Speciation Analysis of Trace Arsenic, Mercury, Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques
Abstract
:1. Introduction
2. Separation Techniques for Speciation Analysis
2.1. Non-Chromatographic Methods
2.1.1. Extraction
2.1.2. Selective Reduction
2.1.3. Miscellaneous Methods
2.2. Chromatographic Hyphenated Techniques
2.2.1. Gas Chromatography
2.2.2. Liquid Chromatography
2.2.3. Capillary Electrophoresis
3. Detection Techniques for Speciation Analysis
3.1. Atomic Spectrometry Methods
3.2. Mass Spectrometry Methods
4. Conclusion and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Dumout, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Vadala, R.; Mottese, A.F.; Bua, G.D.; Salvo, A.; Mallamace, D.; Corsaro, C.; Vasi, S.; Giofre, S.V.; Alfa, M.; Cicero, N.; et al. Statistical analysis of mineral concentration for the geographic identification of garlic samples from Sicily (Italy), Tunisia and Spain. Foods 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Albergamo, A.; Rotondo, A.; Salvo, A.; Pellizzeri, V.; Bua, D.G.; Maggio, A.; Cicero, N.; Dugo, G. Metabolite and mineral profiling of “Violetto di Niscemi” and “Spinoso di Menfi” globe artichokes by H-1-NMR and ICP-MS. Nat. Prod. Res. 2017, 31, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Duffus, J.; Templeton, D.M. Glossary of terms used in toxicokinetics (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 1033–1082. [Google Scholar] [CrossRef] [Green Version]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef] [Green Version]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, Y.; Pervaiz, A.; Kong, L.H.; He, M.C. Comparison of diffusive gradients in thin-films (DGT) and chemical extraction methods for predicting bioavailability of antimony and arsenic to maize. Geoderma 2018, 332, 1–9. [Google Scholar] [CrossRef]
- Aguilar-Carrillo, J.; Herrera, L.; Gutierrez, E.J.; Reyes-Dominguez, I.A. Solid-phase distribution and mobility of thallium in mining-metallurgical residues: Environmental hazard implications. Environ. Pollut. 2018, 243, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Tye, A.M.; Chenery, S.R. Using isotope dilution assays to understand speciation changes in Cd, Zn, Pb and Fe in a soil model system under simulated flooding conditions. Geoderma 2017, 295, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Salvo, A.; La Torre, G.L.; Mangano, V.; Casale, K.E.; Bartolomeo, G.; Santini, A.; Granata, T.; Dugo, G. Toxic inorganic pollutants in foods from agricultural producing areas of Southern Italy: Level and riskassessment. Ecotoxicol. Environ. Saf. 2018, 148, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors—A review. Water Res. 2018, 140, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.L.; Zhang, Z.L.; Zagar, D. Mercury transport and fate models in aquatic systems: A review and synthesis. Sci. Total Environ. 2018, 639, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xu, Y.; Packianathan, C.; Gao, F.; Chen, C.; Zhang, J.; Shen, Q.R.; Rosen, B.P.; Zhao, F.J. Arsenic methylation by a novel ArsM As(III) S-adenosylmethionine methyltransferase that requires only two conserved cysteine residues. Mol. Microbiol. 2018, 107, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, W.L.; Diez, S.; da Silva, C.J.; Ignacio, A.R.A.; Guimaraes, J.R.D. Seasonal changes in peryphytic microbial metabolism determining mercury methylation in a tropical wetland. Sci. Total Environ. 2018, 627, 1345–1352. [Google Scholar] [CrossRef]
- Ruszczynska, A.; Konopka, A.; Kurek, E.; Elguera, J.C.T.; Bulska, E. Investigation of biotransformation of selenium in plants using spectrometric methods. Spectrochim. Acta B 2017, 130, 7–16. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, N.; Ogra, Y. Effect of administration route and dose on metabolism of nine bioselenocompounds. J. Trace Elem. Med. Biol. 2018, 49, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Terol, A.; Ardini, F.; Basso, A.; Grotti, M. Determination of selenium urinary metabolites by high temperature liquid chromatography-inductively coupled plasma mass spectrometry. J. Chromatogr. A 2015, 1380, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Amayo, K.O.; Raab, A.; Krupp, E.M.; Feldmann, J. Identification of arsenolipids and their degradation products in cod-liver oil. Talanta 2014, 118, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Sele, V.; Sloth, J.J.; Holmelid, B.; Valdersnes, S.; Skov, K.; Amlund, H. Arsenic-containing fatty acids and hydrocarbons in marine oils—Determination using reversed-phase HPLC-ICP-MS and HPLC-qTOE-MS. Talanta 2014, 121, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Rumpler, A.; Edmonds, J.S.; Katsu, M.; Jensen, K.B.; Goessler, W.; Raber, G.; Gunnlaugsdottir, H.; Francesconi, K.A. Arsenic-containing long-chain fatty acids in cod-liver oil: A result of biosynthetic infidelity? Angew. Chem. Int. Ed. 2008, 47, 2665–2667. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Acuña, M.; García-Barrera, T.; García-Sevillano, M.A.; Gómez-Ariza, J.L. Speciation of arsenic in marine food (Anemonia sulcata) by liquid chromatography coupled to inductively coupled plasma mass spectrometry and organic mass spectrometry. J. Chromatogr. A 2013, 1282, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Hu, B.; Liu, Q.Q.; Yang, Z.L.; Lu, X.F.; Huang, R.F.; Li, X.F.; Zuidhof, M.J.; Le, X.C. Liquid chromatography combined with atomic and molecular mass spectrometry for speciation of arsenic in chicken liver. J. Chromatogr. A 2014, 1370, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.; Namiesǹik, J. The role of speciation in analytical chemistry. TrAC-Trend Anal. Chem. 2000, 19, 69–79. [Google Scholar] [CrossRef]
- Sadee, B.; Foulkes, M.E.; Hill, S.J. Coupled techniques for arsenic speciation in food and drinking water: A review. J. Anal. Atom. Spectrom. 2015, 30, 102–118. [Google Scholar] [CrossRef]
- Nearing, M.M.; Koch, I.; Reimer, K.J. Complementary arsenic speciation methods: A review. Spectrochim. Acta B 2014, 99, 150–162. [Google Scholar] [CrossRef]
- Huber, J.; Leopold, K. Nanomaterial-based strategies for enhanced mercury trace analysis in environmental and drinking waters. TrAC-Trend Anal. Chem. 2016, 80, 280–292. [Google Scholar] [CrossRef]
- Amde, M.; Yin, Y.G.; Zhang, D.; Liu, J.F. Methods and recent advances in speciation analysis of mercury chemical species in environmental samples: A review. Chem. Speciat. Bioavailab. 2016, 28, 51–65. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Liquid chromatographic analysis of selenium species in plant materials. TrAC-Trend Anal. Chem. 2019, 111, 128–138. [Google Scholar] [CrossRef]
- Pettine, M.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Zboril, R.; Sharma, V.K. A critical review of selenium analysis in natural water samples. Trends Environ. Anal. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; dos Anjos, J.P.; Felix, C.S.A.; da Silva, M.M.; Palacio, E.; Cerda, V. Speciation analysis of antimony in environmental samples employing atomic fluorescence spectrometry—Review. TrAC-Trend Anal. Chem. 2019, 110, 335–343. [Google Scholar] [CrossRef]
