Based on TiO2-Au Nanocomposite. Molecules. 2019; 24(4):656. https://doi.org/10.3390/molecules24040656
Chicago/Turabian StyleWei, Zheng, Xiaoping Cai, Junping Zhang, Junming Fan, Jiangyan Xu, and Liran Xu. 2019. "High Sensitive Immunoelectrochemical Measurement of Lung Cancer Tumor Marker ProGRP Based on TiO2-Au Nanocomposite" Molecules 24, no. 4: 656. https://doi.org/10.3390/molecules24040656
APA StyleWei, Z., Cai, X., Zhang, J., Fan, J., Xu, J., & Xu, L. (2019). High Sensitive Immunoelectrochemical Measurement of Lung Cancer Tumor Marker ProGRP Based on TiO2-Au Nanocomposite. Molecules, 24(4), 656. https://doi.org/10.3390/molecules24040656






