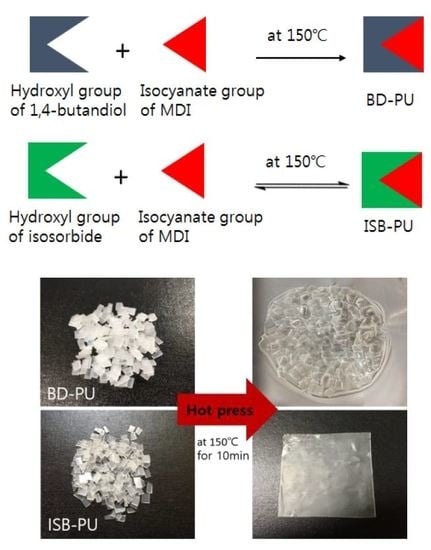
Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of PUEs
2.2. Thermal and Mechanical Properties of PUEs
2.3. Morphologies of PUEs
2.4. Reversibility of PUEs
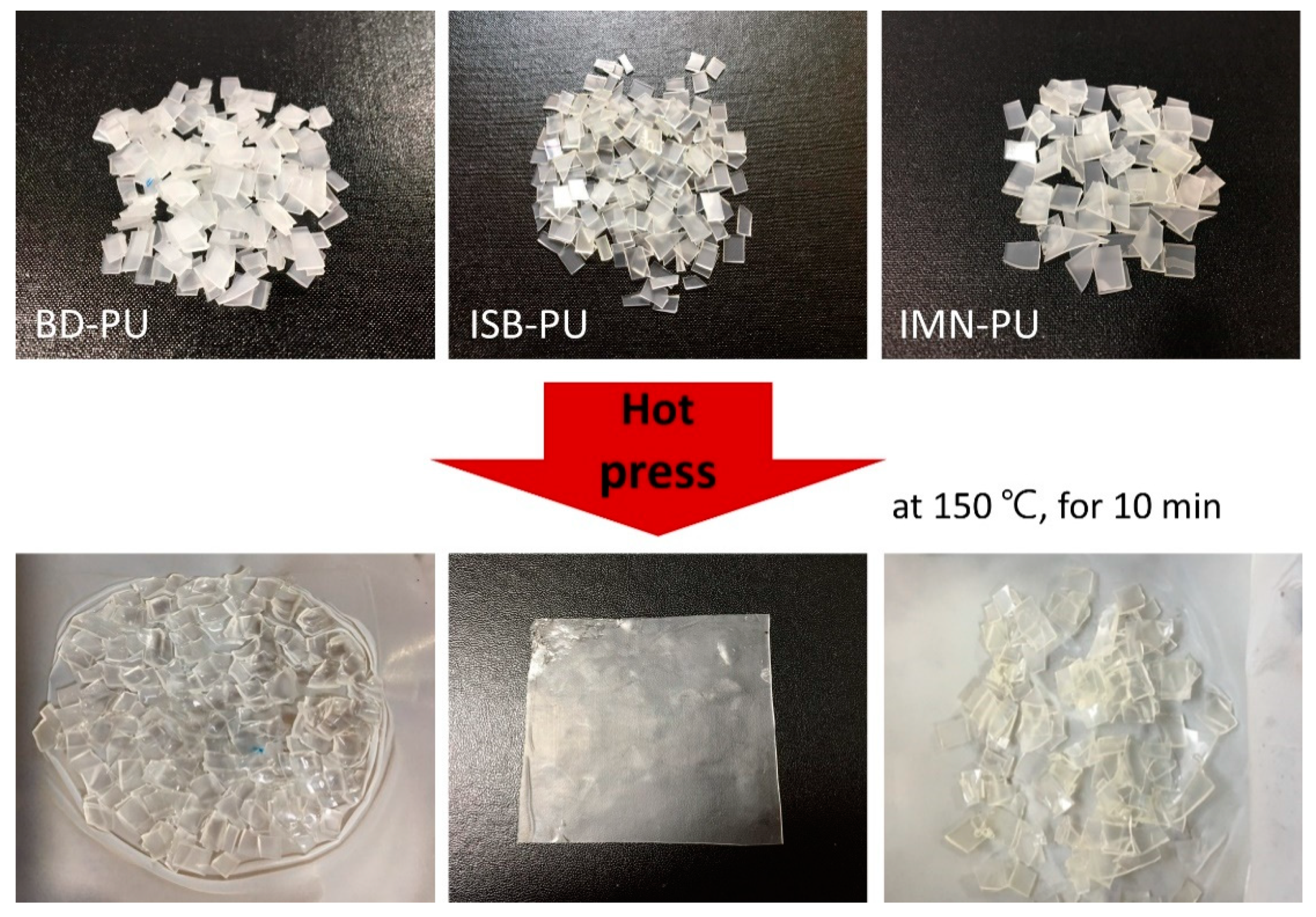
2.5. Reprocessability of ISB-Based PU
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PUEs
4.3. Characterization
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Biesmans, G. Polyurethane Technology & Applications; Lee, S., Ed.; Huntsman International: Everberg, Belgium, 2002. [Google Scholar]
- Hepburn, C. Polyurethane Elastomers, 2nd ed.; Elsevier Applied Science Publishers: London, UK; New York, NY, USA, 1992; ISBN 1581665897. [Google Scholar]
- Eceiza, A.; De La Caba, K.; Kortaberria, G.; Gabilondo, N.; Marieta, C.; Corcuera, M.A.; Mondragon, I. Influence of molecular weight and chemical structure of soft segment in reaction kinetics of polycarbonate diols with 4,4′-diphenylmethane diisocyanate. Eur. Polym. J. 2005, 41, 3051–3059. [Google Scholar] [CrossRef]
- Gisselfält, K.; Helgee, B. Effect of soft segment length and chain extender structure on phase separation and morphology in poly(urethane urea)s. Macromol. Mater. Eng. 2003, 288, 265–271. [Google Scholar] [CrossRef]
- Yilgor, I.; Yilgor, E.; Guler, I.G.; Ward, T.C.; Wilkes, G.L. FTIR investigation of the influence of diisocyanate symmetry on the morphology development in model segmented polyurethanes. Polymer 2006, 47, 4105–4114. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Bruchmann, B.; Lehn, J.M. Dynamers: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [PubMed]
- Wicks, Z.W. Bloked isocyanates. Prog. Org. Coat. 1975, 3, 73–99. [Google Scholar] [CrossRef]
- Rolph, M.S.; Markowska, A.L.J.; Warriner, C.N.; O’Reilly, R.K. Blocked isocyanates: From analytical and experimental considerations to non-polyurethane applications. Polym. Chem. 2016, 7, 7351–7364. [Google Scholar] [CrossRef]
- Sankar, G.; Sultan Nasar, A. Effect of isocyanate structure on deblocking and cure reaction of N-methylaniline-blocked diisocyanates and polyisocyanates. Eur. Polym. J. 2009, 45, 911–922. [Google Scholar] [CrossRef]
- Subramani, S.; Park, Y.J.; Lee, Y.S.; Kim, J.H. New development of polyurethane dispersion derived from blocked aromatic diisocyanate. Prog. Org. Coat. 2003, 48, 71–79. [Google Scholar] [CrossRef]
- Cao, S.; Li, S.; Li, M.; Xu, L.; Ding, H.; Xia, J.; Zhang, M.; Huang, K. A thermal self-healing polyurethane thermoset based on phenolic urethane. Polym. J. 2017, 49, 775–781. [Google Scholar] [CrossRef]