- Miravet, R.; Hernandez-Nataren, E.; Sahuquillo, A.; Rubio, R.; Lopez-Sanchez, J.F. Speciation of antimony in environmental matrices by coupled techniques. TrAC-Trend Anal. Chem. 2010, 29, 28–39. [Google Scholar] [CrossRef]
- Carvalho, D.C.; Coelho, N.M.M.; Melo Coelho, L.; Borges, S.S.S.; Neri, T.S.; Alves, V.N. Strategies to increase selectivity of analytical methods for As, Cr and Se speciation in biological samples: A review. Sample Prep. 2014, 2, 1–12. [Google Scholar] [CrossRef]
- Vieira, M.A.; Grinberg, P.; Bobeda, C.R.R.; Reyes, M.N.M.; Campos, R.C. Non-chromatographic atomic spectrometric methods in speciation analysis: A review. Spectrochim. Acta B 2009, 64, 459–476. [Google Scholar] [CrossRef]
- Gonzalvez, A.; Cervera, M.L.; Armenta, S.; de la Guardia, M. A review of non-chromatographic methods for speciation analysis. Anal. Chim. Acta 2009, 636, 129–157. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.A.; Al-Farawati, R.; Hawas, U.; Shaban, Y. Recent microextraction techniques for determination and chemical speciation of selenium. Open Chem. 2017, 15, 103–122. [Google Scholar] [CrossRef]
- Werner, J.; Grzeskowiak, T.; Zgola-Grzeskowiak, A.; Stanisz, E. Recent trends in microextraction techniques used in determination of arsenic species. TrAC-Trend Anal. Chem. 2018, 105, 121–136. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Lavilla, I.; Bendicho, C. Miniaturized preconcentration methods based on liquid-liquid extraction and their application in inorganic ultratrace analysis and speciation: A review. Spectrochim. Acta B 2009, 64, 1–15. [Google Scholar] [CrossRef]
- Panhwar, A.H.; Tuzen, M.; Kazi, T.G. Ultrasonic assisted dispersive liquid-liquid microextraction method based on deep eutectic solvent for speciation, preconcentration and determination of selenium species (IV) and (VI) in water and food samples. Talanta 2017, 175, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Akramipour, R.; Golpayegani, M.R.; Gheini, S.; Fattahi, N. Speciation of organic/inorganic mercury and total mercury in blood samples using vortex assisted dispersive liquid-liquid microextraction based on the freezing of deep eutectic solvent followed by GFAAS. Talanta 2018, 186, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Haghnazari, L.; Mirzaei, N.; Arfaeinia, H.; Karimyan, K.; Sharafi, H.; Fattahi, N. Speciation of As(III)/As(V) and total inorganic arsenic in biological fluids using new mode of liquid-phase microextraction and electrothermal atomic absorption spectrometry. Biol. Trace Elem. Res. 2018, 183, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.J.; Yan, Y.Y.; Tang, J.; Wu, Y.H.; Zhong, S.S. Speciation of Arsenic(III) and Arsenic(V) based on Triton X-100 hollow fiber liquid phase microextraction coupled with flame atomic absorption spectrometry. Spectrosc. Lett. 2017, 50, 220–226. [Google Scholar] [CrossRef]
- Turker, A.R. Speciation of trace metals and metalloids by solid phase extraction with spectrometric detection: A critical review. Turk. J. Chem. 2016, 40, 847–867. [Google Scholar] [CrossRef]
- Su, C.K.; Chen, W.C. 3D-printed, TiO2 NP-incorporated minicolumn coupled with ICP-MS for speciation of inorganic arsenic and selenium in high-salt-content samples. Microchim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, A.H.; Tuzen, M.; Hazer, B.; Kazi, T.G. Solid phase microextraction method using a novel polystyrene oleic acid imidazole polymer in micropipette tip of syringe system for speciation and determination of antimony in environmental and food samples. Talanta 2018, 184, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hen, B.B.; Zhu, S.Q.; Yu, X.X.; He, M.; Hu, B. Chip-based magnetic solid-phase microextraction online coupled with micro HPLC-ICP-MS for the determination of mercury species in cells. Anal. Chem. 2016, 88, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Tuzen, M.; Kazi, T.G.; Hazer, B. Inorganic arsenic speciation in water samples by miniaturized solid phase microextraction using a new polystyrene polydimethyl siloxane polymer in micropipette tip of syringe system. Talanta 2016, 161, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Nyaba, L.; Matong, J.M.; Dimpe, K.M.; Nomngongo, P.N. Speciation of inorganic selenium in environmental samples after suspended dispersive solid phase microextraction combined with inductively coupled plasma spectrometric determination. Talanta 2016, 159, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.F. Determination of antimony(III) and total antimony by single-drop microextraction combined with electrothermal atomic absorption spectrometry. Anal. Chim. Acta 2007, 585, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Tolessa, T.; Tan, Z.Q.; Yin, Y.G.; Liu, J.F. Single-drop gold nanoparticles for headspace microextraction and colorimetric assay of mercury (II) in environmental waters. Talanta 2018, 176, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.Y.; Zhao, J.Y.; Ren, H.Y.; Wang, J.N.; Hong, Z.X.; Zhang, X. Zwitterion-functionalized polymer microspheres-based solid phase extraction method on-line combined with HPLC-ICP-MS for mercury speciation. Talanta 2019, 196, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Londonio, A.; Hasuoka, P.E.; Pacheco, P.; Gil, R.A.; Smichowski, P. Online solid phase extraction- HPLC-ICP-MS system for mercury and methylmercury preconcentration using functionalised carbon nanotubes for their determination in dietary supplements. J. Anal. Atom. Spectrom. 2018, 33, 1737–1744. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.J.; Hu, X.; Lian, H.Z. Magnetic solid phase extraction for the determination of trace antimony species in water by inductively coupled plasma mass spectrometry. Talanta 2015, 134, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Morita, Y.; Sakuragawa, A.; Isozaki, A. Inorganic speciation of As(III, V), Se(IV, VI) and Sb(III, V) in natural water with GF-AAS using solid phase extraction technology. Talanta 2007, 72, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Zhang, N.; He, M.; Chen, B.B.; Hu, B. Simultaneous speciation analysis of inorganic arsenic, chromium and selenium in environmental waters by 3-(2-aminoethylamino) propyltrimethoxysilane modified multi-wall carbon nanotubes packed microcolumn solid phase extraction and ICP-MS. Talanta 2015, 131, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Zhu, Q.Y.; Mao, L.; Chen, Y.J.; Lian, H.Z.; Hu, X. Preparation of thiol- and amine-bifunctionalized hybrid monolithic column via “one-pot” and applications in speciation of inorganic arsenic. Talanta 2019, 192, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.B.; Hu, L.G.; Hou, X.D.; He, B.; Jiang, G.B. Headspace solid-phase microextraction coupled to miniaturized microplasma optical emission spectrometry for detection of mercury and lead. Anal. Chem. 2018, 90, 3683–3691. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, Q.; Lin, Y.; Tian, Y.F.; Wu, L.; Hou, X.D.; Zheng, C.B. Point discharge optical emission spectrometer as a gas chromatography (GC) detector for speciation analysis of mercury in human hair. Anal. Chem. 2018, 90, 11996–12003. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, Y.; Li, Y.X.; Yang, L.; Hou, X.D.; Feng, X.B.; Zheng, C.B. Ultrasensitive speciation analysis of mercury in rice by headspace solid phase microextraction using porous carbons and gas chromatography-dielectric barrier discharge optical emission spectrometry. Environ. Sci. Technol. 2016, 50, 2468–2476. [Google Scholar] [CrossRef] [PubMed]
