Stability Analysis of Multi Process Parameters for Metal-Organic Chemical Vapor Deposition Reaction Cavity
Abstract
:1. Introduction
2. Problem Formulation
2.1. Governing Equations
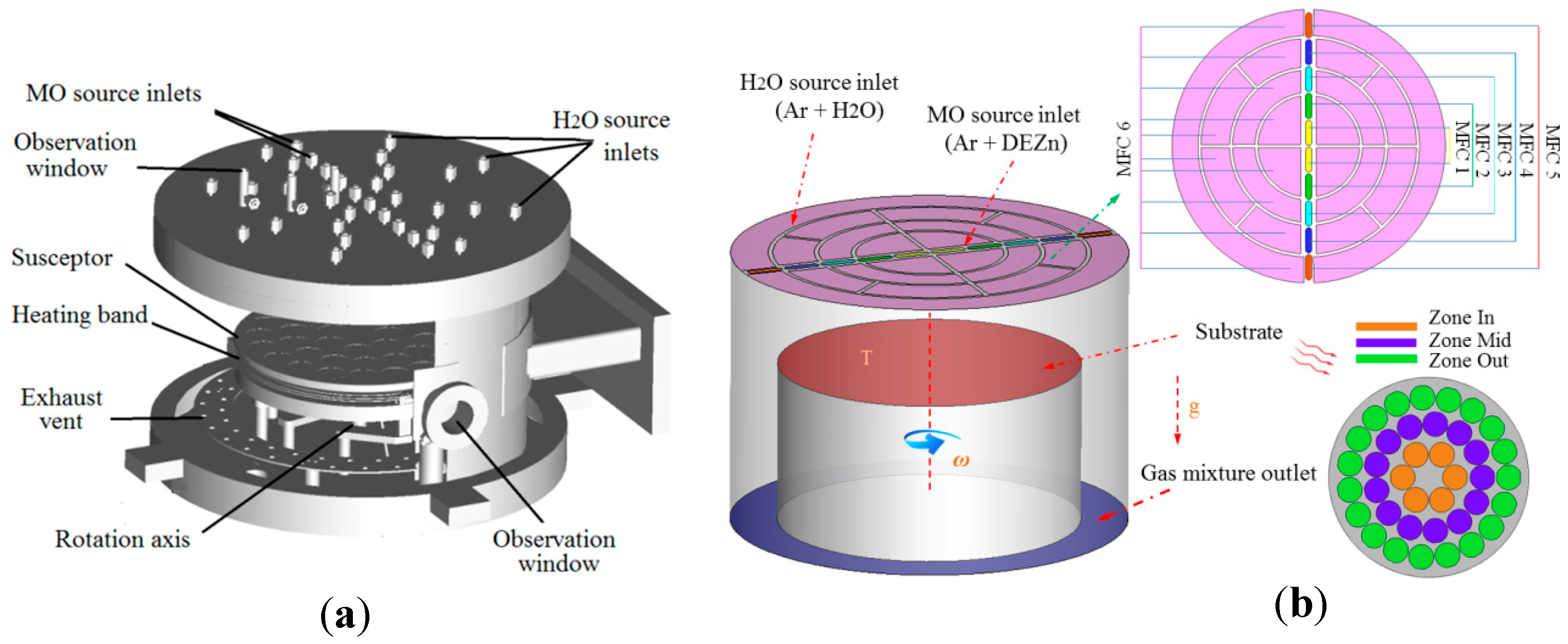
2.2. Geometric Model Description
2.3. Reaction Mechanisms
3. Effect of Process Parameters on Flow Stability in Cavity
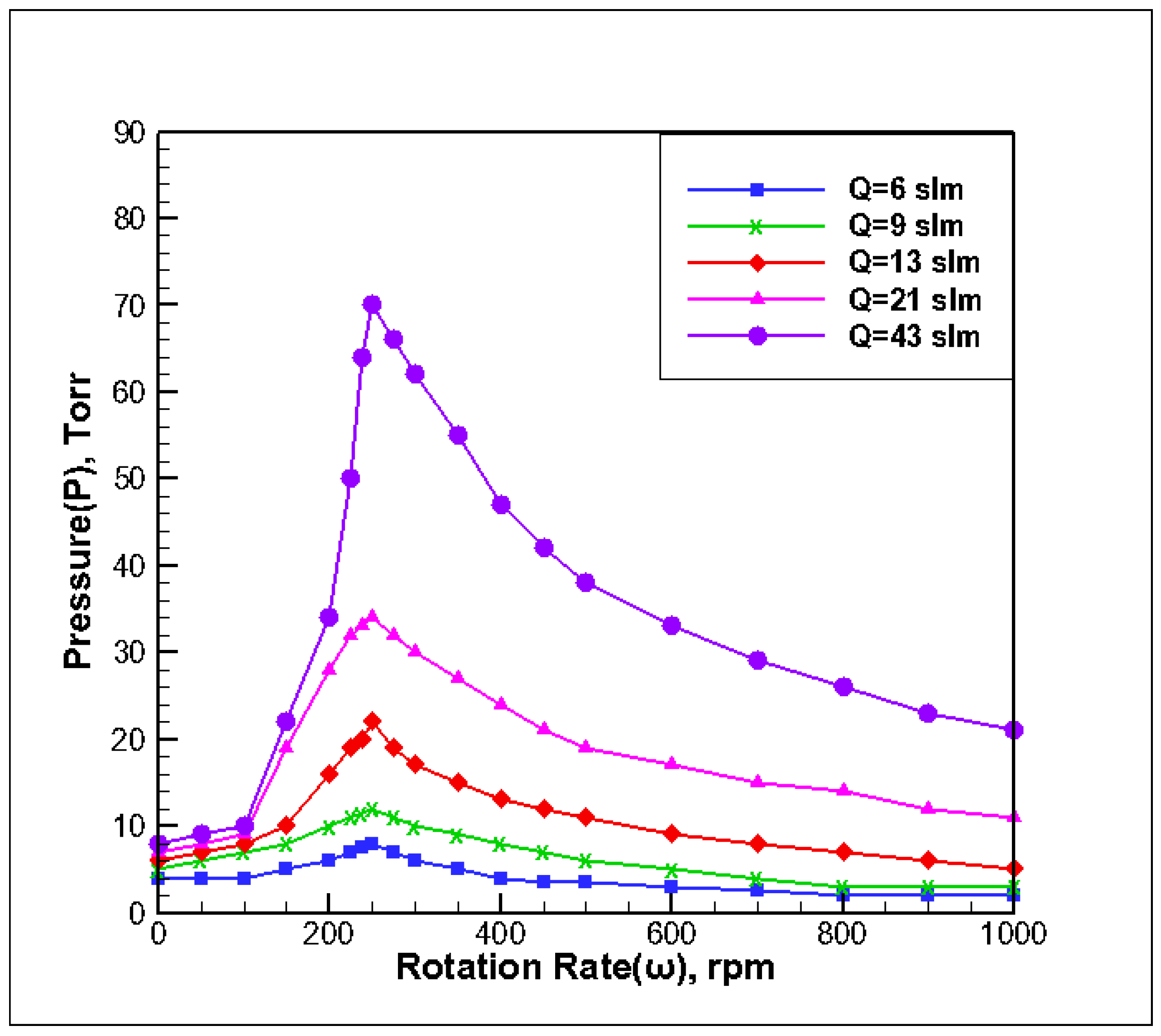
3.1. Effect of Inlet Flow on Stability of Reaction Chamber
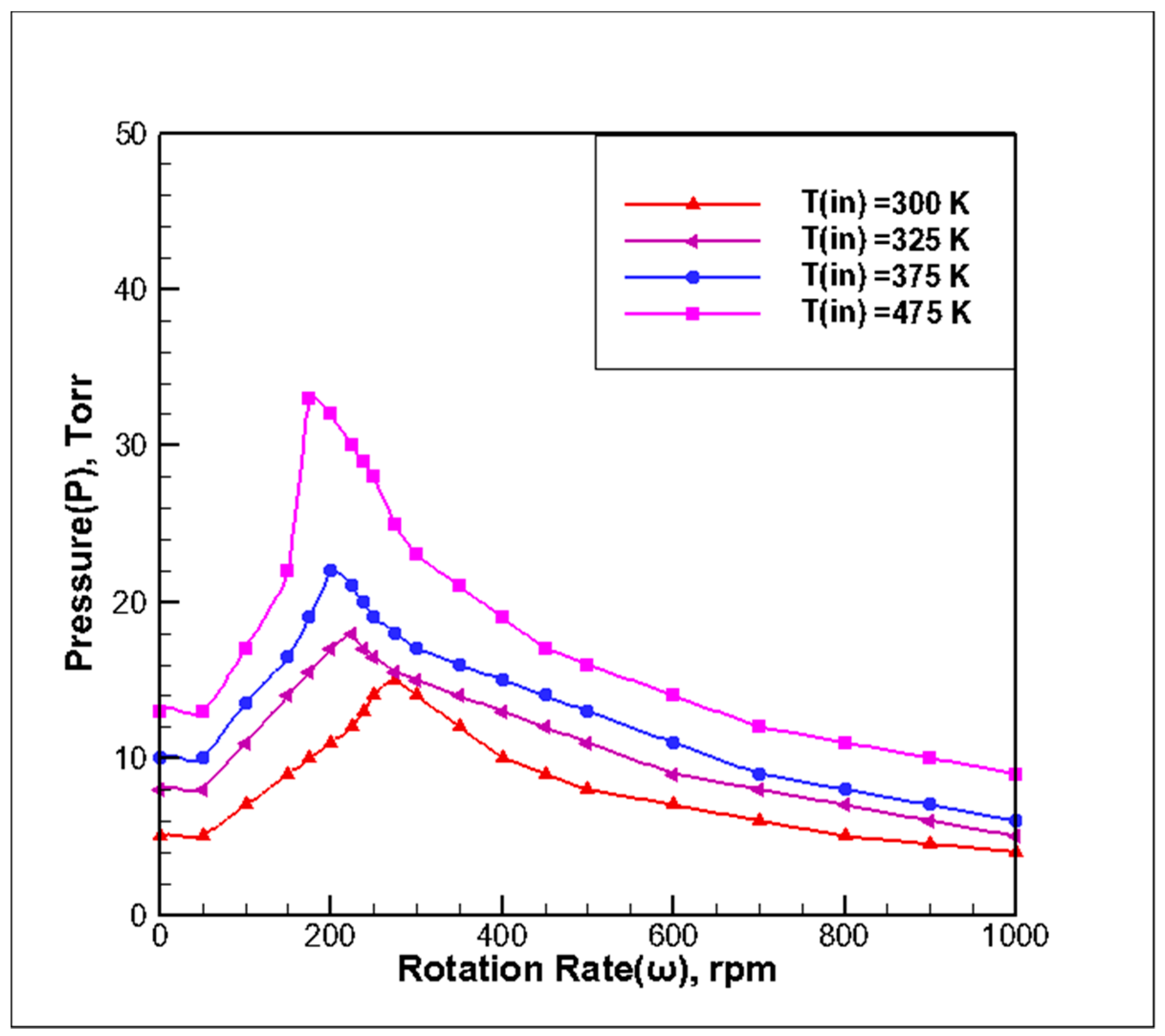
3.2. Effect of Inlet Temperature on Stability of Reaction Chamber
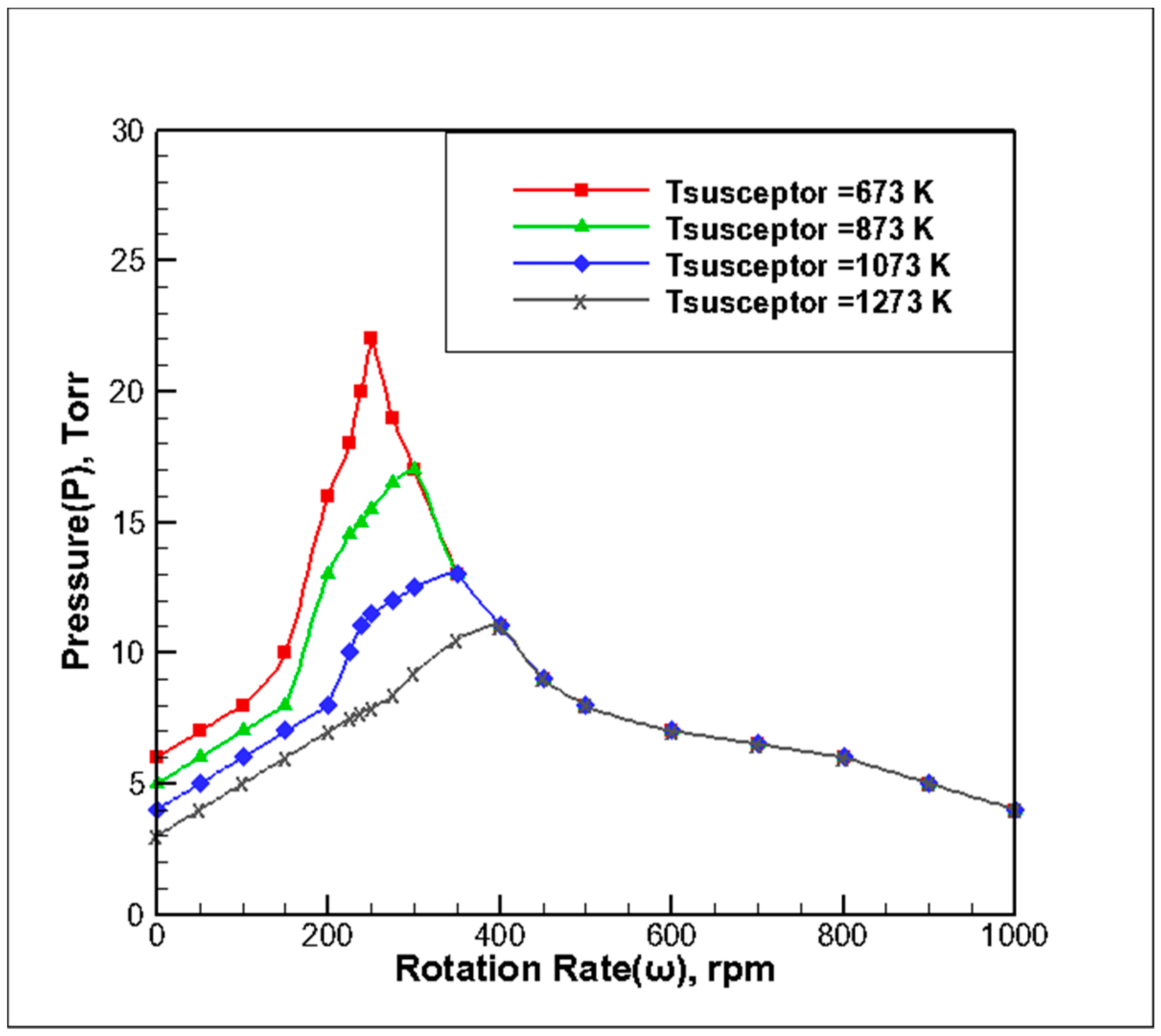
3.3. Effect of Substrate Growth Temperature on the Stability of the Reaction Chamber
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Li, Z.; Zhi, Y.; Su, Y.; Zhang, Y.; Hwang, H.L.; Duan, F.; Zhang, Y. Novel design and performance of the solidly mounted resonator with an AlN-buffered ZnO piezoelectric film. Vacuum 2018, 154, 11–17. [Google Scholar] [CrossRef]
- Qi, H.; Pang, J.; Yang, Z.; Wang, J.; Ma, L.; Schmidt, O.G. Boosting the Photoluminescence of Monolayer MoS2 on High-Density Nanodimer Arrays with Sub-10 nm Gap. Adv. Opt. Mater. 2017. [Google Scholar] [CrossRef]
- Pang, J.; Bachmatiuk, A.; Yin, Y.; Trzebicka, B.; Rummeli, M.H. Applications of Phosphorene and Black Phosphorus in Energy Conversion and Storage Devices. Adv. Ener. Mater. 2018, 8, 1702093. [Google Scholar] [CrossRef]
