Velcrand Functionalized Polyethylene
Abstract
:1. Introduction
2. Results and Discussion
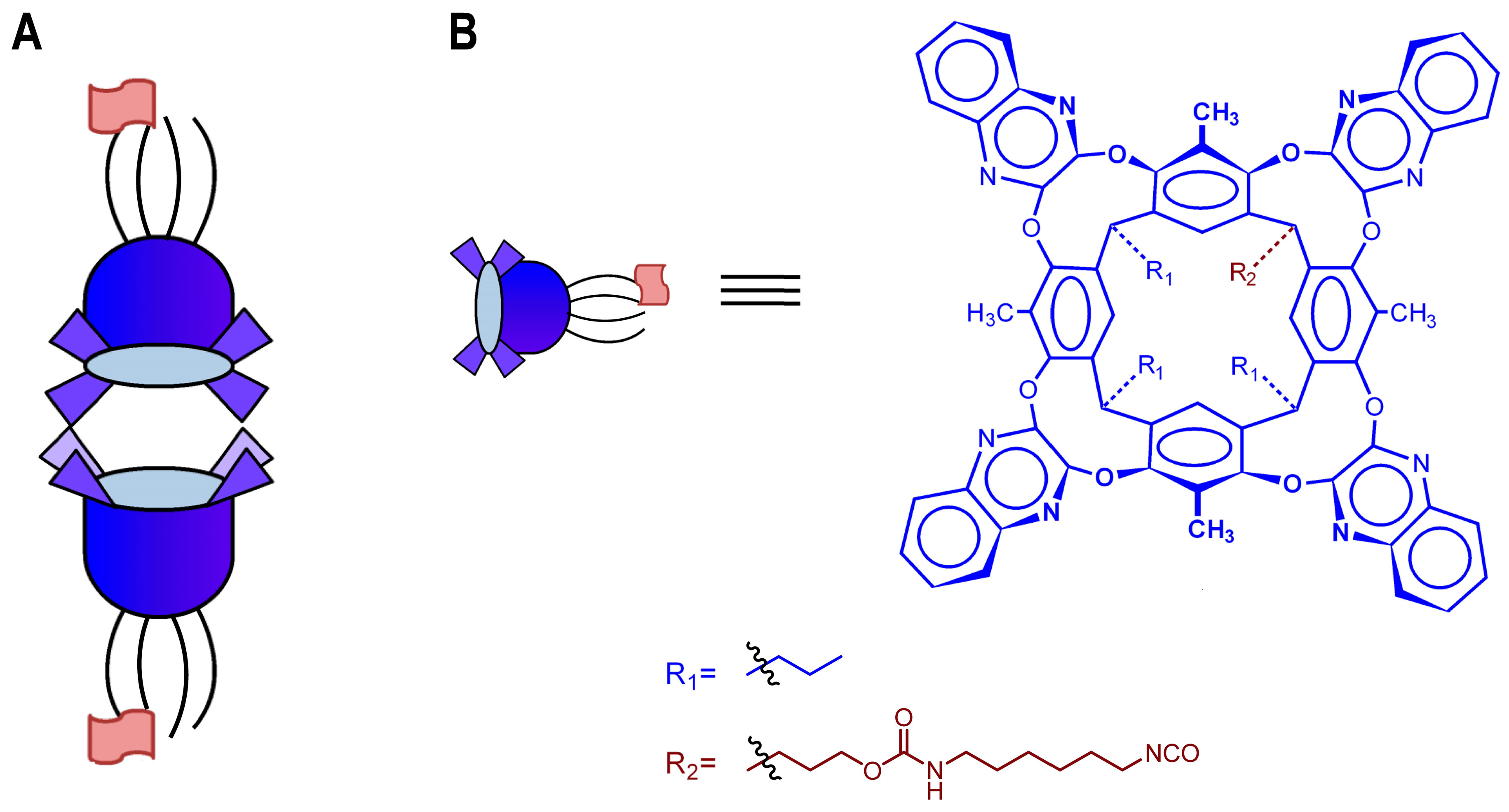
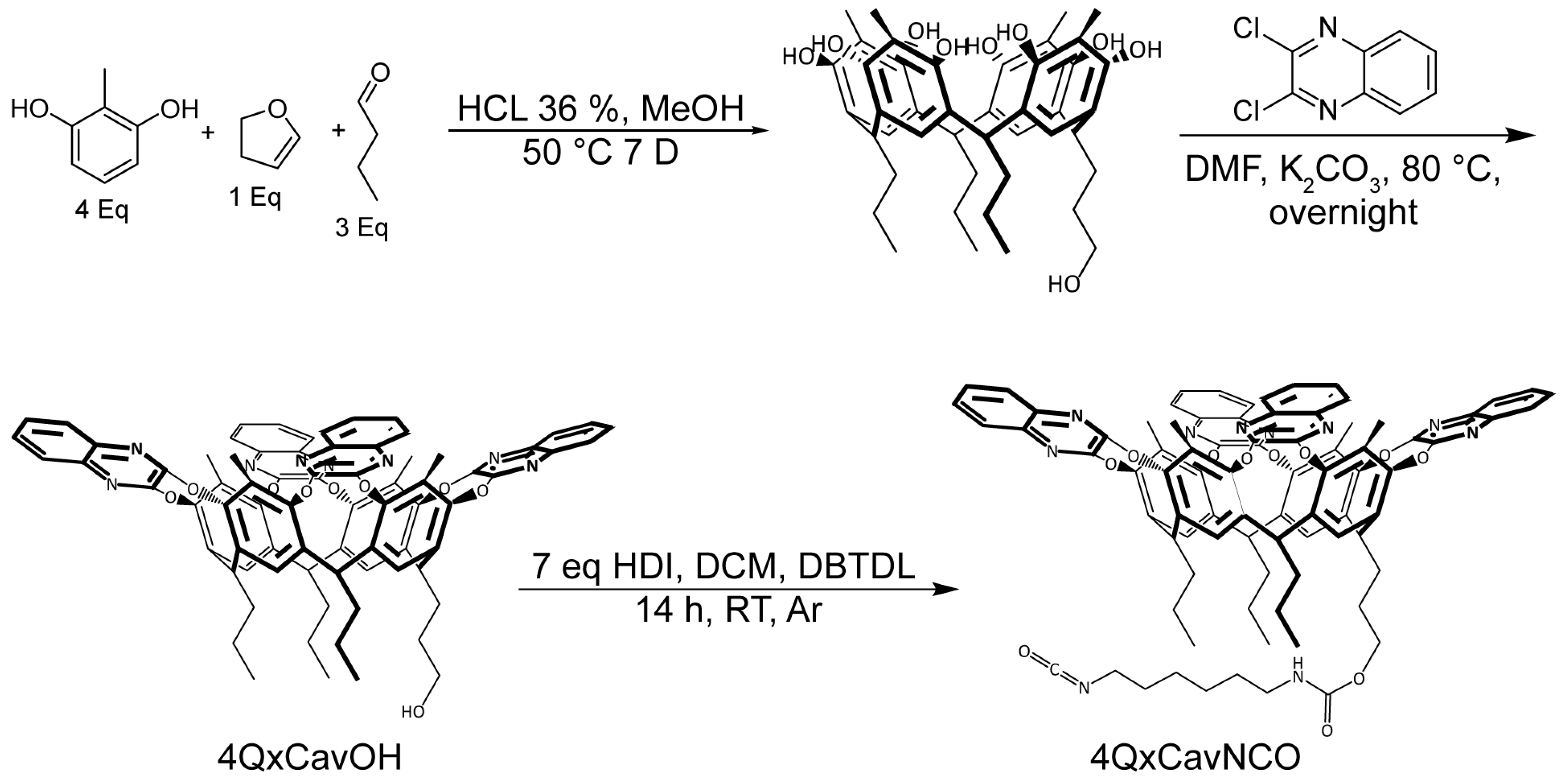
2.1. Design and Synthesis of the Velcrand
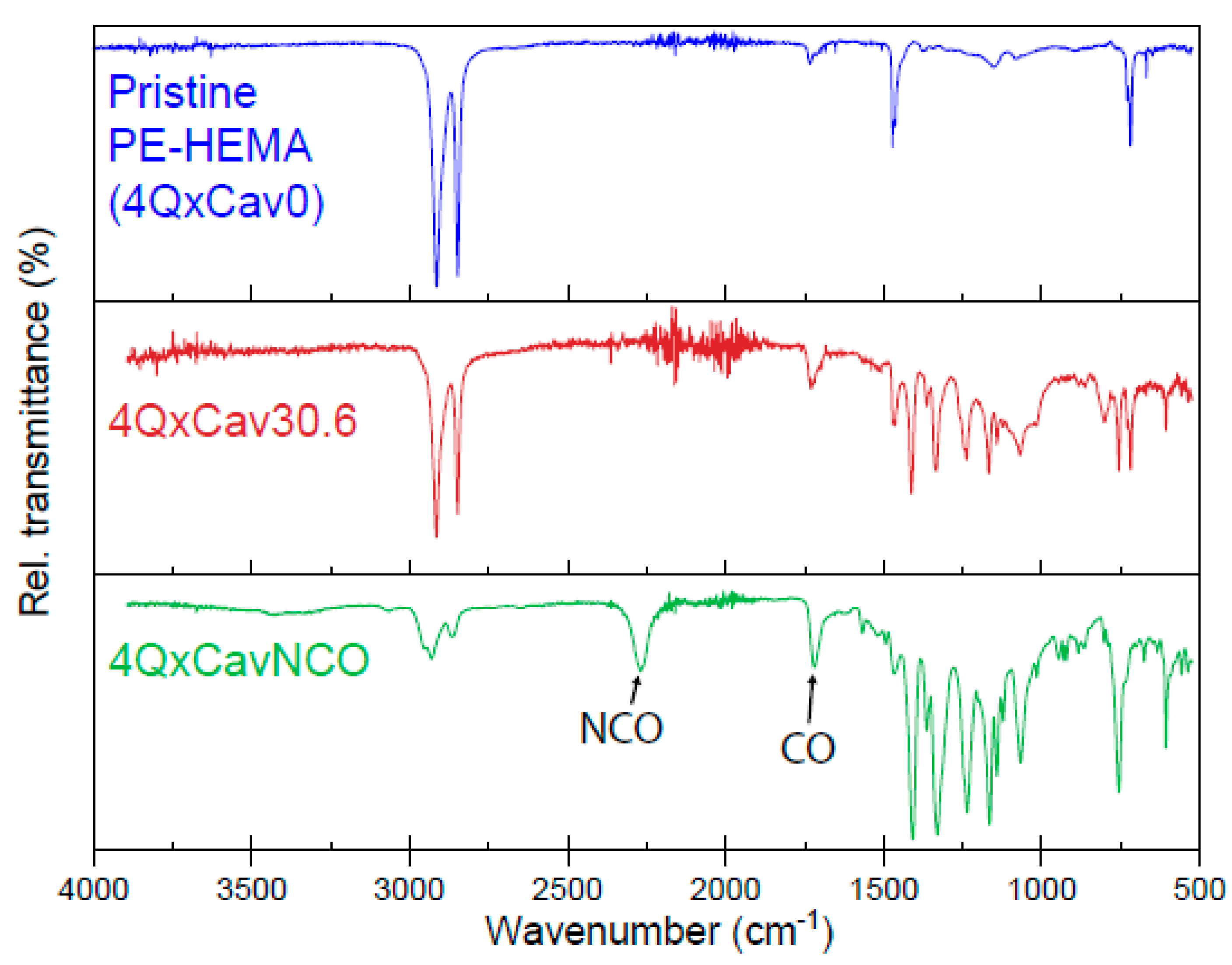
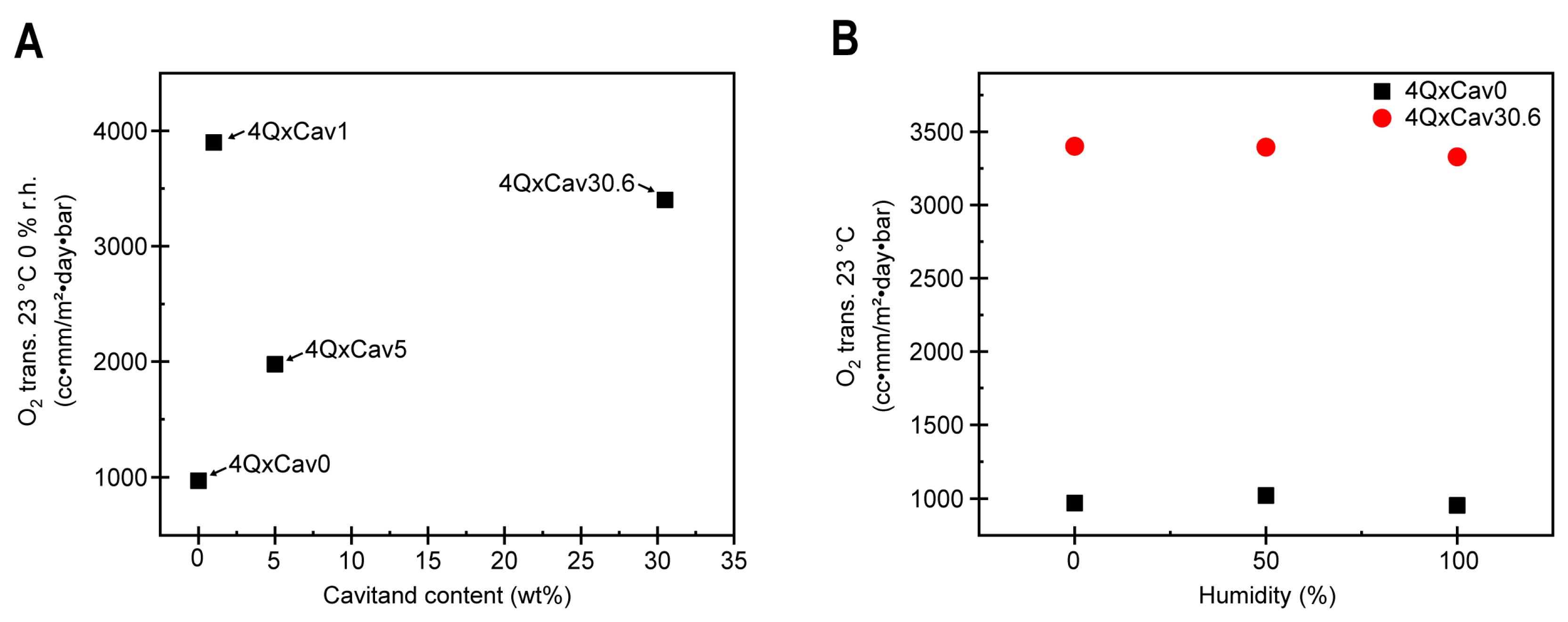
2.2. Synthesis and Characterization of Velcrand Functional Polymer Networks
3. Experimental
3.1. Proton Nuclear Magnetic Resonance (1H-NMR)
3.2. Fourier Transformed Infrared Spectroscopy (FTIR)
3.3. Differential Scanning Calorimetry (DSC)
3.4. Oxygen and Water Transmission
3.5. Synthesis of 4QxCavNCO
3.6. Synthesis of Velcrand Functionalized Polyethylene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Cavitands. In Comprehensive Supramolecular Chemistry II; Atwood, J.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 87–115. [Google Scholar]
- Hooley, R.J.; Rebek, J. Chemistry and catalysis in functional cavitands. Chem. Biol. 2009, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Brenner, E.; Sémeril, D.; Matt, D.; Harrowfield, J. The use of resorcinarene cavitands in metal-based catalysis. Eur. J. Org. Chem. 2017, 2017, 6100–6113. [Google Scholar] [CrossRef]
