Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents
Abstract
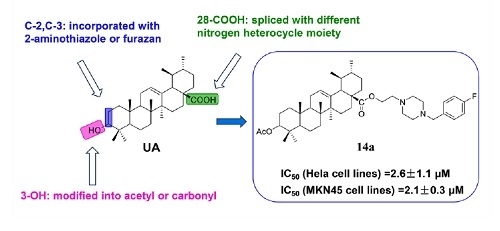
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
3. Materials and Methods
3.1. Materials
3.2. Chemistry
3.2.1. Preparation of Compounds 3, 4 and 6
3.2.2. General Procedure for the Synthesis of Compounds 5a–e
3.2.3. General Procedure for Compounds 8a–c
3.2.4. General Procedure for Compounds 9a–e
3.2.5. General Procedure for Compounds 10 and 11
3.2.6. General Procedure for the Synthesis of Compounds 12a–c
3.2.7. Preparation of Compound 13
3.2.8. General Procedure for Synthesizing Compounds 14a and 14b
3.2.9. General Procedure for Synthesizing Compounds 17 and 18
3.3. Biology
3.3.1. Cell Culture and Treatment
3.3.2. MTT Assay
3.3.3. Flow Cytometric Analysis
3.3.4. Western Blotting Assay
3.3.5. Xenograft Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, Distribution, and Antioxidative Effects of Selected Triterpenes in Mice. J. Agric. Food. Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Gao, Y.; Wang, A.L.; Zhou, X.B.; Zheng, Y.Q.; Zhou, J. Evolution in medicinal chemistry of ursolic acid derivatives as anticancer agents. Eur. J. Med. Chem. 2015, 92, 648–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazakova, O.B.; Giniyatullina, G.V.Y.; Amansarov, E.Y.; Tolstikov, G.A. Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg. Med. Chem. Lett. 2010, 20, 4088–4090. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Cunha, W.R.; de Matos, G.X.; Souza, M.G.; Tozatti, M.G.; Andrade e Silva, M.L.; Martins, C.H.; da Silva, R. Evaluation of the antibacterial activity of the methylene chloride extract of Miconia ligustroides, isolated triterpene acids, and ursolic acid derivatives. Pharm. Biol. 2010, 48, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.X.; Huang, R.Z.; Ye, M.Y.; Pan, Y.M.; Yao, G.Y.; Zhang, Y.; Wang, H.S. Design, synthesis and invitro evaluation of novel ursolic acid derivatives as potential anticancer agents. Eur. J. Med. Chem. 2015, 95, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Cheng, C.H.; Lee, Y.H.; Chang, I.L.; Chen, H.Y.; Hsieh, C.P.; Chueh, P.J. Ursolic acid triggers apoptosis in human osteosarcoma cells via caspase activation and the ERK1/2 MAPK pathway. J. Agric. Food. Chem. 2016, 64, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.J.; Zhou, Y.R.; Bao, B.H.; Jia, M.X.; Zhao, Y.; Zhang, L.; Li, J.X.; He, H.L.; Zhou, X.M. Tryptophan hydroxylase 1 (Tph-1)-targeted bone anabolic agents for osteoporosis. J. Med. Chem. 2014, 57, 4692–4709. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, R.M.P.; Vargas, S.R.; Garcia, B.E.; Gallardo, N.Y. Hypoglycemic activity of constituents from Astianthus viminalis in normal and streptozotocin-induced diabetic mice. J. Nat. Med. 2009, 63, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hong, D.; Zhou, Y.; Zhang, Y.; Shen, Q.; Li, J.Y.; Hu, L.H.; Li, J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochem. Biophis. Acta 2006, 1760, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Ibrahim, S.A.; Jalil, S.; Choudhary, M.I. Ursolic acid: A potent Inhibitor of superoxides produced in the cellular system. Phytother. Res. 2007, 21, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.R.; Jin, J.L.; Li, C.H.; Piao, X.X.; Jin, N.G. Ursolic acid enhances mouse liver regeneration after partial hepatectomy. Pharm Biol. 2012, 50, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Mendes, V.I.S.; Rtholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A.R. Synthesis and cytotoxic activity of novel A-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.H.; Zhou, S.Y.; Qian, Z.Z.; Zhang, H.L.; Qiu, L.H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.S.; Wang, H.Q. Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Xie, X.; Wang, C.; You, J.; Mo, F.; Jin, B.; Chen, J.; Shao, J.; Chen, H.; et al. Dendrimeric anticancer prodrugs for targeted delivery of ursolic acid to folate receptor-expressing cancer cells: Synthesis and biological evaluation. Eur. J. Pharm. Sci. 2015, 70, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, B.; Su, X.; Chen, G.; Li, Y.; Yu, L.; Li, L.; Wei, W. An Ursolic Acid Derived Small Molecule Triggers Cancer Cell Death through Hyperstimulation of Macropinocytosis. J. Med. Chem. 2017, 60, 6638–6648. [Google Scholar] [CrossRef] [PubMed]
- Parrino, B.; Carbone, A.; Spano, V.; Montalbano, A.; Giallombardo, D.; Barraja, P.; Attanzio, A.; Tesoriere, L.; Sissi, C.; Palumbo, M.; et al. Aza-isoindolo and isoindolo-azaquinoxaline derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 94, 367–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrino, B.; Carbone, A.; Ciancimino, C.; Spano, V.; Montalbano, A.; Barraja, P.; Cirrincione, G.; Diana, P.; Sissi, C.; Palumbo, M.; et al. Water-soluble isoindolo[2,1-a]quinoxalin-6-imines: In vitro antiproliferative activity and molecular mechanism(s) of action. Eur. J. Med. Chem. 2015, 94, 149–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Dar, B.A.; Majeed, R.; Hamid, A.; Bhat, B.A. Synthesis and biological evaluation of ursolic acid-triazolyl derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2013, 66, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Yang, S.J.; Jin, L.H.; Hu, D.Y.; Xue, W.; Song, B.A.; Yang, S. Synthesis and cytotoxicity of novel ursolic acid derivatives containing an acyl piperazine moiety. Eur. J. Med. Chem. 2012, 58, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Jin, X.Y.; Li, D.D.; Wang, S.F.; Tao, X.B.; Chen, H. Design, synthesis and in vitro anticancer activity of novel quinoline and oxadiazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2017, 27, 4128–4132. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.W.; Shen, Q.; Yang, F.; Wang, B.; Zou, H.; Li, J.Y.; Li, J.; Tang, J. Synthesis and biological evaluation of heterocyclic ring-substituted maslinic acid derivatives as novel inhibitors of protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2009, 19, 6618–6622. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, X.; Wu, K.; Wang, Y.; Shi, W.; Li, Y.; Yin, S. Synthesis and Antitumor Activities of 3’-Substituted Propanolursolates. Chin. J. Org. Chem. 2012, 32, 703–708. [Google Scholar] [CrossRef]
- Khan, M.S.Y.; Ahmad, S.; Yadav, M.R.; Jindal, D.P. Synthesis and study of azatriterpenes. J. Indian Chem. Soc. 1990, 67, 330–331. [Google Scholar]
