Purification and Structural Analysis of the Effective Anti-TMV Compound ε-Poly-l-lysine Produced by Streptomyces ahygroscopicus
Abstract
:1. Introduction
2. Results
2.1. Isolation, Morphological and Cultural Characteristics of Streptomyces STZ
2.2. Physiological and Biochemical Characteristics
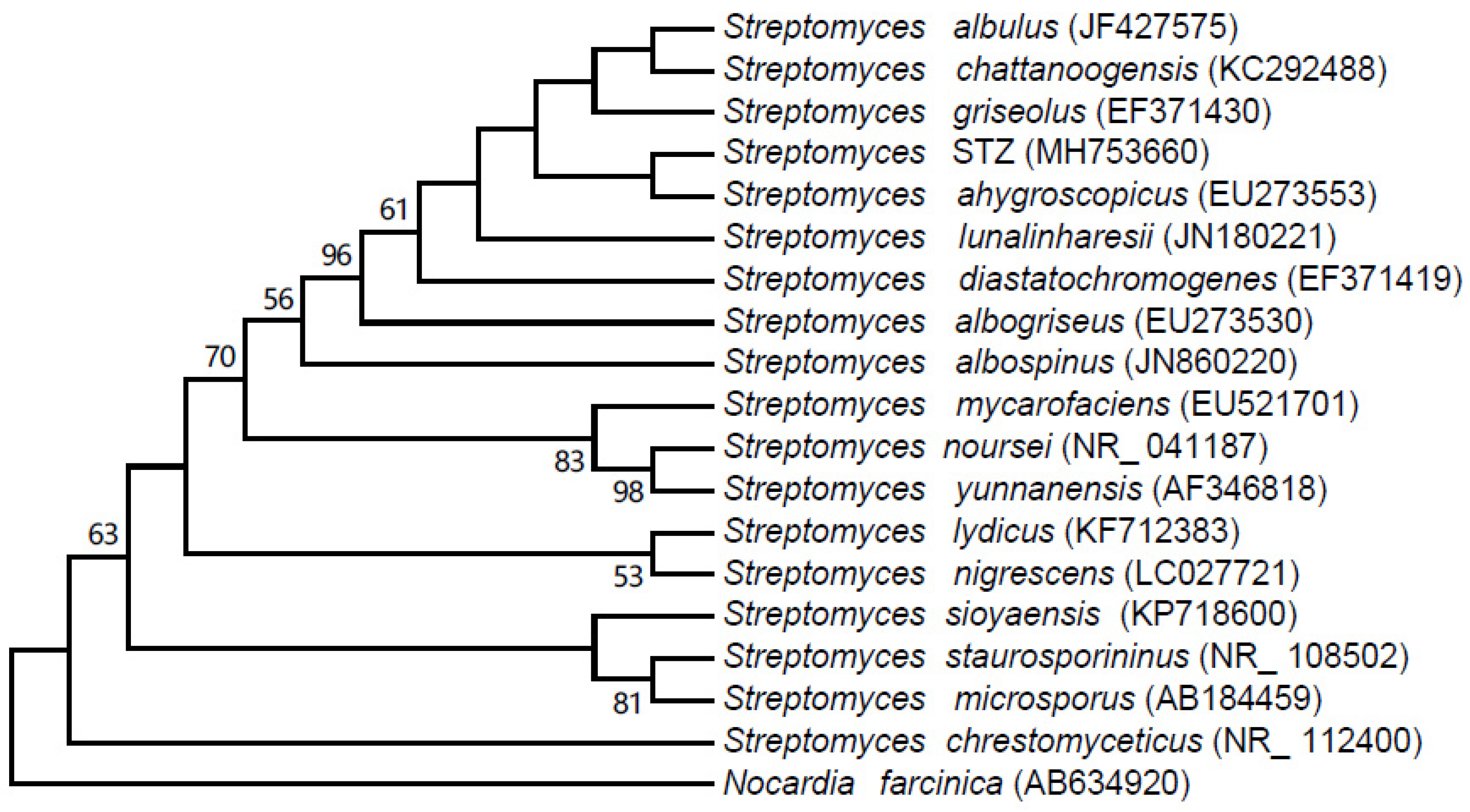
2.3. 16S rRNA Sequence Analysis
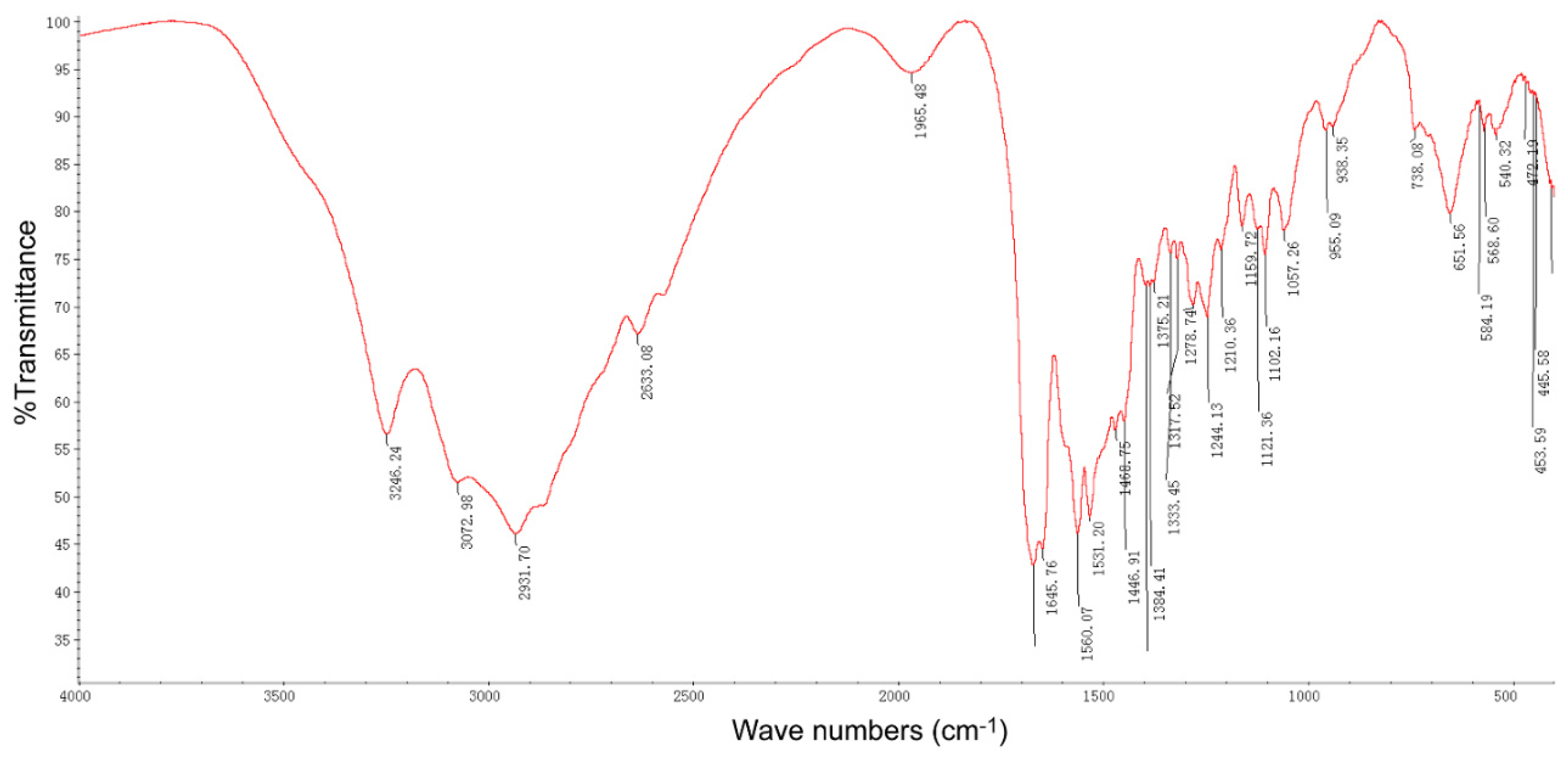
2.4. Purification and Structure Elucidation of the Pure Compound
2.5. MALDI-TOF-MS Analysis of the Active Compound
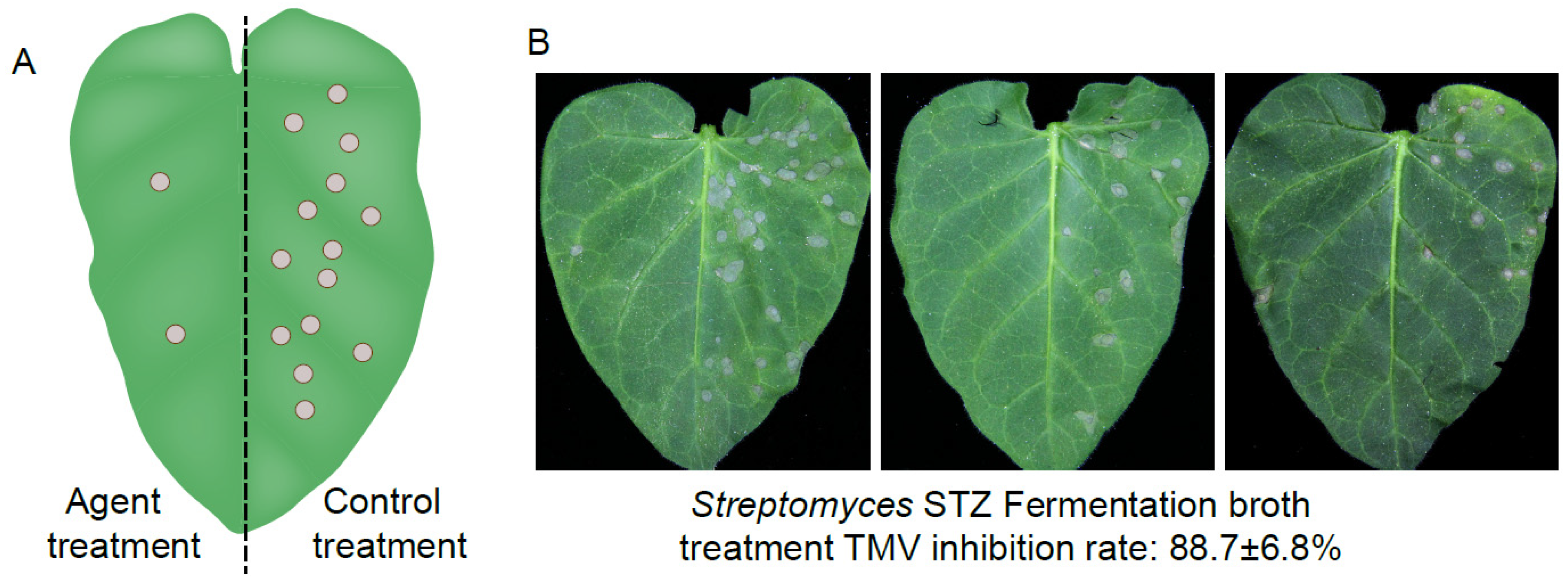
2.6. Protective and Curative Activities of the Compound ε-PL Against TMV
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Isolation of Streptomyces Strains
4.2. Fermentation
4.3. Anti-TMV Activity Screening of Streptomyces Strains
4.4. Taxonomic Characterization of the Strain STZ
4.5. Separation and Purification
4.6. Structure Determination for the Pure Compound
4.7. Molecular Mass Determination of the Pure Compound
4.8. Protection Effect of the Compound Against TMV In Vivo
4.9. Curative Effect of the Compound Against TMV In Vivo
Author Contributions
Funding
Conflicts of Interest
References
- Scholthof, K.G.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Fraile, A.; Garcia-Arenal, F. Tobamoviruses as Models for the Study of Virus Evolution. Adv. Virus Res. 2018, 102, 89–117. [Google Scholar] [PubMed]
- Roossinck, M.J. Plants, viruses and the environment: Ecology and mutualism. Virology 2015, 479–480, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N. Sustainability and agricultural and food chemistry. J. Agric. Food Chem. 2011, 59, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.G.; Luo, Y.; Qin, S.R.; Xi, L.; Wan, B.; Du, L.F. Induction of systemic resistance against tobacco mosaic virus by Ningnanmycin in tobacco. Pestic. Biochem. Physiol. 