- Vorobyeva, N.A.; Rumyantseva, M.N.; Vasiliev, R.B.; Kozlovskiy, V.F.; Soshnikova, Y.M.; Filatova, D.G.; Zaytsev, V.B.; Zaytseva, A.V.; Gaskov, A.M. Doping effects on electrical and optical properties of spin-coated ZnO thin films. Vacuum 2015, 114, 198–204. [Google Scholar] [CrossRef]
- Gröttrup, J.; Postica, V.; Smazna, D.; Hoppe, M.; Kaidas, V.; Mishra, Y.K.; Lupan, O.; Adelung, R. UV detection properties of hybrid ZnO tetrapod 3-D networks. Vacuum 2017, 146, 492–500. [Google Scholar] [CrossRef]
- Ji, G.; Lv, H.; Wang, M.; Shang, C.; Zhang, H.; Du, Y. FeCo/ZnO composites with enhancing microwave absorbing properties: Effect of hydrothermal temperature and time. RSC Adv. 2014, 4, 57529–57533. [Google Scholar]
- Jaramillo, P.; Navío, J.A.; Hidalgo, M.C. Silver-modified ZnO highly UV-photoactive. J. Photoch. Photobio. A 2018, 356, 112–122. [Google Scholar] [CrossRef]
- Fortunato, E.M.C.; Barquinha, P.M.C.; Pimentel, A.; Goncalves, A.M.F.; Marques, A.N.J.S.; Martins, R.F.P.; Pereira, L.M.N. Wide-bandgap high-mobility ZnO thin-film transistors produced at room temperature. Appl. Phys. Lett. 2004, 85, 2541–2543. [Google Scholar] [CrossRef]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, G. ZnO Nanostructures for Dye-Sensitized Solar Cells. Adv. Mater. 2010, 21, 4087–4108. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, T.S.; Lubguban, J.A.; White, H.W.; Kim, B.J.; Park, Y.S.; Youn, C.J. Next generation of oxide photonic devices: ZnO-based ultraviolet light emitting diodes. Appl. Phys. Lett. 2006, 88, 153504. [Google Scholar] [CrossRef]