- Mirabaud, A.; Mulatier, J.-C.; Martinez, A.; Dutasta, J.-P.; Dufaud, V. Merging host-guest chemistry and organocatalysis for the chemical valorization of CO2. Catal. Today 2017, 281, 387–391. [Google Scholar] [CrossRef]
- Vidal, D.; Costas, M.; Lledó, A. A deep cavitand receptor functionalized with Fe(II) and Mn(II) aminopyridine complexes for bioinspired oxidation catalysis. ACS Catal. 2018, 8, 3667–3672. [Google Scholar] [CrossRef]
- Pinalli, R.; Dalcanale, E.; Ugozzoli, F.; Massera, C. Resorcinarene-based cavitands as building blocks for crystal engineering. CrystEngComm 2016, 18, 5788–5802. [Google Scholar] [CrossRef]
- Pochorovski, I.; Diederich, F. Development of redox-switchable resorcin[4]arene cavitands. Acc. Chem. Res. 2014, 47, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Pinalli, R.; Brancatelli, G.; Pedrini, A.; Menozzi, D.; Hernández, D.; Ballester, P.; Geremia, S.; Dalcanale, E. The origin of selectivity in the complexation of N-methyl amino acids by tetraphosphonate cavitands. J. Am. Chem. Soc. 2016, 138, 8569–8580. [Google Scholar] [CrossRef] [PubMed]
- Brancatelli, G.; Dalcanale, E.; Pinalli, R.; Geremia, S. Probing the Structural Determinants of Amino Acid Recognition: X-Ray Studies of Crystalline Ditopic Host-Guest Complexes of the Positively Charged Amino Acids, Arg, Lys, and His with a Cavitand Molecule. Molecules 2018, 23, 3368. [Google Scholar] [CrossRef] [PubMed]
