Antitrypanosomal Activity of Sesquiterpene Lactones from Helianthus tuberosus L. Including a New Furanoheliangolide with an Unusual Structure
Abstract
:1. Introduction
2. Results and Discussion
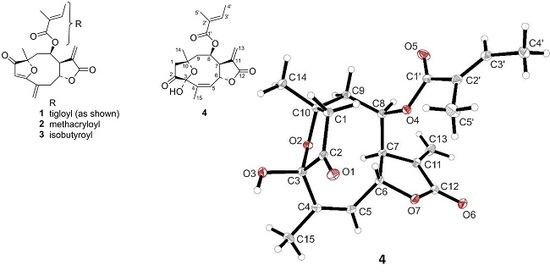
2.1. Isolation of STLs from H. tuberosus and Structure Elucidation of a New Furanoheliangolide
2.2. Antiprotozoal and Cytotoxic Activity of the Isolated STLs
3. Materials and Methods
3.1. General Methods
3.1.1. Ultra-High Performance Liquid Chromatography/Mass Spectrometry (UHPLC/MS)
3.1.2. Preparative HPLC
3.1.3. NMR Spectroscopy
3.1.4. X-ray Diffractometry
3.2. Plant Material
3.3. Extraction and Isolation
3.4. X-ray Crystallographic Analysis of Compound 4
3.5. Analytical Data
3.6. Biological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, T.J.; Da Costa, F.B.; Lopes, N.P.; Kaiser, M.; Brun, R. In Silico prediction and experimental evaluation of furanoheliangolide sesquiterpene lactones as potent agents against Trypanosoma brucei rhodesiense. Antimicrob. Agents Chemother. 2014, 58, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Spring, O. Sesquiterpene lactones from Helianthus tuberosus. Phytochemistry 1991, 30, 519–522. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Khalid, S.A.; Romanha, A.J.; Alves, T.M.; Biavatti, M.W.; Brun, R.; Da Costa, F.B.; de Castro, S.L.; Ferreira, V.F.; de Lacerda, M.V. The Potential of Secondary Metabolites from Plants as Drugs or Leads against Protozoan Neglected Diseases—Part I. Curr. Med. Chem. 2012, 19, 2128–2175. [Google Scholar] [CrossRef] [PubMed]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Anti-trypanosomatid elemanolide sesquiterpene lactones from Vernonia lasiopus O. Hoffm. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Antiprotozoal sesquiterpene lactones and other constituents from Tarchonanthus camphoratus and Schkuhria pinnata. J. Nat. Prod. 2018, 81, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kimani, M.N.; Matasyoh, J.C.; Kaiser, M.; Brun, R.; Schmidt, T.J. Sesquiterpene lactones from Vernonia cinerascens Sch. Bip. and their in vitro antitrypanosomal activity. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Kimani, N.M.; Matasyoh, J.C.; Kaiser, M.; Nogueira, M.S.; Trossini, G.H.G.; Schmidt, T.J. Complementary quantitative structure-activity relationship models for the antitrypanosomal activity of sesquiterpene lactones. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Nour, A.M.M.; Khalid, S.A.; Kaiser, M.; Brun, R. Quantitative Structure-Antiprotozoal activity relationships of Sesquiterpene Lactones. Molecules 2009, 14, 2062–2076. [Google Scholar] [CrossRef] [PubMed]
- APEX3 (2016), SAINT (2015), SADABS (2014) and XP–Interactive Molecular Graphics (2015); Bruker AXS Inc.: Madison, WI, USA, 2014.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Mabry, T.A. Furanoheliangolides from Helianthus schweinitzii. Phytochemistry 1984, 23, 2557–2559. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–4 are available from the authors. |


| Position | δC | δH | Mult. | J (Hz) |
|---|---|---|---|---|
| 1 | 45.4 | 3.142 | d | 19.9 |
| 2.684 | d | 19.9 | ||
| 2 | 210.2 | -- | ||
| 3 | 99.5 | -- | ||
| 4 | 138.9 | -- | ||
| 5 | 131.2 | 5.704 | dq | 6.5; 1.5 |
| 6 | 76.6 | 5.131 | ddq | 6.5; 1.5; 1.5 |
| 7 | 48.7 | 3.612 | m | |
| 8 | 77.2 | 5.275 | ddd | 3; 4; 1 |
| 9 | 43.4 | 2.370 | dd | 3.3; 16.2 |
| 2.292 | dd | 4.3; 16.2 | ||
| 10 | 78.7 | -- | ||
| 11 | 138.2 | -- | ||
| 12 | 169.4 | -- | ||
| 13 | 124.8 | 6.329 | d | 2.5 |
| 5.742 | d | 2.2 | ||
| 14 | 32.5 | 1.506 | s | |
| 15 | 20.1 | 1.939 | dd[t] | 1.5; 1.5 |
| 1′ | 166.6 | -- | ||
| 2′ | 127.9 | -- | ||
| 3′ | 139.6 | 6.758 | 1.4; 7.0 | |
| 4′ | 14.8 | 1.787 | dq | 1.1; 7.0 |
| 5′ | 12.23 | 1.766 | dq | ≈1.2 |
| 3-OH | -- | 2.724 | br s |
| Compound | Tbr | Tc | Ld | Pf | L6 |
|---|---|---|---|---|---|
| 1 a | 0.015 ± 0.003 | 3.7 ± 1.3 | n.t. | 1.0 ± 0.2 | 1.2 ± 0.5 |
| 2 | 0.077 ± 0.002 | 1.6 b | 0.83 ± 0.47 | 1.4 ± 0.1 | 0.52 ± 0.13 |
| 3 | 0.26 ± 0.10 | 3.1 b | 0.37 ± 0.19 | 0.83 ± 0.04 | 0.88 ± 0.26 |
| 4 | 0.92 ± 0.26 | 5.7 ± 0.6 | 2.0 ± 0.3 | 5.5 ± 0.3 | 3.9 ± 1.2 |
| Pos. Contr. | 0.008 ± 0.003 c | 1.45 ± 0.18 | 1.09 ± 0.21 e | 0.0012 ± 0.003 f | 0.024 ± 0.002 g |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galkina, A.; Krause, N.; Lenz, M.; Daniliuc, C.G.; Kaiser, M.; Schmidt, T.J. Antitrypanosomal Activity of Sesquiterpene Lactones from Helianthus tuberosus L. Including a New Furanoheliangolide with an Unusual Structure. Molecules 2019, 24, 1068. https://doi.org/10.3390/molecules24061068
Galkina A, Krause N, Lenz M, Daniliuc CG, Kaiser M, Schmidt TJ. Antitrypanosomal Activity of Sesquiterpene Lactones from Helianthus tuberosus L. Including a New Furanoheliangolide with an Unusual Structure. Molecules. 2019; 24(6):1068. https://doi.org/10.3390/molecules24061068
Chicago/Turabian StyleGalkina, Anna, Nico Krause, Mairin Lenz, Constantin G. Daniliuc, Marcel Kaiser, and Thomas J. Schmidt. 2019. "Antitrypanosomal Activity of Sesquiterpene Lactones from Helianthus tuberosus L. Including a New Furanoheliangolide with an Unusual Structure" Molecules 24, no. 6: 1068. https://doi.org/10.3390/molecules24061068
APA StyleGalkina, A., Krause, N., Lenz, M., Daniliuc, C. G., Kaiser, M., & Schmidt, T. J. (2019). Antitrypanosomal Activity of Sesquiterpene Lactones from Helianthus tuberosus L. Including a New Furanoheliangolide with an Unusual Structure. Molecules, 24(6), 1068. https://doi.org/10.3390/molecules24061068