2014, 111, 14–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.X.; Wu, Y.H.; Du, C.M.; Song, Y. Control of tomato virus disease with Cytosintetidemycin. Pesticides 2004, 43, 534–536. [Google Scholar]
- Zhu, C.Y.; Wu, Y.H.; Wang, C.M.; Zhao, X.X.; Wang, Y.H.; Du, C.M. Inhibition of Cytosinpeptidemycin on tobacco mosaic virus. Plant Prot. 2005, 31, 52–54. [Google Scholar]
- Yu, L.; Wang, W.L.; Zeng, S.; Chen, Z.; Yang, A.M.; Shi, J.; Zhao, X.Z.; Song, B.A. Label-free quantitative proteomics analysis of Cytosinpeptidemycin responses in southern rice black-streaked dwarf virus-infected rice. Pestic. Biochem. Physiol. 2018, 147, 20–26. [Google Scholar] [CrossRef]
- Tan, Q.W.; Fang, P.H.; Ni, J.C.; Gao, F.L.; Chen, Q.J. Metabolites Produced by an Endophytic Phomopsis sp. and their anti-TMV activity. Molecules 2017, 22, 2073. [Google Scholar] [CrossRef]
- Han, L.R.; Zhang, G.Q.; Miao, G.P.; Zhang, X.; Feng, J.T. Streptomyces kanasensis sp. nov., an antiviral glycoprotein producing Streptomyces isolated from forest soil around Kanas Lake of China. Curr. Microbiol. 2015, 71, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Matsuoka, H.; Iwamoto, T.; Sakai, H. Antimicrobial action of epsilon-poly-l-lysine. J. Antibiot. 1984, 37, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Neda, K.; Sakurai, T.; Takahashi, M.; Ashiuchi, M.; Ohgushi, M. Two-generation reproduction study with teratology test of ε-poly-l-lysine by dietary administration in rats. Jpn. Pharmacol. Ther. 1999, 27, 1139–1159. [Google Scholar]
- Hiraki, J. Basic and applied studies on ε-poly-l-lysine. J. Antibact. Antifung. Agents 1995, 23, 349–354. [Google Scholar]