- Ghang, Y.-J.; Perez, L.; Morgan, M.A.; Si, F.; Hamdy, O.M.; Beecher, C.N.; Larive, C.K.; Julian, R.R.; Zhong, W.; Cheng, Q.; et al. Anionic deep cavitands enable the adhesion of unmodified proteins at a membrane bilayer. Soft Matter 2014, 10, 9651–9656. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, N.; Biavardi, E.; Bordiga, D.; Candiani, G.; Alessandri, I.; Bergese, P.; Dalcanale, E. Probing lysine mono-methylation in histone H3 tail peptides with an abiotic receptor coupled to a non-plasmonic resonator. Nanoscale 2017, 9, 8639–8646. [Google Scholar] [CrossRef] [PubMed]
- Clément, P.; Korom, S.; Struzzi, C.; Parra, E.J.; Bittencourt, C.; Ballester, P.; Llobet, E. Deep cavitand self-assembled on Au NPs-MWCNT as highly sensitive benzene sensing interface. Adv. Funct. Mater. 2015, 25, 4011–4020. [Google Scholar] [CrossRef]
- Tudisco, C.; Fragalà, M.E.; Giuffrida, A.E.; Bertani, F.; Pinalli, R.; Dalcanale, E.; Compagnini, G.; Condorelli, G.G. Hierarchical route for the fabrication of cavitand-modified nanostructured ZnO fibers for volatile organic compound detection. J. Phys. Chem. C 2016, 120, 12611–12617. [Google Scholar] [CrossRef]