- Ma, Y.-T.; Yang, Y.; Cai, P.; Sun, D.-Y.; Sánchez-Murcia, P.A.; Zhang, X.-Y.; Jia, W.-Q.; Lei, L.; Guo, M.; Gago, F.; et al. A Series of Enthalpically Optimized Docetaxel Analogues Exhibiting Enhanced Antitumor Activity and Water Solubility. J. Nat. Prod. 2018, 81, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, Q.; Wang, N.; Yang, Y.; Liu, J.; Yu, G.; Yang, X.; Xu, H.; Wang, H. A complex micellar system co-delivering curcumin with doxorubicin against cardiotoxicity and tumor growth. Int. J. Nanomed. 2018, 13, 4549–4561. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Sun, D.; Zhang, J.; Xie, X.; Wu, X.; Fang, W.; Tian, J.; Yan, C.; Wang, H.; Fu, F. Lx2-32c, a novel semi-synthetic taxane, exerts antitumor activity against prostate cancer cells in vitro and in vivo. Acta Pharm. Sin. B 2017, 7, 52–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.J.; Tong, J.; Zeng, F.Y.; Guo, M.; Li, Y.H.; Wang, H.; Wang, P. Nicotinic ACh receptor α7 inhibits PDGF-induced migration of vascular smooth muscle cells by activating mitochondrial deacetylase sirtuin 3. Br. J. Pharmacol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guan, D.; Lei, L.; Lu, J.; Liu, J.Q.; Yang, G.; Yan, C.; Zhai, R.; Tian, J.; Bi, Y.; et al. H6, a novel hederagenin derivative, reverses multidrug resistance in vitro and in vivo. Toxicol. Appl. Pharm. 2018, 341, 98–105. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |





| Compound | Inhibition Rates a | IC50 (µM) b | ||
|---|---|---|---|---|
| Hela | MKN45 | Hela | MKN45 | |
| UA | 13% | 18% | 15.1 ± 2.7 | 16.7 ± 1.4 |
| 5a | 1% | 31% | >20 | >20 |
| 5b | 2% | 35% | >20 | >20 |
| 5c | 16% | 45% | >20 | >20 |
| 5d | 3% | 31% | NTc | NT |
| 5e | 2% | 32% | NT | NT |
| 7 | 1% | 35% | NT | NT |
| 9a | 59% | 51% | 9.8 ± 1.8 | 7.4 ± 1.9 |
| 9b | 34% | 29% | >20 | >20 |
| 9c | 61% | 55% | 9.3 ± 0.5 | 7.9 ± 0.2 |
| 9d | 9% | 10% | 16.2 ± 0.8 | 19.3 ± 0.3 |
| 9e | 24% | 27% | NT | NT |
| 12a | 81% | 77% | 6.4 ± 0.3 | 6.4 ± 0.1 |
| 12b | 89% | 75% | 7.3 ± 0.2 | 6.2 ± 0.5 |
| 12c | 8% | 0% | NT | NT |
| 13 | 58% | 61% | 8.5 ± 0.3 | 8.9 ± 0.1 |
| 14a | 89% | 84% | 2.6 ± 1.1 | 2.1 ± 0.3 |
| 14b | 16% | 7% | NT | NT |
| 17 | 3% | 3% | NT | NT |
| 18 | 90% | 86% | 3.9 ± 0.6 | 4.5 ± 0.2 |
| CDDP d | 77% | 62% | 15.1 ± 0.9 | 2.8 ± 0.1 |
| Group (mg/kg) | Number (Initial/End) | Body Weight (g) | Tumor Weight (g) | ||
|---|---|---|---|---|---|
| Initial | End | g | IR (%) | ||
| Control | 5/5 | 20.52 ± 0.99 | 20.56 ± 2.11 | 2.12 ± 0.40 | |
| Cisplatin 3 | 5/5 | 20.80 ± 1.34 | 21.70 ± 1.32 | 0.88 ± 0.12 * | 58.49 |
| 14a 50 | 5/5 | 21.08 ± 1.04 | 20.48 ± 0.89 | 1.20 ± 0.59 * | 43.40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lei, L.; Liu, Z.; Wang, H.; Meng, Q. Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents. Molecules 2019, 24, 877. https://doi.org/10.3390/molecules24050877
Wang W, Lei L, Liu Z, Wang H, Meng Q. Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents. Molecules. 2019; 24(5):877. https://doi.org/10.3390/molecules24050877
Chicago/Turabian StyleWang, Wenzhi, Lei Lei, Zhi Liu, Hongbo Wang, and Qingguo Meng. 2019. "Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents" Molecules 24, no. 5: 877. https://doi.org/10.3390/molecules24050877
APA StyleWang, W., Lei, L., Liu, Z., Wang, H., & Meng, Q. (2019). Design, Synthesis, and Biological Evaluation of Novel Nitrogen Heterocycle-Containing Ursolic Acid Analogs as Antitumor Agents. Molecules, 24(5), 877. https://doi.org/10.3390/molecules24050877