- Mester, Z. Gas phase sampling of volatile (organo)metallic compounds above solid samples. J. Anal. Atom. Spectrom. 2002, 17, 868–871. [Google Scholar] [CrossRef]
- Zhang, W.F.; Hu, Y.A.; Cheng, H.F. Optimization of microwave-assisted extraction for six inorganic and organic arsenic species in chicken tissues using response surface methodology. J. Sep. Sci. 2015, 38, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Zounr, R.A.; Tuzen, M.; Khuhawar, M.Y. Ultrasound assisted deep eutectic solvent based on dispersive liquid liquid microextraction of arsenic speciation in water and environmental samples by electrothermal atomic absorption spectrometry. J. Mol. Liq. 2017, 242, 441–446. [Google Scholar] [CrossRef]
- Shirkhanloo, H.; Khaligh, A.; Mousavi, H.Z.; Rashidi, A. Ultrasound assisted-dispersive-ionic liquid-micro-solid phase extraction based on carboxyl-functionalized nanoporous graphene for speciation and determination of trace inorganic and organic mercury species in water and caprine blood samples. Microchim. J. 2017, 130, 245–254. [Google Scholar] [CrossRef]
- Altunay, N.; Gurkan, R. Separation/preconcentration of ultra-trace levels of inorganic Sb and Se from different sample matrices by charge transfer sensitized ion-pairing using ultrasonic-assisted cloud point extraction prior to their speciation and determination by hydride generation AAS. Talanta 2016, 159, 344–355. [Google Scholar] [PubMed]
- Lou, C.G.; Liu, W.Q.; Liu, X.D. Quantitative analysis of arsenic speciation in guano and ornithogenic sediments using microwave-assisted extraction followed by high-performance liquid chromatography coupled to hydride generation atomic fiuorescence spectrometry. J. Chromatogr. B 2014, 969, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Velez, A.A.; Hinojosa-Reyes, L.; Villanueva-Rodriguez, M.; Caballero-Quintero, A.; Hernandez-Ramirez, A.; Guzman-Mar, J.L. Speciation analysis of organoarsenic compounds in livestock feed by microwave-assisted extraction and high performance liquid chromatography coupled to atomic fluorescence spectrometry. Food Chem. 2017, 232, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.P.; Yan, L.Z.; Huang, H.L.; Deng, B.Y. Selenium speciation in radix puerariae using ultrasonic assisted extraction combined with reversed phase high performance liquid chromatography-inductively coupled plasma-mass spectrometry after magnetic solid-phase extraction with 5-sulfosalicylic acid functionalized magnetic nanoparticles. Spectrochim. Acta B 2016, 122, 172–177. [Google Scholar]
- Musil, S.; Petursdottir, A.H.; Raab, A.; Gunnlaugsdottir, H.; Krupp, E.; Feldmann, J. Speciation without chromatography using selective hydride generation: Inorganic arsenic in rice and samples of marine origin. Anal. Chem. 2014, 86, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Welna, M.; Pohl, P. Potential of the hydride generation technique coupled to inductively coupled plasma optical emission spectrometry for non-chromatographic As speciation. J. Anal. Atom. Spectrom. 2017, 32, 1766–1779. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.Y.; Wang, X.; Yang, L.; Yang, H.Y.; Tang, Y.Y.; Luo, H.; Xiong, X.L.; Jiang, X.; Huang, K. Speciation of mercury by hydride generation ultraviolet atomization-atomic fluorescence spectrometry without chromatographic separation. Microchem. J. 2018, 143, 228–233. [Google Scholar] [CrossRef]
- Vieira, M.A.; Ribeiro, A.S.; Curtius, A.J.; Sturgeon, R.E. Determination of total mercury and methylmercury in biological samples by photochemical vapor generation. Anal. Bioanal. Chem. 2007, 388, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Mendez, H.; Lavilla, I.; Bendicho, C. Mild sample pretreatment procedures based on photolysis and sonolysis-promoted redox reactions as a new approach for determination of Se(IV), Se(VI) and Se(-II) in model solutions by the hydride generation technique with atomic absorption and fluorescence detection. J. Anal. Atom. Spectrom. 2004, 19, 1379–1385. [Google Scholar]
- Chen, Y.W.; Zhou, M.D.; Tong, J.; Belzile, N. Application of photochemical reactions of Se in natural waters by hydride generation atomic fluorescence spectrometry. Anal. Chim. Acta 2005, 545, 142–148. [Google Scholar] [CrossRef]
- Chen, Y.W.; Zhou, X.L.; Tong, J.; Truong, Y.; Belzile, N. Photochemical behavior of inorganic and organic selenium compounds in various aqueous solutions. Anal. Chim. Acta 2005, 545, 149–157. [Google Scholar] [CrossRef]
- Shuvaeva, O.V.; Gustaytis, M.A.; Anoshin, G.N. Mercury speciation in environmental solid samples using thermal release technique with atomic absorption detection. Anal. Chim. Acta 2008, 621, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kaercher, L.E.; Goldschmidt, F.; Paniz, J.N.G.; Flores, É.M.M.; Dressler, V.L. Determination of inorganic and total mercury by vapor generation atomic absorption spectrometry using different temperatures of the measurement cell. Spectrochim. Acta Part B 2005, 60, 705–710. [Google Scholar] [CrossRef]
- Liao, M.X.; Deng, T.L. Arsenic species analysis in porewaters and sediments using hydride generation atomic fluorescence spectrometry. J. Environ. Sci. 2006, 18, 995–999. [Google Scholar] [CrossRef]
- Xi, J.C.; He, M.C.; Wang, K.P.; Zhang, G.Z. Comparison of masking agents for antimony speciation analysis using hydride generation atomic fluorescence spectrometry. Front. Environ. Sci. Eng. 2015, 9, 970–978. [Google Scholar] [CrossRef]
- Teran-Baamonde, J.; Bouchet, S.; Tessier, E.; Amouroux, D. Development of a large volume injection method using a programmed temperature vaporization injector-gas chromatography hyphenated to ICP-MS for the simultaneous determination of mercury, tin and lead species at ultra-trace levels in natural waters. J. Chromatogr. A 2018, 1547, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Giraaldez, I.; Ruiz-Azcona, P.; Vidal, A.; Morales, E. Speciation of selenite and selenoamino acids in biota samples bdual stir bar sorptive extraction-single desorption-capillary gas chromatography/mass spectrometry. Microchim. J. 2015, 122, 197–204. [Google Scholar] [CrossRef]
- Rahman, G.M.M.; Wolle, M.M.; Fahrenholz, T.; Kingston, H.M.; Pamuku, M. Measurement of mercury species in whole blood using speciated isotope dilution methodology integrated with microwave-enhanced solubilization and spike equilibration, headspace-solid-phase microextraction, and GC-ICP-MS analysis. Anal. Chem. 2014, 86, 6130–6137. [Google Scholar] [CrossRef] [PubMed]