- Fan, X.M.; Lian, J.S.; Guo, Z.X.; Lu, H.J. Microstructure and photoluminescence properties of ZnO thin films grown by PLD on Si(111) substrates. Appl. Surf. Sci. 2005, 239, 176–181. [Google Scholar] [CrossRef]
- Dewan, S.; Tomar, M.; Kapoor, A.K.; Tandon, R.P.; Gupta, V. Luminescence studies of laser MBE grown GaN on ZnO nanostructures[C]//Society of Photo-Optical Instrumentation Engineers. SPIE 2017, 30. [Google Scholar] [CrossRef]
- Yang, X.D.; Su, S.C.; Xu, Y.; Mei, T. Effect of Thickness on the Structural, Electrical and Optical Properties of ZnO Films Deposited by MBE. Adv. Mater. Res. 2011, 311–313, 1271–1276. [Google Scholar] [CrossRef]
- Liu, Y.; Gorla, C.R.; Liang, S.; Emanetoglu, N.; Lu, Y.; Shen, H.; Wraback, M. Ultraviolet detectors based on epitaxial ZnO films grown by MOVCD. J. Electron. Mater. 2000, 29, 69–74. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Bang, K.H.; Hwang, D.K.; Jeong, M.C.; Sohn, K.S.; Myoung, J.M. Comparative studies on structural and optical properties of ZnO films grown on c-plane sapphire and GaAs (001) by MOCVD. Solid State Commun. 2003, 126, 623–627. [Google Scholar] [CrossRef]
- Heydari, G.M.; Majkic, G.; Galstyan, E.; Xu, A.; Zhang, Y.; Li, X.F.; Selvamanickam, V. Control of in-field performance of 25 mol.% Zr-added REBCO superconductor tapes. Physica C 2018, 553, 26–32. [Google Scholar] [CrossRef]