- Hamano, Y. Occurrence, biosynthesis, biodegradation, and industrial and medical applications of a naturally occurring epsilon-poly-l-lysine. Biosci. Biotechnol. Biochem. 2011, 75, 1226–1233. [Google Scholar] [CrossRef]
- Shima, S.; Sakai, H. Poly-l-lysine Produced by Streptomyces. Part III. Chemical Studies. Agric. Biol. Chem. Chem. 1981, 45, 2503–2508. [Google Scholar] [CrossRef]
- Maeda, S.; Kunimoto, K.K.; Sasaki, C.; Kuwae, A.; Hanaic, K. Characterization of microbial poly (ε-l-lysine) by FT-IR, Raman and solid state 13C-NMR spectroscopies. J. Mol. Struct. 2003, 655, 149–155. [Google Scholar] [CrossRef]
- Maeda, S.; Mori, T.; Sasaki, C.; Kunimoto, K.; Kuwae, A.; Hanai, K. Structural investigation of microbial poly (ε-l-lysine) derivatives with azo dyes by solid-state 13C and 15N NMR. Polym. Bull. 2005, 53, 259–267. [Google Scholar] [CrossRef]
- Shima, S.; Sakai, H. Polylysine produced by Streptomyces. Agric. Biol. Chem. 1977, 41, 1807–1809. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kobayashi, K. Streptomyces roseoverticillatus produces two different poly(amino acid)s: Lariat-shaped gamma-poly (l-glutamic acid) and epsilon-poly(l-lysine). Microbiology 2009, 155, 2988–2993. [Google Scholar] [CrossRef] [PubMed]
- Takehara, M.; Hibino, A.; Saimura, M.; Hirohara, H. High-yield production of short chain length poly (ε-l-ly sine) consisting of 5–20 residues by Streptomyces aureofaciens, and its antimicrobial activity. Biotechnol. Lett. 2010, 32, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, L.; Chen, X.S.; Liao, L.J.; Li, F.; Mao, Z.G. Isolation and characterization of a novel ε-poly-l-lysine producing strain: Streptomyces griseofuscus. J. Ind. Microbiol. Biotechnol. 2011, 38, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Han, Q.; Feng, J.L.; Tian, W.L.; Mo, H.Z. Antibacterial characteristics and mechanisms of ε-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control 2014, 43, 22–27. [Google Scholar] [CrossRef]