- Gajdosechova, Z.; Pagliano, E.; Zborowski, A.; Mester, Z. Headspace in-tube microextraction and GC-ICP-MS determination of mercury species in petroleum hydrocarbons. Energy Fuels 2018, 32, 10493–10501. [Google Scholar] [CrossRef]
- Cai, Y.; Monsalud, S.; Jaffé, R.; Jones, R.D. Gas chromatographic determination of organomercury following aqueous derivatization with sodium tetraethylborate and sodium tetraphenylborate: Comparative study of gas chromatography coupled with atomic fluorescence spectrometry, atomic emission spectrometry and mass spectrometry. J. Chromatogr. A 2000, 876, 147–155. [Google Scholar] [PubMed]
- Nevado, J.J.B.; Martín-Doimeadios, R.C.R.; Krupp, E.M.; Bernardo, F.J.G.; Fariñas, N.R.; Moreno, M.J.; Wallace, D.; Ropero, M.J.P. Comparison of gas chromatographic hyphenated techniques for mercury speciation analysis. J. Chromatogr. A 2011, 1218, 4545–4551. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Kang, J.H.; Jung, H.J.; Ma, S.Y. Inorganic arsenic contents in ready-to-eat rice products and various Korean rice determined by a highly sensitive gas chromatography-tandem mass spectrometry. Food Chem. 2018, 240, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Jung, H.J.; Jung, M.Y. One step derivatization with British Anti-Lewsite in combination with gas chromatography coupled to triple-quadrupole tandem mass spectrometry for the fast and selective analysis of inorganic arsenic in rice. Anal. Chim. Acta 2016, 934, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Gionfriddo, E.; Naccarato, A.; Sindona, G.; Tagarelli, A. A reliable solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry method for the assay of selenomethionine and selenomethylselenocysteine in aqueous extracts: Difference between selenized and not-enriched selenium potatoes. Anal. Chim. Acta 2012, 747, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, E.; Sillanpaa, M.; Najafi, N.M. Headspace hollow fiber protected liquid-phase microextraction combined with gas chromatography-mass spectroscopy for speciation and determination of volatile organic compounds of selenium in environmental and biological samples. J. Chromatogr. A 2011, 118, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Maher, W.A.; Craig, P.J.; Jenkins, R.O. Speciation of volatile antimony compounds in culture headspace gases of Cryptococcus humicolus using solid phase microextraction and gas chromatography-mass spectrometry. Appl. Organomet. Chem. 2002, 16, 287–293. [Google Scholar] [CrossRef]
- Rekhi, H.; Rani, S.; Sharma, N.; Malik, A.K. A review on recent applications of high-performance liquid chromatography in metal determination and speciation analysis. Crit. Rev. Anal. Chem. 2017, 47, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Lee, W.B.; Kim, D.; Lee, Y.; Nam, S.H. An alternative analytical method for determining arsenic species in rice by using ion chromatography and inductively coupled plasma-mass spectrometry. Food Chem. 2019, 270, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.L.; Ju, M.Y.; Zhu, X.X.; Gan, H.; Gu, R.L.; Wu, Z.N.; Meng, Z.Y.; Dou, G.F. Pharmacokinetic properties of arsenic species after intravenous and intragastrical administration of arsenic trioxide solution in cynomolgus macaques using HPLC-ICP-MS. Molecules 2019, 24, 241. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.X.; Zhang, G.W.; Wang, J.T.; Zhong, Y.P.; Huang, Z.G. Determination of arsenic species in ophiocordyceps sinensis from major habitats in China by HPLC-ICP-MS and the edible hazard assessment. Molecules 2018, 23, 1012. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Kim, C.K.; Lee, K.S.; Min, H.S.; Lee, J.H. Study on the analytical method of arsenic species in marine samples by ion chromatography coupled with mass spectrometry. Microchem. J. 2018, 143, 16–20. [Google Scholar] [CrossRef]
- Doker, S.; Yilmaz, M. Speciation of arsenic in spring, well, and tap water by high-performance liquid chromatography-inductively coupled plasma-mass spectrometry. Anal. Lett. 2018, 51, 254–264. [Google Scholar] [CrossRef]
- Yu, H.M.; Du, H.; Wu, L.; Li, R.L.; Sun, Q.; Hou, X.D. Trace arsenic speciation analysis of bones by high performance liquid chromatography-inductively coupled plasma mass spectrometry. Microchim. J. 2018, 141, 176–180. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.M.; Zhang, X.Q.; Dong, R.; Yu, W.J.; Liu, Y.F.; Guo, Z.M.; Liang, X.M.; Zhu, J.H. Enzyme-assisted extraction and liquid chromatography-inductively coupled plasma mass spectrometry for the determination of arsenic species in fish. J. Chromatogr. A 2018, 1573, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Shen, L.H.; Liu, J.H.; Xu, Z.G.; Wang, Y.C. Coupling nanoliter high-performance liquid chromatography to inductively coupled plasma mass spectrometry for arsenic speciation. J. Sep. Sci. 2018, 41, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Landero, J.A.; Novo, D.L.; Duarte, F.A.; Mesko, M.F.; Caruso, J.A.; Flores, E.M.M. A feasible method for As speciation in several types of seafood by LC-ICP-MS/MS. Food Chem. 2018, 255, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Esperanza, M.G.; Barrientos, E.Y.; Wrobel, K.; Aguilar, F.J.A.; Escobosa, A.R.; Wrobel, K. Determination of total arsenic and speciation analysis in Mexican maize tortillas by hydride generation-microwave plasma atomic emission spectrometry and high performance liquid chromatography-inductively coupled plasma-mass spectrometry. Anal. Methods 2017, 9, 2059–2068. [Google Scholar] [CrossRef]
- Chen, S.Z.; Guo, Q.Z.; Liu, L.P. Determination of arsenic species in edible mushrooms by high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry. Food Anal. Method 2017, 10, 740–748. [Google Scholar] [CrossRef]
- Han, T.T.; Ji, H.W.; Li, H.X.; Cui, H.; Song, T.; Duan, X.J.; Zhu, Q.L.; Cai, F.; Zhang, L. Speciation analysis of arsenic compounds in seafood by ion chromatography-atomic fluorescence spectrometry. J. Ocean Univ. China 2017, 16, 455–460. [Google Scholar] [CrossRef]
- Schmidt, L.; Landero, J.A.; Santos, R.F.; Mesko, M.F.; Mello, P.A.; Flores, E.M.M.; Caruso, J.A. Arsenic speciation in seafood by LC-ICP-MS/MS: Method development and influence of culinary treatment. J. Anal. Atom. Spectrom. 2017, 32, 1490–1499. [Google Scholar] [CrossRef]
- Firat, M.; Bakirdere, S.; Sel, S.; Chormey, D.S.; Elkiran, O.; Erulas, F.; Turak, F. Arsenic speciation in water and biota samples at trace levels by ion chromatography inductively coupled plasma-mass spectrometry. Int. J. Environ. Anal. Chem. 2017, 97, 684–693. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, Y.; Su, Y.A.; Luo, Y.T.; Shih, T.T.; Sun, Y.C. Nanocomposite-coated microfluidic-based photocatalyst-assisted reduction device to couple high-performance liquid chromatography and inductively coupled plasma-mass spectrometry for online determination of inorganic arsenic species in natural water. Anal. Chem. 2017, 89, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, S.; Bakirdere, S.; Ataman, O.Y. Speciation of arsenic in fish by high-performance liquid chromatography-inductively coupled plasma-mass spectrometry. Anal. Lett. 2016, 49, 2501–2512. [Google Scholar] [CrossRef]