- Heydari, G.M.; Gleason, K. Device Fabrication Based on oxidative Chemical Vapor Deposition (oCVD) Synthesis of Conducting Polymers and Related Conjugated Organic Materials. Adv. Mater. Interfaces 2019, 6, 1801564. [Google Scholar] [CrossRef]
- Chandrakala, C.; Sravanthi, P.; Bharath, S.R.; Arockiasamy, S.; Johnson, M.G.; Nagaraja, K.S.; Jeyaraj, B. Synthesis, structure, vapour pressure and deposition of ZnO thin film by plasma assisted MOCVD technique using a novel precursor bis[(pentylnitrilomethylidine) (pentylnitrilomethylidine-μ-phenalato)]dizinc(II). J. Mol. Struct. 2017, 1130, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Pu, Y.; Chen, Y.F.; Mo, C.L. MOCVD growth of ZnO films on Si(111) substrate using a thin AlN buffer layer. J. Cryst. Growth 2005, 284, 459–463. [Google Scholar] [CrossRef]
- Nicolay, S.; Faÿ, S.; Ballif, C. Growth Model of MOCVD Polycrystalline ZnO. Cryst. Growth Des. 2009, 9, 4957–4962. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Ye, Z.; Xu, W.; Chen, F.; Zhou, X.; Zhao, B.; Zhu, L.; Lu, J. p-Type conduction in phosphorus-doped ZnO thin films by MOCVD and thermal activation of the dopant. Appl. Surf. Sci. 2006, 252, 7953–7956. [Google Scholar]
- Creighton, J.R.; Wang, G.T.; Breiland, W.G.; Coltrin, M.E. Nature of the parasitic chemistry during AlGaInN OMVPE. J. Cryst. Growth 2004, 261, 204–213. [Google Scholar] [CrossRef]