- Erice, A.; Ruiz de Luzuriaga, A.; Matxain, J.M.; Ruipérez, F.; Asua, J.M.; Grande, H.J.; Rekondo, A. Reprocessable and recyclable crosslinked poly(urea-urethane)s based on dynamic amine/urea exchange. Polymer 2018, 145, 127–136. [Google Scholar] [CrossRef]
- Zheng, K.; Tian, Y.; Fan, M.; Zhang, J.; Cheng, J. Recyclable, shape-memory, and self-healing soy oil-based polyurethane crosslinked by a thermoreversible Diels–Alder reaction. J. Appl. Polym. Sci. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Lee, A.; Deng, Y. Green polyurethane from lignin and soybean oil through non-isocyanate reactions. Eur. Polym. J. 2015, 63, 67–73. [Google Scholar] [CrossRef]
- Liu, W.; Xie, T.; Qiu, R. Biobased Thermosets Prepared from Rigid Isosorbide and Flexible Soybean Oil Derivatives. ACS Sustain. Chem. Eng. 2017, 5, 774–783. [Google Scholar] [CrossRef]
- Blache, H.; Méchin, F.; Rousseau, A.; Fleury, É.; Pascault, J.P.; Alcouffe, P.; Jacquel, N.; Saint-Loup, R. New bio-based thermoplastic polyurethane elastomers from isosorbide and rapeseed oil derivatives. Ind. Crops Prod. 2018, 121, 303–312. [Google Scholar] [CrossRef]
- Li, C.; Dai, J.; Liu, X.; Jiang, Y.; Ma, S.; Zhu, J. Green Synthesis of a Bio-Based Epoxy Curing Agent from Isosorbide in Aqueous Condition and Shape Memory Properties Investigation of the Cured Resin. Macromol. Chem. Phys. 2016, 217, 1439–1447. [Google Scholar] [CrossRef]
- Charlon, M.; Heinrich, B.; Matter, Y.; Couzigné, E.; Donnio, B.; Avérous, L. Synthesis, structure and properties of fully biobased thermoplastic polyurethanes, obtained from a diisocyanate based on modified dimer fatty acids, and different renewable diols. Eur. Polym. J. 2014, 61, 197–205. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.P. Polymers from renewable 1,4:3,6-dianhydrohexitols (isosorbide, isomannide and isoidide): A review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Yokoe, M.; Keigo, A.O.I.; Okada, M. Biodegradable polymers based on renewable resources. VII. Novel random and alternating copolycarbonates from 1,4:3,6-dianhydrohexitols and aliphatic diols. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 2312–2321. [Google Scholar] [CrossRef]
- Chatti, S.; Bortolussi, M.; Bogdal, D.; Blais, J.C.; Loupy, A. Microwave-assisted polycondensation of aliphatic diols of isosorbide with aliphatic disulphonylesters via phase-transfer catalysis. Eur. Polym. J. 2004, 40, 561–577. [Google Scholar] [CrossRef]
- Wu, J.; Eduard, P.; Thiyagarajan, S.; Noordover, B.A.J.; Van Es, D.S.; Koning, C.E. Semi-aromatic polyesters based on a carbohydrate-derived rigid diol for engineering plastics. ChemSusChem 2015, 8, 67–72. [Google Scholar] [CrossRef]