- Jia, X.Y.; Gong, D.R.; Wang, J.N.; Huang, F.Y.; Duan, T.C.; Zhang, X. Arsenic speciation in environmental waters by a new specific phosphine modified polymer microsphere preconcentration and HPLC-ICP-MS determination. Talanta 2016, 160, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.S.; Dai, J.Y.; Zhao, H.Y.; Zhu, J.; Luo, L.; Zhang, R.; Zhang, Z.; Li, L. Synthesis of MoS2 nanosheets for mercury speciation analysis by HPLC-UV-HG-AFS. RSC Adv. 2018, 8, 18364–18371. [Google Scholar] [CrossRef]
- Liu, H.; Luo, J.Y.; Ding, T.; Gu, S.Y.; Yang, S.H.; Yang, M.H. Speciation analysis of trace mercury in sea cucumber species of apostichopus japonicus using high-performance liquid chromatography Conjunction with inductively coupled plasma mass spectrometry. Biol. Trace Elem. Res. 2018, 186, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Zhang, F.P.; Jiao, B.Y.; Rao, J.Y.; Leng, G. Automated dispersive liquid-liquid microextraction coupled to high performance liquid chromatography-cold vapour atomic fluorescence spectroscopy for the determination of mercury species in natural water samples. J. Chromatogr. A 2017, 1493, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.H.; Jiang, S.J.; Sahayam, A.C.; Huang, Y.L. Speciation of mercury in fish oils using liquid chromatography inductively coupled plasma mass spectrometry. Microchem. J. 2017, 133, 556–560. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Chen, B.B.; He, M.; Huang, T.; Hu, B. Speciation of mercury in water and fish samples by HPLC-ICP-MS after magnetic solid phase extraction. Talanta 2017, 171, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.H.; Zhang, S.X.; Wang, M.L. Directly-thiolated graphene based organic solvent-free cloud point extraction-like method for enrichment and speciation of mercury by HPLC-ICP-MS. Microchem. J. 2017, 132, 299–307. [Google Scholar] [CrossRef]
- Zhang, S.X.; Luo, H.; Zhang, Y.Y.; Li, X.Y.; Liu, J.S.; Xu, Q.; Wang, Z.H. In situ rapid magnetic solid-phase extraction coupled with HPLC-ICP-MS for mercury speciation in environmental water. Microchem. J. 2016, 126, 25–31. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, Y.S.; Li, P.; Xue, M.; Pei, F.; Yang, W.J.; Ma, N.; Hu, Q.H. Simultaneous determination of arsenic and mercury species in rice by ion-pairing reversed phase chromatography with inductively coupled plasma mass spectrometry. Food Chem. 2016, 213, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, S.; Baker, P.; Crouch, A. Determination of mercury in selected polluted sediments using HPLC-ICP-MS in Westbank Area, Western Cape, South Africa. S. Afr. J. Chem. 2016, 69, 124–131. [Google Scholar] [CrossRef]
- Bakirdere, S.; Volkan, M.; Ataman, O.Y. Selenium speciation in chicken breast samples from inorganic and organic selenium fed chickens using high performance liquid chromatography-inductively coupled plasma-mass spectrometry. J. Food Compos. Anal. 2018, 71, 28–35. [Google Scholar] [CrossRef]
- Hu, T.; Liu, L.P.; Chen, S.Z.; Wu, W.L.; Xiang, C.G.; Guo, Y.B. Determination of selenium species in cordyceps militaris by high-performance liquid chromatography coupled to hydride generation atomic fluorescence spectrometry. Anal. Lett. 2018, 51, 2316–2330. [Google Scholar] [CrossRef]
- Gao, H.H.; Chen, M.X.; Hu, X.Q.; Chai, S.S.; Qin, M.L.; Cao, Z.Y. Separation of selenium species and their sensitive determination in rice samples by ion-pairing reversed-phase liquid chromatography with inductively coupled plasma tandem mass spectrometry. J. Sep. Sci. 2018, 41, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.; Yamini, Y. Inorganic selenium speciation in water and biological samples by three phase hollow fiber-based liquid phase microextraction coupled with HPLC-UV. New J. Chem. 2017, 41, 2378–2385. [Google Scholar] [CrossRef]
- Wiktor, L.; Barbara, M.; Dariusz, K.; Piotr, K.; Danuta, B. Study on Speciation of As, Cr, and Sb in bottled flavored drinking water samples using advanced analytical techniques IEC/SEC-HPLC/ICP-DRC-MS and ESI-MS/MS. Molecules 2019, 24, 668. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Lorenc, W.; Baralkiewicz, D. Study of the impact of bottles material and color on the presence of As-III, As-V, Sb-III, Sb-V and Cr-VI in matrix-rich mineral water—Multielemental speciation analysis by HPLC/ICP-DRC-MS. Microchem. J. 2017, 132, 1–7. [Google Scholar] [CrossRef]
- Lin, Y.A.; Jiang, S.J.; Sahayam, A.C. Determination of antimony compounds in waters and juices using ion chromatography-inductively coupled plasma mass spectrometry. Food Chem. 2017, 230, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, M.; Komorowicz, I.; Barałkiewicz, D. New procedure for multielemental speciation analysis of five toxic species: As(III), As(V), Cr(VI), Sb(III) and Sb(V) in drinking water samples by advanced hyphenated technique HPLC/ICP-DRC-MS. Anal. Chim. Acta 2016, 920, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, W.; Astudillo, F.; Bravo, M.; Cereceda-Balic, F.; Vidal, V.; Palomo-Marin, M.R.; Rueda-Holgado, F.; Pinilla-Gil, E. Antimony speciation in soils, sediments and volcanic ashes by microwave extraction and HPLC-HG-AFS detection. Microchem. J. 2016, 129, 111–116. [Google Scholar] [CrossRef]
- Jablonska-Czapla, M.; Szopa, S. Arsenic, antimony and chromium speciation using HPLC-ICP-MS in selected river ecosystems of Upper Silesia, Poland—A preliminary study and validation of methodology. Water Sci. Technol.-Water Supply 2016, 16, 354–361. [Google Scholar] [CrossRef]
- Wei, C.J.; Liu, J.X. A new hydride generation system applied in determination of arsenic species with ion chromatography–hydride generation-atomic fluorescence spectrometry (IC–HG-AFS). Talanta 2007, 73, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Han, C.; Cheng, H.Y.; Wang, Y.C.; Liu, J.H.; Xua, Z.G.; Hu, L. Rapid speciation analysis of mercury in seawater and marine fish by cation exchange chromatography hyphenated with inductively coupled plasma mass spectrometry. J. Chromatogr. A 2013, 1314, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Yanga, G.P. Selenium speciation in bay scallops by high performance liquid chromatography separation and inductively coupled plasma mass spectrometry detection after complete enzymatic extraction. J. Chromatogr. A 2014, 1325, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, R.; Soeroes, C.; Ipolyi, I.; Fodor, P.; Thomaidis, N.S. Determination of arsenic species in seafood samples from the Aegean Sea by liquid chromatography-(photo-oxidation)-hydride generation-atomic fluorescence spectrometry. Anal. Chim. Acta 2005, 547, 109–118. [Google Scholar] [CrossRef]
- Sánchez-Rodas, D.; Mellano, F.; Martínez, F.; Palencia, P.; Giráldez, I.; Morales, E. Speciation analysis of Se-enriched strawberries (Fragaria ananassa Duch) cultivated on hydroponics by HPLC-TR-HG-AFS. Microchem. J. 2016, 127, 120–124. [Google Scholar] [CrossRef]