- Mazumder, S.; Lowry, S.A. The importance of predicting rate-limited growth for accurate modeling of commercial MOCVD reactors. J. Cryst. Growth 2001, 224, 165–174. [Google Scholar] [CrossRef]
- Theodoropoulos, C.; Mountziaris, T.J.; Moffat, H.K.; Han, J. Design of gas inlets for the growth of gallium nitride by metalorganic vapor phase epitaxy. J. Cryst. Growth 2000, 217, 65–81. [Google Scholar] [CrossRef]
- Parikh, R.P.; Adomaitis, R.A. An overview of gallium nitride growth chemistry and its effect on reactor design: Application to a planetary radial-flow CVD system. J. Cryst. Growth 2006, 286, 259–278. [Google Scholar] [CrossRef]
- Kadinski, L.; Merai, V.; Parekh, A.; Ramer, J.; Armour, E.A.; Stall, R.; Gurary, A.; Galyukov, A.; Makarov, Y. Computational analysis of GaN/InGaN deposition in MOCVD vertical rotating disk reactors. J. Cryst. Growth 2004, 261, 175–181. [Google Scholar] [CrossRef]
- Mitrovic, B.; Gurary, A.; Kadinski, L. On the flow stability in vertical rotating disc MOCVD reactors under a wide range of process parameters. J. Cryst. Growth 2006, 287, 656–663. [Google Scholar] [CrossRef]
- Mitrovic, B.; Gurary, A.; Quinn, W. Process conditions optimization for the maximum deposition rate and uniformity in vertical rotating disc MOCVD reactors based on CFD modeling. J. Cryst. Growth 2007, 303, 323–329. [Google Scholar] [CrossRef]