- Yoon, W.J.; Hwang, S.Y.; Koo, J.M.; Lee, Y.J.; Lee, S.U.; Im, S.S. Synthesis and characteristics of a biobased high-T g terpolyester of isosorbide, ethylene glycol, and 1,4-cyclohexane dimethanol: Effect of ethylene glycol as a chain linker on polymerization. Macromolecules 2013, 46, 7219–7231. [Google Scholar] [CrossRef]
- Varkey, E.C.; Sreekumar, K. Isosorbide based chiral polyurethanes: Optical and thermal studies. J. Mater. Sci. 2010, 45, 1912–1920. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Kasmi, N.; Tsanaktsis, V.; Doulakas, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. Synthesis and characterization of bio-based polyesters: Poly(2-methyl-1,3-propylene-2,5-furanoate), Poly(isosorbide-2,5-furanoate), Poly(1,4-cyclohexanedimethylene-2,5-furanoate). Materials 2017, 10, 801. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. The effect of gamma radiation on molecular stability and mechanical properties of biodegradable polyurethanes for medical applications. Polym. Degrad. Stab. 2003, 79, 465–474. [Google Scholar] [CrossRef]
- Kang, H.; Li, M.; Tang, Z.; Xue, J.; Hu, X.; Zhang, L.; Guo, B. Synthesis and characterization of biobased isosorbide-containing copolyesters as shape memory polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 7877–7886. [Google Scholar] [CrossRef]
- Gorna, K.; Gogolewski, S. Biodegradable porous polyurethane scaffolds for tissue repair and regeneration. Biomed. Mater. Res. Part A 2006, 79, 128–138. [Google Scholar] [CrossRef]
- Dirlikov, S.K.; Schneider, C.J. Polyurethane based on 1;4-3:6 dianhydrohexitol. U.S. Patent 4,443,563A, 17 April 1984. [Google Scholar]
- Javni, I.; Bilić, O.; Bilić, N.; Petrović, Z.S.; Eastwood, E.A.; Zhang, F.; Ilavský, J. Thermoplastic polyurethanes with isosorbide chain extender. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Javni, I.; Bilić, O.; Bilić, N.; Petrović, Z.S.; Eastwood, E.A.; Zhang, F.; Ilavský, J. Thermoplastic polyurethanes with controlled morphology based on methylenediphenyldiisocyanate/isosorbide/butanediol hard segments. Polym. Int. 2015, 64, 1607–1616. [Google Scholar] [CrossRef]
- Cognet-Georjon, E.; Méchin, F.; Pascault, J.P. New polyurethanes based on 4,4′-diphenylmethane diisocyanate and 1,4:3,6 dianhydrosorbitol, 2. Synthesis and properties of segmented polyurethane elastomers. Macromol. Chem. Phys. 1996, 197, 3593–3612. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Luo, M.; Xing, J.; Wu, J.; Pan, H.; Ruan, C.; Luo, Y. Incorporating isosorbide as the chain extender improves mechanical properties of linear biodegradable polyurethanes as potential bone regeneration materials. RSC Adv. 2017, 7, 13886–13895. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.I.; Park, H.S.; Park, J.H.; Knowles, J.C.; Gong, M.S. Application of high-strength biodegradable polyurethanes containing different ratios of biobased isomannide and poly (ε-caprolactone) diol. J. Bioact. Compat. Polym. 2013, 28, 274–288. [Google Scholar] [CrossRef]