- Chu, Y.L.; Jiang, S.J. Speciation analysis of arsenic compounds in edible oil by ion chromatography–inductively coupled plasma mass spectrometry. J. Chromatogr. A 2011, 1218, 5175–5179. [Google Scholar] [CrossRef] [PubMed]
- Wolle, M.M.; Rahman, G.M.M.; Kingston, H.M.; Pamuku, M. Speciation analysis of arsenic in prenatal and children’s dietary supplements using microwave-enhanced extraction and ion chromatography–inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2014, 818, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszek, J.; Kwiatkowski, P.; Ruzik, L. Speciation analysis and bioaccessibility evaluation of trace elements in goji berries (Lycium Barbarum L.). J. Chromatogr. A 2017, 1492, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.Y.; Wang, L.; Ma, L.; Yang, Z.G. Speciation analysis of six arsenic species in marketed shellfish: Extraction optimization and health risk assessment. Food Chem. 2018, 244, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.H.; Mar, J.L.G.; Rahman, G.M.M.; Seybert, B. Simultaneous determination of arsenic and selenium species in fish tissues using microwave–assisted enzymatic extraction and ion chromatography–inductively coupled plasma mass spectrometry. Talanta 2009, 78, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Nan, K.; He, M.; Chen, B.B.; Chen, Y.J.; Hu, B. Arsenic speciation in tree moss by mass spectrometry based hyphenated techniques. Talanta 2018, 183, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Sentkowska, A.; Pyrzynska, K. Hydrophilic interaction liquid chromatography in the speciation analysis of selenium. J. Chromatogr. B 2018, 1074, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Le, X.C.; Li, X.F.; Lai, V.; Ma, M.; Yalcin, S.; Feldmann, J. Simultaneous speciation of selenium and arsenic using elevated temperature liquid chromatography separation with inductively coupled plasma mass spectrometry detection. Spectrochim. Acta B 1998, 53, 899–909. [Google Scholar] [CrossRef]
- Ammann, A.A. Arsenic speciation by gradient anion exchange narrow bore ion chromatography and high resolution inductively coupled plasma mass spectrometry detection. J. Chromatogr. A 2010, 1217, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Milstein, L.S.; Essader, A.; Pellizzari, E.D.; Fernando, R.A.; Raymer, J.H.; Levine, K.E.; Akinbo, O. Development and application of a robust speciation method for determination of six arsenic compounds present in human urine. Environ. Health Perspect. 2003, 111, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Timerbaev, A.R. Element speciation analysis using capillary electrophoresis: Twenty years of development and applications. Chem. Rev. 2013, 113, 778–812. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; He, B.; Yun, Z.J.; Sun, J.; Jiang, G.B. Speciation analysis of arsenic compounds by capillary electrophoresis on-line coupled with inductively coupled plasma mass spectrometry using a novel interface. J. Chromatogr. A 2013, 1304, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.H.; Liu, J.Y.; Lu, W.H.; Gao, F.F.; Wang, L.Y.; Ma, J.P.; Liu, H.T.; Liao, C.Y.; Chen, L.X. Speciation analysis of mercury by dispersive solid-phase extraction coupled with capillary electrophoresis. Electrophoresis 2018, 39, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.F.; Li, J.H.; Lu, W.H.; Wen, Y.Y.; Cai, X.Q.; You, J.M.; Ma, J.P.; Ding, Y.J.; Chen, L.X. Speciation analysis of mercury in water samples by dispersive liquid-liquid microextraction coupled to capillary electrophoresis. Electrophoresis 2014, 35, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Li, P.J.; He, M.; Chen, B.B.; Hu, B. Automated dynamic hollow fiber liquid-liquid-liquid microextraction combined with capillary electrophoresis for speciation of mercury in biological and environmental samples. J. Chromatogr. A 2015, 1415, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kovachev, N.; Aguirre, M.Á.; Hidalgo, M.; Simitchiev, K.; Stefanova, V.; Kmetov, V.; Canals, A. Elemental speciation by capillary electrophoresis with inductively coupled plasma spectrometry: A new approach by Flow Focusing® nebulization. Microchem. J. 2014, 117, 27–33. [Google Scholar] [CrossRef]
- Liu, L.H.; Yun, Z.J.; He, B.; Jiang, G.B. Efficient interface for online coupling of capillary electrophoresis with inductively coupled plasma-mass spectrometry and its application in simultaneous speciation analysis of arsenic and selenium. Anal. Chem. 2014, 86, 8167–8175. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, H.; Ślachciński, M. Development of a new hybrid technique for inorganic arsenic speciation analysis by microchip capillary electrophoresis coupled with hydride generation microwave induced plasma spectrometry. Microchem. J. 2012, 102, 61–67. [Google Scholar] [CrossRef]
- Timerbaev, A.R. Element speciation analysis by capillary electrophoresis: What are the hints on becoming a standard analytical methodology. Anal. Chim. Acta 2001, 433, 165–180. [Google Scholar] [CrossRef]
- Clough, R.; Harrington, C.F.; Hill, S.J.; Madrid, Y.; Tyson, J.F. Atomic spectrometry update: Review of advances in elemental speciation. J. Anal. Atom. Spectrom. 2018, 33, 1103–1149. [Google Scholar] [CrossRef]
- D’Ulivo, A.; Baiocchi, C.; Pitzalis, E.; Onor, M.; Zamboni, R. Chemical vapor generation for atomic spectrometry. A contribution to the comprehension of reaction mechanisms in the generation of volatile hydrides using borane complexes. Spectrochim. Acta B 2004, 59, 471–486. [Google Scholar] [CrossRef]
- Grijalba, A.C.; Fiorentini, E.F.; Martinez, L.D.; Wuilloud, R.G. A comparative evaluation of different ionic liquids for arsenic species separation and determination in wine varietals by liquid chromatography-hydride generation atomic fluorescence spectrometry. J. Chromatogr. A 2016, 1462, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Xiao, Z.M.; Jia, Z.; Tian, J.; Liu, X.L.; Fan, X. Quantitative determination of arsenic species in feed using liquid chromatography-hydride generation atomic fluorescence spectrometry. Chin. J. Anal. Chem. 2018, 46, 537–542. [Google Scholar]
- Wang, Y.; Li, Y.Q.; Lv, K.; Chen, X.L.; Yu, X.Y. A simple and sensitive non-chromatographic method for quantification of four arsenic species in rice by hydride generation-atomic fluorescence spectrometry. Spectrochim. Acta B 2018, 149, 197–202. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Miro, M.; Kolev, S.D. A novel on-line organic mercury digestion method combined with atomic fluorescence spectrometry for automatic mercury speciation. Talanta 2018, 189, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Grijalba, A.C.; Fiorentini, E.F.; Wuilloud, R.G. Ionic liquid-assisted separation and determination of selenium species in food and beverage samples by liquid chromatography coupled to hydride generation atomic fluorescence spectrometry. J. Chromatogr. A 2017, 1491, 117–125. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.S.; Silva, L.O.B.; Santos, A.F.; da Silva, E.G.P.; dos Santos, W.N.L. Analytical strategies for determination and environmental impact assessment of inorganic antimony species in natural waters using hydride generation atomic fluorescence spectrometry (HG-AFS). J. Braz. Chem. Soc. 2018, 29, 185–190. [Google Scholar] [CrossRef]