- Mitrovic, B.; Parekh, A.; Ramer, J.; Merai, V.; Armour, E.A.; Kadinski, L.; Gurary, A. Reactor design optimization based on 3D modeling of nitrides deposition in MOCVD vertical rotating disc reactors. J. Cryst. Growth 2006, 289, 708–714. [Google Scholar] [CrossRef]
- Liu, S.M.; Gu, S.L.; Zhu, S.M.; Ye, J.D.; Liu, W.; Zhou, X.; Zhang, R.; Shi, Y.; Zheng, Y.D. Modeling analysis of the MOCVD growth of ZnO film. J. Cryst. Growth 2007, 299, 303–308. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chen, C.T. Mathematical modeling and optimal design of an MOCVD reactor for GaAs film growth. J. Taiwan Inst. Chem. Eng. 2014, 45, 254–267. [Google Scholar] [CrossRef]
- Zhi, Z.; Fang, H.; Han, Y.; Jiang, Z.; Jiang, Z.; Gan, Z. Influencing factors of GaN growth uniformity through orthogonal test analysis. Appl. Therm. Eng. 2015, 91, 53–61. [Google Scholar]
- Zhi, Z.; Fang, H.; Yao, Q.; Han, Y.; Gan, Z. Species transport and chemical reaction in a MOCVD reactor and their influence on the GaN growth uniformity. J. Cryst. Growth 2016, 454, 87–95. [Google Scholar]
- Maejima, K.; Fujita, S. Chemical vapor reactions of ZnO growth by metal-organic vapor phase epitaxy. J. Cryst. Growth 2006, 293, 305–310. [Google Scholar] [CrossRef]
- Ye, J.D.; Gu, S.L.; Liu, W.; Zhu, S.M.; Kwong, D.L. Competitive adsorption and two-site occupation effects in metal-organic chemical vapor deposition of ZnO. Appl. Phys. Lett. 2007, 90, 241108. [Google Scholar] [CrossRef]