- Li, H.; He, C.; Li, G.G.; Zhang, Z.Q.; Li, B.Q.; Tian, S.P. The modes of action of epsilon-polylysine (ε-PL) against Botrytis cinerea in jujube fruit. Postharvest Biol. Technol. 2019, 147, 1–9. [Google Scholar] [CrossRef]
- Ye, R.S.; Xu, H.Y.; Wan, C.X.; Peng, S.S.; Wang, L.J.; Xu, H.; Aguilar, Z.; Xiong, Y.H.; Zeng, Z.L.; Wei, H. Antibacterial activity and mechanism of action of e-poly-l-lysine. Biochem. Biophys. Res. Commun. 2013, 439, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Shima, S.; Fukuhara, Y.; Sakai, H. Inactivation of bacteriophages by ε-poly-l-lysine produced by Streptomyces. Agric. Biol. Chem. 1982, 46, 1917–1919. [Google Scholar]
- El-Sersy, N.A.; Abdelwahab, A.E.; Abouelkhiir, S.S.; Abou-Zeid, D.M.; Sabry, S. Antibacterial and anticancer activity of ε-poly-l-lysine (ε-PL) produced by a marine Bacillus subtilis sp. J. Basic Microbiol. 2012, 52, 513–522. [Google Scholar] [CrossRef]
- Hiraki, J. ε-Polylysine: Its development and utilization. Fine Chem. 2000, 29, 18–25. [Google Scholar]
- Hiraki, J.; Ichikawa, T.; Ninomiya, S.; Seki, H.; Uohama, K.; Seki, H.; Kimura, S.; Yanagimoto, Y.; Barnett, J.W. Use of ADME studies to confirm the safety of ε-polylysine as a preservative in food. Regul. Toxicol. Pharmacol. 2003, 37, 328–340. [Google Scholar] [CrossRef]
- Yamanaka, K.; Hamano, Y. Biotechnological production of poly-epsilon-l-lysine for food and medical applications. In Amino-Acid Homopolymers Occurring in Nature; Hamano, Y., Ed.; Springer: Berlin, Germany, 2010; pp. 61–75. [Google Scholar]
- Shih, I.L.; Van, Y.T.; Shen, M.H. Biomedical applications of chemically and microbiologically synthesized poly (glutamicacid) and poly(lysine). Mini-Rev. Med. Chem. 2004, 4, 179–188. [Google Scholar] [CrossRef]