- de Quadros, D.P.C.; Campanella, B.; Onor, M.; Bramanti, E.; Borges, D.L.G.; D’Ulivo, A. Mercury speciation by high-performance liquid chromatography atomic fluorescence spectrometry using an integrated microwave/UV interface. Optimization of a single step procedure for the simultaneous photo-oxidation of mercury species and photo-generation of Hg0. Spectrochim. Acta B 2014, 101, 312–319. [Google Scholar]
- Ai, X.; Wang, Y.; Hou, X.D.; Yang, L.; Zheng, C.B.; Wu, L. Advanced oxidation using Fe3O4 magnetic nanoparticles and its application in mercury speciation analysis by high performance liquid chromatography-cold vapor generation atomic fluorescence spectrometry. Analyst 2013, 138, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Chen, Y.J.; Tsai, Y.N. Determination of urinary arsenic species using an on-line nano-TiO2 photooxidation device coupled with microbore LC and hydride generation-ICP-MS system. Microchem. J. 2007, 86, 140–145. [Google Scholar] [CrossRef]
- Yu, X.P.; Deng, T.L.; Guo, Y.F.; Wang, Q. Arsenic species analysis in freshwater using liquid chromatography combined to hydride generation atomic fluorescence spectrometry. J. Anal. Chem. 2014, 69, 83–88. [Google Scholar] [CrossRef]
- Musil, S.; Matoušek, T. On-line pre-reduction of pentavalent arsenicals by thioglycolic acid for speciation analysis by selective hydride generation–cryotrapping–atomic absorption spectrometry. Spectrochim. Acta B 2008, 63, 685–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caylak, O.; Elci, S.G.; Hol, A.; Akdogan, A.; Divrikli, U.; Elci, L. Use of an aminated Amberlite XAD-4 column coupled to flow injection cold vapour generation atomic absorption spectrometry for mercury speciation in water and fish tissue samples. Food Chem. 2019, 274, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Chen, P.; Cao, L.; Xu, G.L.; Yang, S.Y.; Fang, Y.; Wang, G.Z.; Hong, X.C. Selenium speciation in rice samples by magnetic ionic liquid-based up-and-down-shaker-assisted dispersive liquid-liquid microextraction coupled to graphite furnace atomic absorption spectrometry. Food Anal. Method 2017, 10, 1653–1660. [Google Scholar] [CrossRef]
- dos Santos, Q.O.; Silva, M.M.; Lemos, V.A.; Ferreira, S.L.C.; de Andrade, J.B. An online preconcentration system for speciation analysis of arsenic in seawater by hydride generation flame atomic absorption spectrometry. Microchem. J. 2018, 143, 175–180. [Google Scholar] [CrossRef]
- Maratta, A.; Carrizo, B.; Bazan, V.L.; Villafane, G.; Martinez, L.D.; Pacheco, P. Antimony speciation analysis by hydride trapping on hybrid nanoparticles packed in a needle trap device with electro-thermal atomic absorption spectrometry determination. J. Anal. Atom. Spectrom. 2018, 33, 2195–2202. [Google Scholar] [CrossRef]
- Welna, M.; Pohl, P.; Szymczycha-Madeja, A. Non-chromatographic speciation of inorganic arsenic in rice by hydride generation inductively coupled plasma optical emission spectrometry. Food Anal. Method 2019, 12, 581–594. [Google Scholar] [CrossRef]
- Mo, J.M.; Li, Q.; Guo, X.H.; Zhang, G.X.; Wang, Z. Flow injection photochemical vapor generation coupled with miniaturized solution-cathode glow discharge atomic emission spectrometry for determination and speciation analysis of mercury. Anal. Chem. 2017, 89, 10353–10360. [Google Scholar] [CrossRef] [PubMed]
- Dundar, M.S.; Kaptan, F.; Caner, C.; Altundag, H. Speciation of antimony using dithizone ligand via cloud point extraction and determination by USN-ICP-OES. Atom. Spectrosc. 2018, 39, 100–105. [Google Scholar]
- Marcinkowska, M.; Baralkiewicz, D. Multielemental speciation analysis by advanced hyphenated technique—HPLC/ICP-MS: A review. Talanta 2016, 161, 177–204. [Google Scholar] [CrossRef] [PubMed]
- Popp, M.; Hann, S.; Koellensperger, G. Environmental application of elemental speciation analysis based on liquid or gas chromatography hyphenated to inductively coupled plasma mass spectrometry—A review. Anal. Chim. Acta 2010, 668, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Terol, A.; Marcinkowska, M.; Ardini, F.; Grotti, M. Fast determination of toxic arsenic species in food samples using narrow-bore high-performance liquid-chromatography inductively coupled plasma mass spectrometry. Anal. Sci. 2016, 32, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Zhang, W.W.; Wang, Y.C.; Liu, J.H. Interfacing nanoliter liquid chromatography and inductively coupled plasma mass spectrometry with an in-column high-pressure nebulizer for mercury speciation. J. Chromaatogr. A 2018, 1575, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Bolea-Fernandez, E.; Balcaen, L.; Resano, M.; Vanhaecke, F. Interference-free determination of ultra-trace concentrations of arsenic and selenium using methyl fluoride as a reaction gas in ICP-MS/MS. Anal. Bioanal. Chem. 2015, 407, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Stiboller, M.; Raber, G.; Gjengedal, E.L.F.; Eggesbo, M.; Francesconi, K.A. Quantifying inorganic arsenic and other water-soluble arsenic species in human milk by HPLC/ICPMS. Anal. Chem. 2017, 89, 6266–6272. [Google Scholar] [CrossRef] [PubMed]
- Izabela, K.; Adam, S.; Danuta, B. Total arsenic and arsenic species determination in freshwater fish by ICP-DRC-MS and HPLC/ICP-DRC-MS techniques. Molecules 2019, 24, 607. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Komorowicz, I.; Baralkiewicz, D. Study on multielemental speciation analysis of Cr(VI), As(III) and As(V) in water by advanced hyphenated technique HPLC/ICP-DRC-MS. Fast and reliable procedures. Talanta 2015, 144, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Bolea-Fernandez, E.; Balcaen, L.; Resano, M.; Vanhaecke, F. Overcoming spectral overlap via inductively coupled plasma-tandem mass spectrometry (ICPMS/MS). A tutorial review. J. Anal. Atom. Spectrom. 2017, 32, 1660–1679. [Google Scholar] [CrossRef]
- Balcaen, L.; Bolea-Fernandez, E.; Resano, M.; Vanhaecke, F. Inductively coupled plasma-tandem mass spectrometry (ICP-MS/MS): A powerful and universal tool for the interference-free determination of (ultra) trace elements—A tutorial review. Anal. Chim. Acta 2015, 894, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, P.; Alonso, J.I.G. Recent advances in isotope dilution analysis for elemental speciation. J. Anal. Atom. Spectrom. 2010, 25, 239–259. [Google Scholar] [CrossRef]
- Jeong, J.S.; Lee, J.; Park, Y.N. Quantitative speciation of selenium in human blood serum and urine with AE-RP- and AF-HPLC-ICP/MS. Bull. Korean Chem. Soc. 2013, 34, 3817–3824. [Google Scholar] [CrossRef]