- Park, J.; Jung, T.H.; Lee, J.H.; Kim, H.S.; Park, J.S. The growth behavior and properties of atomic layer deposited zinc oxide films using hydrogen peroxide (H2O2) and ozone (O3) oxidants. Ceram. Int. 2015, 41, 1839–1845. [Google Scholar] [CrossRef]
- Kim, Y.S.; Won, Y.S. Investigation on Reaction Pathways for ZnO Formation from Diethylzinc and Water during Chemical Vapor Deposition. Bull. Korean Chem. Soc. 2009, 30, 7. [Google Scholar]
- Jian, L.; Gan, H.; Xu, Y.; Wang, C.; Feng, L.G.; Gang, W. Chemical reaction-transport model of oxidized diethylzinc based on quantum mechanics and computational fluid dynamics approaches. RSC Adv. 2018, 8, 1116–1123. [Google Scholar] [Green Version]
- Jian, L.; Gan, H.; Xu, Y.; Wang, C.; Gu, F.L.; Gang, W. Chemical reaction-transport model of diethylzinc hydrolysis in a vertical MOCVD reactor. Appl. Therm. Eng. 2018, 136, 108–177. [Google Scholar]
- Li, J.; Gan, H.; Xu, Y.; Wang, C.; Pei, Y.; Gu, F.L.; Wang, G. Chemical reaction mechanism of ZnO grown by DEZn and N2O in MOCVD. Crystengcomm 2018, 20, 6775–6785. [Google Scholar] [CrossRef]
- Atkins, P.; Paula, D.J. Atkins’ Physical Chemistry, 9rd ed.; Oxford University Press: Oxford, UK, 2010; pp. 800–811. [Google Scholar]
- ANSYS Fluent 14 Theory Guide, US. Available online: https://www.mendeley.com/catalogue/ansys-fluent-theory-guide/ (accessed on 28 February 2019).