- Brunette, C.M.; Hsu, S.L.; MacKnight, W.J. Hydrogen-Bonding Properties of Hard-Segment Model Compounds in Polyurethane Block Copolymers. Macromolecules 1982, 77, 71–77. [Google Scholar] [CrossRef]
- Coleman, M.M.; Skrovanek, D.J.; Hu, J. Hydrogen Bonding in Polymer Blends. 1. FTIR Studies of Urethane-Ether Blends. Macromolecules 1988, 21, 59–65. [Google Scholar] [CrossRef]
- Wang, C.; Feve, M.; Lam, T.M.Y.; Pascault, J.P. FTIR Analysis of Hydrogen Bonding in Amorphous Linear Aromatic Polyurethanes. I. Influence of Temperature. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1305–1313. [Google Scholar] [CrossRef]
- Wong, C.S.; Badri, K.H. Chemical Analyses of Palm Kernel Oil-Based Polyurethane Prepolymer. Mater. Sci. Appl. 2012, 3, 78–86. [Google Scholar] [CrossRef]
- Tien, Y.I.; Wei, K.H. Hydrogen bonding and mechanical properties in segmented montmorillonite/polyurethane nanocomposites of different hard segment ratios. J. Polym. Sci. 2001, 42, 3213–3221. [Google Scholar] [CrossRef]
- Hanna, J.G.; Siggia, S.; Chemical, O.M. Primary and Secondary Hydroxyl Group Content of Polypropylene Glycols. J. Polym. Sci. 1962, 56, 297–304. [Google Scholar] [CrossRef]
- Kojio, K.; Nakashima, S.; Furukawa, M. Microphase-separated structure and mechanical properties of norbornane diisocyanate-based polyurethanes. Polymer 2007, 48, 997–1004. [Google Scholar] [CrossRef]
- Frick, A.; Rochman, A. Characterization of TPU-elastomers by thermal analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Hossieny, N.; Shaayegan, V.; Ameli, A.; Saniei, M.; Park, C.B. Characterization of hard-segment crystalline phase of thermoplastic polyurethane in the presence of butane and glycerol monosterate and its impact on mechanical property and microcellular morphology. Polymer 2017, 112, 208–218. [Google Scholar] [CrossRef]
- Beniah, G.; Heath, W.H.; Jeon, J.; Torkelson, J.M. Tuning the properties of segmented polyhydroxyurethanes via chain extender structure. J. Appl. Polym. Sci. 2017, 134, 1–10. [Google Scholar] [CrossRef]
- Beniah, G.; Uno, B.E.; Lan, T.; Jeon, J.; Heath, W.H.; Scheidt, K.A.; Torkelson, J.M. Tuning nanophase separation behavior in segmented polyhydroxyurethane via judicious choice of soft segment. Polymer 2017, 110, 218–227. [Google Scholar] [CrossRef]
- Pedrazzoli, D. Understanding phase separation and morphology in thermoplastic polyurethanes nanocomposites. Polymer 2016, 90, 256–263. [Google Scholar] [CrossRef]