- Castillo, A.; Boix, C.; Fabregat, N.; Roig-Navarro, A.F.; Rodriguez-Castrillon, J.A. Rapid screening of arsenic species in urine from exposed human by inductively coupled plasma mass spectrometry with germanium as internal standard. J. Anal. Atom. Spectrom. 2012, 27, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Petursdottir, A.H.; Gunnlaugsdottir, H. Selective and fast screening method for inorganic arsenic in seaweed using hydride generation inductively coupled plasma mass spectrometry (HG-ICP-MS). Microchem. J. 2019, 144, 45–50. [Google Scholar] [CrossRef]
- Matousek, T.; Wang, Z.F.; Douillet, C.; Musil, S.; Styblo, M. Direct speciation analysis of arsenic in whole blood and blood plasma at low exposure levels by hydride generation-cryotrapping-inductively coupled plasma mass spectrometry. Anal. Chem. 2017, 89, 9633–9637. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.N.; Lin, C.H.; Hsu, I.H.; Sun, Y.C. Sequential photocatalyst-assisted digestion and vapor generation device coupled with anion exchange chromatography and inductively coupled plasma mass spectrometry for speciation analysis of selenium species in biological samples. Anal. Chim. Acta 2014, 806, 165–171. [Google Scholar] [CrossRef] [PubMed]
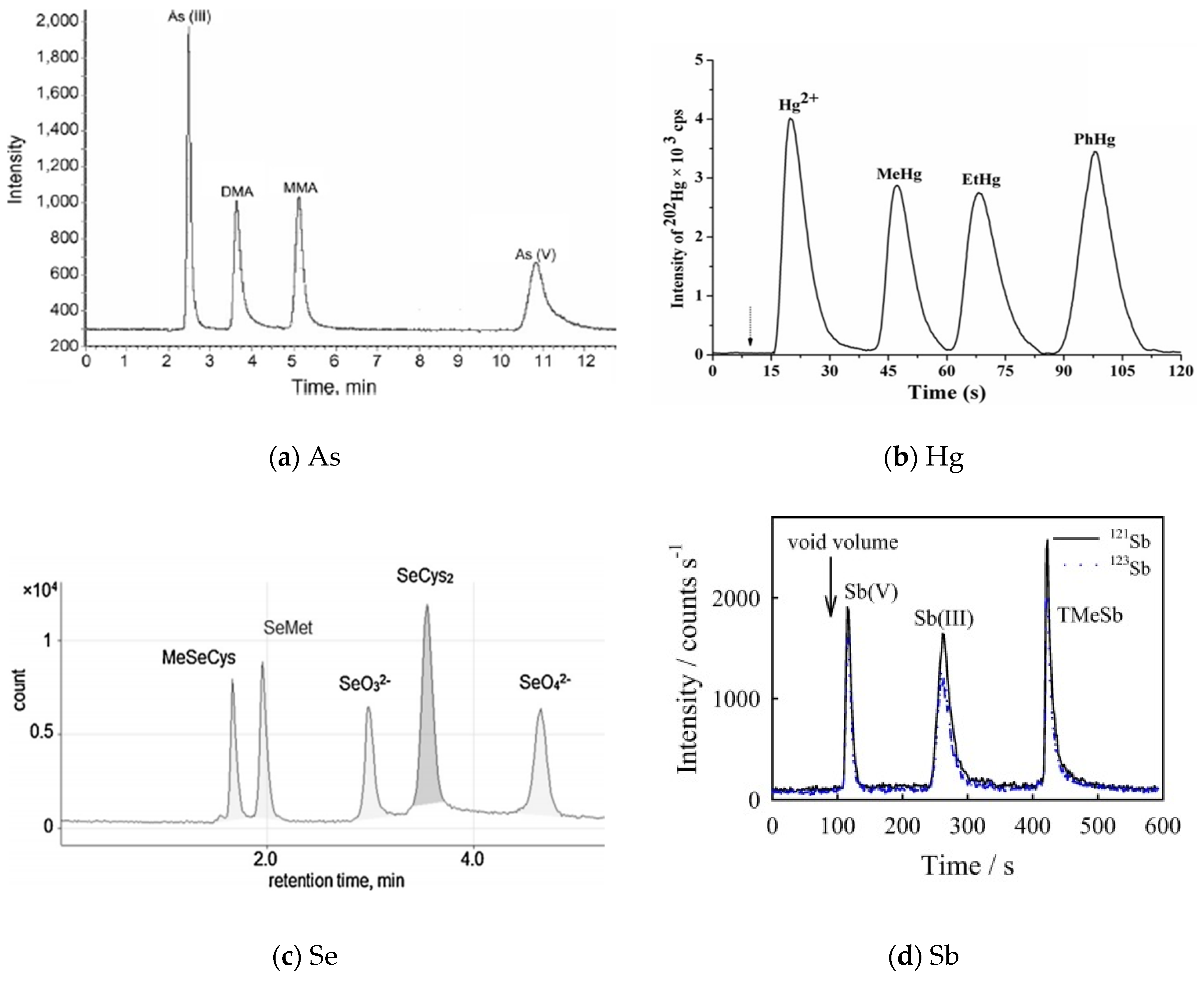
| Element | Species | Abbreviation | Chemical Formula |
|---|---|---|---|
| As | Arsenite (arsenous acid) | As(III) | As(OH)3 |
| Arsenate (arsenic acid) | As(V) | AsO(OH)3 | |
| Monomethylarsenate (Monomethylarsonic acid) | MMA | CH3AsO(OH)2 | |
| Dimethylarsonate (Dimethylarsinic acid) | DMA | (CH3)2AsO(OH) | |
| Trimethylarsinic oxide | TMAO | (CH3)3AsO | |
| Arsenocholine | AsC |  | |
| Arsenobetaine | AsB | 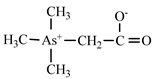 | |
| Arsenosugars | AsS | 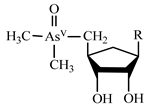 | |
| Roxarsone | Rox | 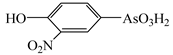 | |
| Arsenolipids a | 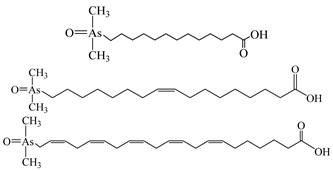 | ||
| Hg | Inorganic bivalent mercury | Hg(II) | Hg2+ |
| Methylmercury | MeHg | CH3Hg+ | |
| Dimethylmercury | DMeHg | (CH3)2Hg | |
| Ethylmercury | EtHg | CH3CH2Hg+ | |
| Diethylmercury | DEtHg | (CH3CH2)2Hg | |
| Methylethylmercury | MEtHg | (CH3CH2)(CH3)Hg | |
| Phenylmercury | PhHg | C6H5Hg+ | |
| Sb | Antimonite (Antimonous acid) | Sb(III) | Sb(OH)3 |
| Antimonate (Antimonic acid) | Sb(V) | SbO(OH)3 | |
| Methylantimate (Methylantimonic acid) | MMSb | CH3SbO(OH)2 | |
| Dimethylantimate (Dimethylantimonic acid) | DMSb | (CH3)2SbO(OH) | |
| Trimethylantimony dichloride | TMSbCl2 | (CH3)3SbCl2 | |
| Se | Selenite | Se(IV) | H2SeO3 |
| Selenate | Se(VI) | H2SeO4 | |
| Selenomethionine | SeMet | CH3SeCH2CH2CH(NH2)COOH | |
| Selenocysteine | SeCys | HSeCH2CH(NH2)COOH | |
| Se-methylselenocysteine | SeMeCys | CH3SeCH2CH(NH2)COOH | |
| Selenoethionine | SeEt | CH3CH2SeCH2CH2CH(NH2)COOH | |
| Selenocystine | SeCys2 | HOOCCH(NH2)CH2Se-SeCH2CH(NH2)COOH | |
| Selenohomolanthionine | SeHLan | HOOCCH(NH2)CH2CH2SeCH2CH2CH(NH2)COOH | |
| Trimethylselenonium ion | TMSe+ | (CH3)3Se+ | |
| Selenocyanate | SeCN− | N≡C―Se− | |
| Selenosugar 1 | SeSug 1 | 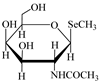 | |
| Selenosugar 2 | SeSug 2 |  | |
| Selenosugar 3 | SeSug 3 |  |
| Element | Species | Column | Detector | Matrix | Ref. |
|---|---|---|---|---|---|
| As | As(III), As(V), DMA, MMA | Hamilton PRP-X100 | ICP-MS | Rice | [91] |
| As(III), As(V), DMA, MMA | Agilent ZORBAX SB-Aq | ICP-MS | Cynomolgus macaques | [92] | |
| As(III), As(V), DMA, MMA, AsB, AsC | Dionex IonPac AS19 | ICP-MS | Ophiocordyceps sinensis | [93] | |
| As(III), As(V), DMA, MMA, AsB | Hamilton PRP-X100 | MS | Marine samples | [94] | |
| As(III), As(V) | Hamilton PRP-X100 | ICP-MS | Spring, well, and tap water | [95] | |
| As(III), As(V), DMA, MMA, AsB, AsC | Dionex IonPac AS7 | ICP-MS | Bones | [96] | |
| As(III), As(V), DMA, MMA, AsB, AsC | Dionex IonPac AS7 | ICP-MS | Fish | [97] | |
| As(III), As(V), DMA, MMA | Homemade capillary columns | ICP-MS | Human urine | [98] | |
| As(III), As(V), DMA, MMA, AsB | Hamilton PRP-X10 | ICP-MS/MS | Seafood | [99] | |
| As(III), As(V) | Hamilton PRP-X100 | ICP-MS | Mexican maize tortillas | [100] | |
| As(III), As(V), DMA, MMA, AsB, AsC | Dionex IonPac AS19 | ICP-MS | Edible Mushrooms | [101] | |
| As(III), As(V), DMA, MMA, AsB | Hamilton PRP-X100 | HG-AFS | Seafood | [102] | |
| As(III), As(V), DMA, MMA, AsB | Hamilton PRP-X10 | ICP-MS/MS | Seafood | [103] | |
| As(III), As(V), AsB | Dionex IonPac AS9-HC | ICP-MS | Water and biota samples | [104] | |
| As(III), As(V) | Hamilton PRP-X100 | ICP-MS | Natural water | [105] | |
| DMA, AsB | Spheris S5SCX | ICP-MS | Fish | [106] | |
| As(III), As(V), DMA, MMA | Hamilton PRP-X100 | ICP-MS | Environmental waters | [107] | |
| Hg | Hg(II), MeHg, EtHg | ZORBAX SB-C18 | ICP-MS | Surface water, seawater | [51] |
| Hg(II), MeHg, EtHg | Athena-C18 | HG-AFS | Environmental and biological samples | [108] | |
| Hg(II), MeHg, EtHg | ZORBAX SB-C18 | ICP-MS | Sea Cucumber | [109] | |
| Hg(II), MeHg, EtHg | Venusil MP-C18 | CV-AFS | Natural water | [110] | |
| Hg(II), MeHg, EtHg | PerkinElmer C8 | ICP-MS | Fish oils | [111] | |
| Hg(II), MeHg, PhHg | Hypersil ODS2 C18 | ICP-MS | Water and fish samples | [112] | |
| Hg(II), MeHg | CLC-ODS C18 | ICP-MS | Water | [113] | |
| Hg(II), MeHg | CLC-ODS C18 | ICP-MS | Water | [114] | |
| Hg(II), MeHg, EtHg | Agilent Eclipse plus C18 | ICP-MS | Rice | [115] | |
| Hg(II), MeHg, EtHg | Synergi Hydro-RP C18 | ICP-MS | Polluted sediments | [116] | |
| Se | Se(IV), Se(VI), SeMet, SeCys | Spheris S5 SAX | ICP-MS | Chicken breast | [117] |
| Se(IV), Se(VI), SeMet, SeCys | Hamilton PRP-X100 | HG-AFS | Cordyceps militaris | [118] | |
| Se(IV), Se(VI), SeMet, SeCys, SeMeCys | StableBond C18 | ICP-MS | Rice | [119] | |
| Se(IV), Se(VI) | ODS-3 | UV-Vis | Water and biological samples | [120] | |
| Sb | Sb(III), Sb(V) | Hamilton PRP-X100 | ICP-MS | Bottled flavored drinking water | [121] |
| Sb(III), Sb(V) | Hamilton PRP-X100 | ICP-MS | Matrix-rich mineral water | [122] | |
| Sb(III), Sb(V), TMSbCl2 | Hamilton PRP-X100 | ICP-MS | Waters, juices | [123] | |
| Sb(III), Sb(V) | Hamilton PRP-X100 | ICP-MS | Drinking water | [124] | |
| Sb(III), Sb(V) | Hamilton PRP-X100 | HG-AFS | Soils, sediments, volcanic ashes | [125] | |
| Sb(III), Sb(V) | Hamilton PRP-X100 | ICP-MS | Sediments, water | [126] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Liu, C.; Guo, Y.; Deng, T. Speciation Analysis of Trace Arsenic, Mercury, Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques. Molecules 2019, 24, 926. https://doi.org/10.3390/molecules24050926
Yu X, Liu C, Guo Y, Deng T. Speciation Analysis of Trace Arsenic, Mercury, Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques. Molecules. 2019; 24(5):926. https://doi.org/10.3390/molecules24050926
Chicago/Turabian StyleYu, Xiaoping, Chenglong Liu, Yafei Guo, and Tianlong Deng. 2019. "Speciation Analysis of Trace Arsenic, Mercury, Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques" Molecules 24, no. 5: 926. https://doi.org/10.3390/molecules24050926
APA StyleYu, X., Liu, C., Guo, Y., & Deng, T. (2019). Speciation Analysis of Trace Arsenic, Mercury, Selenium and Antimony in Environmental and Biological Samples Based on Hyphenated Techniques. Molecules, 24(5), 926. https://doi.org/10.3390/molecules24050926