- Pinalli, R.; Dalcanale, E. Supramolecular sensing with phosphonate cavitands. Acc. Chem. Res. 2013, 46, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Environmental gas sensing with cavitands. Chem Eur. J. 2018, 24, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Azov, V.A.; Beeby, A.; Cacciarini, M.; Cheetham, A.G.; Diederich, F.; Frei, M.; Gimzewski, J.K.; Gramlich, V.; Hecht, B.; Jaun, B.; et al. Resorcin[4]arene Cavitand-Based Molecular Switches. Adv. Funct. Mater. 2006, 16, 147–156. [Google Scholar] [CrossRef]
- Moran, J.R.; Karbach, S.; Cram, D.J. Cavitands: Synthetic Molecular Vessels. J. Am. Chem. Soc. 1982, 104, 5826–5828. [Google Scholar] [CrossRef]
- Cram, D.J.; Choi, H.-J.; Bryant, J.A.; Knobler, C.B. Solvophobic and Entropic Driving Forces for Forming Velcaplexes, Which are Four-Fold, Lock-Key Dimers in Organic Media. J. Am. Chem. Soc. 1992, 114, 7748–7765. [Google Scholar] [CrossRef]
- Pirondini, L.; Stendardo, A.G.; Geremia, S.; Campagnolo, M.; Samorì, P.; Rabe, J.P.; Fokkens, R.; Dalcanale, E. Dynamic Materials via Metal-Directed and Solvent-Driven Self-Assembly of Cavitands. Angew. Chem. Int. Ed. 2003, 42, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.