- Schön, P.; Bagdi, K.; Molnár, K.; Markus, P.; Pukánszky, B.; Vancso, G.J. Quantitative mapping of elastic moduli at the nanoscale in phase separated polyurethanes by AFM. Eur. Polym. J. 2011, 47, 692–698. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. Small-angle scattering analysis of hard-microdomain structure and microphase mixing in polyurethane elastomers. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 1883–1913. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Galembos, A.F.; Leung, L.M. Compression-Molded Polyurethane Block Copolymers. 1. Microdomain Morphology and Thermomechanical Properties. Macromolecules 1992, 25, 6195–6204. [Google Scholar] [CrossRef]
- Nasar, A.S.; Kalaimani, S. Synthesis and studies on forward and reverse reactions of phenol-blocked polyisocyanates: an insight into blocked isocyanates. RSC Adv. 2016, 6, 76802–76812. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |










| Sample Code | Composition (Molar Ratio) | Average Molecular Weight | ||||||
|---|---|---|---|---|---|---|---|---|
| Prepolymer | Chain Extender | Mn | Mw | Ð | ||||
| PTMEG | MDI | BD | ISB | IMN | (g/mol) | (g/mol) | ||
| BD–PU | 1 | 2 | 1 | - | - | 27,000 | 45,000 | 1.67 |
| ISB–PU | 1 | 2 | - | 1 | - | 23,000 | 44,000 | 1.91 |
| IMN–PU | 1 | 2 | - | - | 1 | 16,000 | 34,000 | 2.12 |
| Sample Code | DSC | DMA | ||||
|---|---|---|---|---|---|---|
| Tgs (°C) | Tgh (°C) | Tmh (°C) | ΔHmh (J/g) | Tgs (°C) | Tflow (°C) | |
| BD–PU | −50 | 58 | 151–183 | 4.7 | −30 | 151 |
| ISB–PU | −51 | 57 | 147–198 | 4.6 | −29 | 145 |
| IMN–PU | −60 | 47 | 151–199 | 7.2 | −44 | 168 |
| Sample Code | Young’s Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| BD–PU | 8 (±0.3) | 36 (±2.1) | 570 (±30.1) |
| ISB–PU | 14 (±0.8) | 48 (±3.4) | 462 (±49.0) |
| IMN–PU | 39 (±1.2) | 24 (±2.1)) | 518 (±21.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-N.; Lee, D.-W.; Ryu, H.; Song, G.-S.; Lee, D.-S. Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules 2019, 24, 1061. https://doi.org/10.3390/molecules24061061
Kim H-N, Lee D-W, Ryu H, Song G-S, Lee D-S. Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules. 2019; 24(6):1061. https://doi.org/10.3390/molecules24061061
Chicago/Turabian StyleKim, Han-Na, Dae-Woo Lee, Hoon Ryu, Gwang-Seok Song, and Dai-Soo Lee. 2019. "Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds" Molecules 24, no. 6: 1061. https://doi.org/10.3390/molecules24061061
APA StyleKim, H.-N., Lee, D.-W., Ryu, H., Song, G.-S., & Lee, D.-S. (2019). Preparation and Characterization of Isosorbide-Based Self-Healable Polyurethane Elastomers with Thermally Reversible Bonds. Molecules, 24(6), 1061. https://doi.org/10.3390/molecules24061061