| No. | Reaction | A | |
|---|---|---|---|
| G1 | Zn(CH2CH3)2 + H2O → Zn(CH2CH3)2·H2O | Coll. | 0 |
| G2 | Zn(CH2CH3)2·H2O → C2H6 + Zn(CH2CH3)OH | 2.96 × 1012 | 28.2 |
| G3 | Zn(CH2CH3)OH + H2O → Zn(OH)2 + C2H6 | 1.1 × 1014 | 39.8 |
| G4 | Zn(CH2CH3)2 + 2H2O → H2O ·Zn(CH2CH3)2·H2O | Coll. | 0 |
| G5 | H2O ·Zn(CH2CH3)2·H2O →C2H6·Zn(CH2CH3)OH·H2O | 8.97 × 1012 | 28.3 |
| G6 | C2H6·Zn(CH2CH3)OH·H2O → Zn(OH)2 + 2C2H6 | 8.03 × 1013 | 38.6 |
| G7 | 3Zn(OH)2 → Zn3(OH)6 | Coll. | 0 |
| G8 | Zn3(OH)6 → Zn3O5H4 + H2O | 2.42 × 1013 | 39.2 |
| G9 | Zn3O5H4 → Zn3O4H2 + H2O | 1.67 × 1012 | 30.6 |
| G10 | Zn3O4H2 → Zn3O3 + H2O | 1.08 × 1012 | 17.3 |
| No. | Reaction | ||
|---|---|---|---|
| S1 | Zn(CH2CH3)2·H2O→ZnO+2C2H6 | s = 1 | 8.36 |
| S2 | H2O·Zn(CH2CH3)2·H2O→ZnO+2C2H6+H2O | s = 1 | 8.36 |
| S3 | Zn(CH2CH3)OH → ZnO + C2H6 | s = 1 | 0 |
| S4 | C2H6·Zn(CH2CH3)OH·H2O→ ZnO + 2C2H6 + H2O | s = 1 | 0 |
| S5 | Zn (OH)2 → ZnO + H2O | s = 1 | 0 |
| S6 | Zn3(OH)6 → 3ZnO + 3H2O | s = 1 | 0 |
| S7 | Zn3O5H4 → 3ZnO + 2H2O | s = 1 | 0 |
| S8 | Zn3O4H2 → 3ZnO + H2O | s = 1 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wu, Z.; Xu, Y.; Pei, Y.; Wang, G. Stability Analysis of Multi Process Parameters for Metal-Organic Chemical Vapor Deposition Reaction Cavity. Molecules 2019, 24, 876. https://doi.org/10.3390/molecules24050876
Li J, Wu Z, Xu Y, Pei Y, Wang G. Stability Analysis of Multi Process Parameters for Metal-Organic Chemical Vapor Deposition Reaction Cavity. Molecules. 2019; 24(5):876. https://doi.org/10.3390/molecules24050876
Chicago/Turabian StyleLi, Jian, Ziling Wu, Yifeng Xu, Yanli Pei, and Gang Wang. 2019. "Stability Analysis of Multi Process Parameters for Metal-Organic Chemical Vapor Deposition Reaction Cavity" Molecules 24, no. 5: 876. https://doi.org/10.3390/molecules24050876