-J.; Paek, K. Molecular Engineering. Part 14. Self-Assembled Oligovelcraplexes by π-π Stacking Interaction and Metal Coordination. Bull. Korean Chem. Soc. 2007, 28, 1440–1442. [Google Scholar] [CrossRef] [Green Version]
- Tancini, F.; Rampazzo, E.; Dalcanale, E. Interplay Between Cyclization and Polymerization in Ditopic Cavitand Monomers. Aust. J. Chem. 2010, 63, 646–652. [Google Scholar] [CrossRef]
- Ihm, H.; Ahn, J.-S.; Lah, M.S.; Ko, Y.H.; Paek, K. Oligobisvelcraplex: Self-Assembled Linear Oligomer by Solvophobic π−π Stacking Interaction of Bisvelcrands Based on Resorcin[4]arene. Org. Lett. 2004, 6, 3893–3896. [Google Scholar] [CrossRef] [PubMed]
- Lagaron, J.M.; Catalá, R.; Gavara, R. Structural characteristics defining high barrier properties in polymeric materials. Mater. Sci. Technol. 2004, 20, 1–7. [Google Scholar] [CrossRef]
- Moran, J.R.; Ericson, J.L.; Dalcanale, E.; Bryant, J.A.; Knobler, C.B.; Cram, D.J. Vases and kites as cavitands. J. Am. Chem. Soc. 1991, 113, 5707–5714. [Google Scholar] [CrossRef]
- Cormier, C.M.; Wunderlich, B. Heat of fusion of polyethylene. J. Polym. Sci. 1967, 5, 987–988. [Google Scholar] [CrossRef] [Green Version]
- Hirschmann, R.P.; Kniseley, R.N.; Fassel, V.A. The infrared spectra of alkyl isocyanates. Spectrochim. Acta 1965, 21, 2125–2133. [Google Scholar] [CrossRef]