- Shih, I.L.; Shen, M.H.; Van, Y.T. Microbial synthesis of poly (ε-lysine) and its various applications. Bioresour. Technol. 2006, 97, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.C.; Singh, A.; Pandey, A.K.; Mishra, A. Review on production and medical applications of ε-polylysine. Biochem. Eng. J. 2012, 65, 70–78. [Google Scholar] [CrossRef]
- Tsao, P.H.; Leben, C.; Keitt, G.W. An enrichment method for isolating Streptomyces that produce diffusible antifungal antibiotics. Phytopathology 1960, 50, 88–89. [Google Scholar]
- Gooding, G.V.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285–1287. [Google Scholar] [PubMed]
- Song, B.A.; Zhang, H.P.; Wang, H.; Yang, S.; Jin, L.H.; Hu, D.Y. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891. [Google Scholar] [CrossRef] [PubMed]
- Shirling, E.B.; Gottlieb, D. Methods for characterisation of Streptomyces species. Int. J. Syst. Bacteriol. 1966, 16, 313–340. [Google Scholar] [CrossRef]
- Jones, K.L. Fresh isolates of Streptomyces in which the presence of sporogenous aerial mycelia is a fluctuating characteristic. J. Bacteriol. 1949, 57, 141–145. [Google Scholar] [PubMed]
- Waksman, S.A. The Streptomyces: Their nature, occurrence, activities, and importance. J. Am. Med. Assoc. 1950, 144, 505–506. [Google Scholar]
- Gottlieb, D. An evolution of criteria and procedures used in the description and bcharacterization of Streptomyces. Appl. Microbiol. 1961, 9, 55–65. [Google Scholar] [PubMed]
- Holding, A.J.; Collee, J.G. Chapter I routine biochemical tests. Methods Microbiol. 1971, 6A, 1–32. [Google Scholar]