- Hauke, F.; Myles, A.J.; Rebek, J. Lower rim mono-functionalization of resorcinarenes. Chem. Commun. 2005, 4164–4166. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of isocyanate functionalized velcrand and functionalized polymers are available from the authors. |



| Entry | HEMA a (mol%) | 4QxCavNCO (wt%) | OH-Group Funcionalization (mol%) | Tm b (°C) | Xc d (%) |
|---|---|---|---|---|---|
| 4QxCav0 | 0.91 | - | - | 107.4 | 30.2 |
| 4QxCav1 | 0.91 | 1 | 3 | 107.5 | 29.3 |
| 4QxCav5 | 0.91 | 5 | 16 | 106.0 | 25.4 |
| 4QxCav30.6 | 0.91 | 30.6 c | 100 | 96.8 | 11.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tellers, J.; Vachon, J.; Soliman, M.; Dalcanale, E.; Pinalli, R. Velcrand Functionalized Polyethylene. Molecules 2019, 24, 902. https://doi.org/10.3390/molecules24050902
Tellers J, Vachon J, Soliman M, Dalcanale E, Pinalli R. Velcrand Functionalized Polyethylene. Molecules. 2019; 24(5):902. https://doi.org/10.3390/molecules24050902
Chicago/Turabian StyleTellers, Jonathan, Jérôme Vachon, Maria Soliman, Enrico Dalcanale, and Roberta Pinalli. 2019. "Velcrand Functionalized Polyethylene" Molecules 24, no. 5: 902. https://doi.org/10.3390/molecules24050902