- Sharma, A.D.; Singh, J. A nonenzymatic method to isolate genomic DNA from bacteria and Streptomyces. Anal. Biochem. 2005, 337, 354–356. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA gene. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the compound is available from the authors. |


| Tests | Result | Tests | Result |
|---|---|---|---|
| Biochemical tests: | Carbon source utilization: | ||
| Cellulose decomposition | − | d-glucose | + |
| Starch hydrolysis | + | Sucrose | + |
| Gelatin hydrolysis | + | l-arabinose | + |
| Nitrate reduction | + | Starch | + |
| H2S production | − | Raffinose | − |
| NaCl tolerance (%, w/v): | d-fructose | + | |
| 5 | + | Maltose | + |
| 7 | + | d-Xylose | + |
| 10 | − | Galactose | + |
| Growth temperature (°C): | d-mannitol | + | |
| 20 | + | Inositol | + |
| 28 | + | l-rhamnose | − |
| 37 | + | ||
| 50 | − |
| Compound Treatment | Protective Effect | Curative Effect |
|---|---|---|
| 500 μg/mL | 71.4 ± 1.7% | 59.3 ± 2.9% |
| 1000 μg/mL | 84.2 ± 3.2% | 68.7 ± 3.4% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Liu, H.; Xia, Z.; Zhao, X.; Wu, Y.; An, M. Purification and Structural Analysis of the Effective Anti-TMV Compound ε-Poly-l-lysine Produced by Streptomyces ahygroscopicus. Molecules 2019, 24, 1156. https://doi.org/10.3390/molecules24061156
Chen J, Liu H, Xia Z, Zhao X, Wu Y, An M. Purification and Structural Analysis of the Effective Anti-TMV Compound ε-Poly-l-lysine Produced by Streptomyces ahygroscopicus. Molecules. 2019; 24(6):1156. https://doi.org/10.3390/molecules24061156
Chicago/Turabian StyleChen, Jianguang, He Liu, Zihao Xia, Xiuxiang Zhao, Yuanhua Wu, and Mengnan An. 2019. "Purification and Structural Analysis of the Effective Anti-TMV Compound ε-Poly-l-lysine Produced by Streptomyces ahygroscopicus" Molecules 24, no. 6: 1156. https://doi.org/10.3390/molecules24061156





