Polyphenols and Alkaloids in Byproducts of Longan Fruits (Dimocarpus Longan Lour.) and Their Bioactivities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polyphenols in Different Parts of Longan Fruit
2.1.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
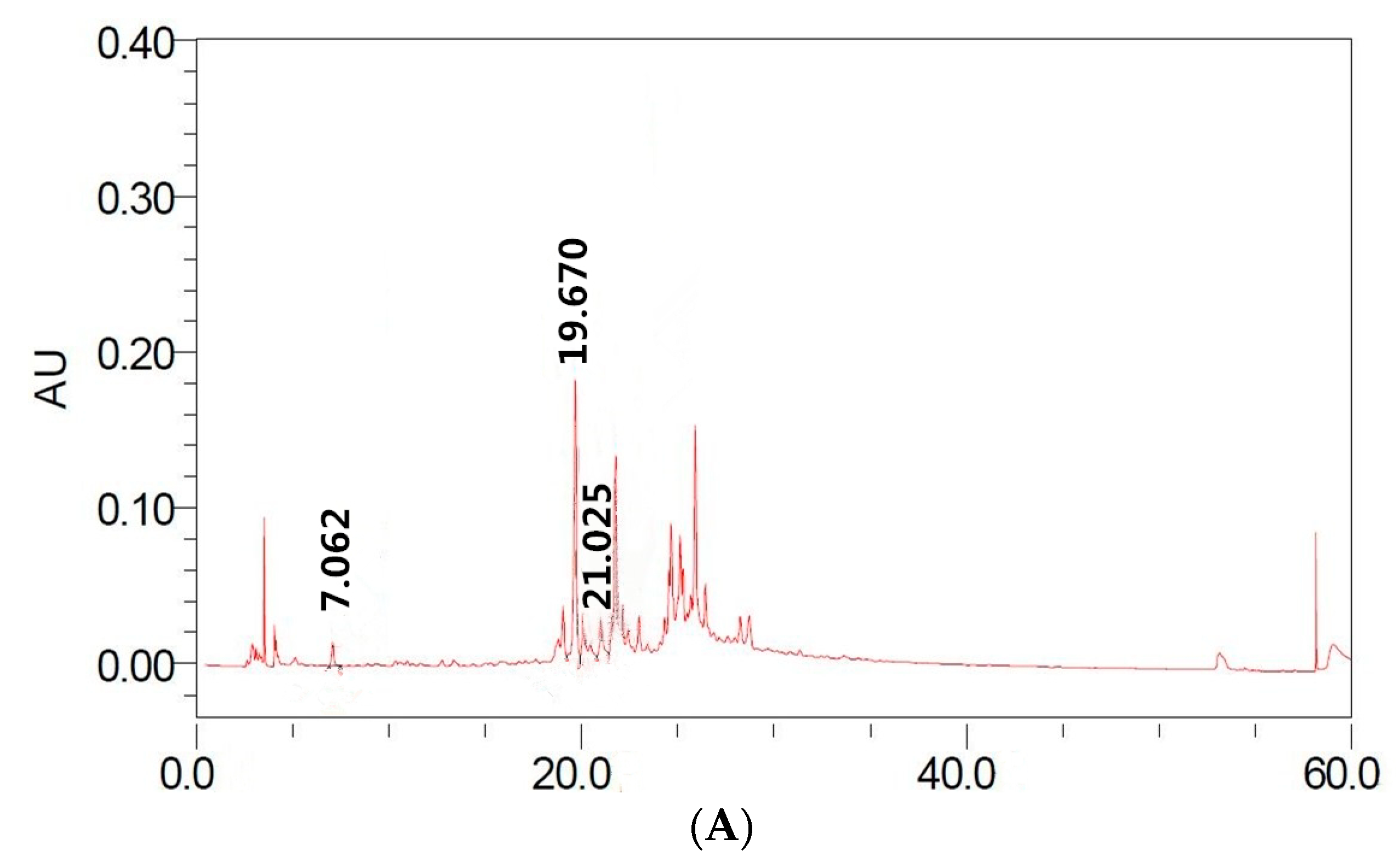
2.1.2. Identification and Quantification of Individual Polyphenolic Compounds
2.2. Total Alkaloid Content (TAC) in Different Parts of Longan Fruit
2.3. Functional Activities of Polyphenolic and Alkaloid Extracts in Longan Byproducts
2.3.1. Antioxidant Activities
2.3.2. Nitrite Scavenging Activities
2.3.3. Anti-Hyperglycemic Activities
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Longan Fruit Samples
3.3. Determination of Bioactive Substances in Different Parts of Longan Fruit
3.3.1. Determination of Total Phenolic Content (TPC)
3.3.2. Determination of Total Flavonoid Content (TFC)
3.3.3. Determination of Total Alkaloid Content (TAC)
3.4. Identification of Major Polyphenolic Compounds in Longan Byproducts
3.5. Bioactivities of Longan Byproducts
3.5.1. Antioxidant Activities
DPPH Free Radical Scavenging Activity
Oxygen Radical Absorbance Capacity (ORAC)
3.5.2. Nitrite Scavenging Activities in Simulated Gastric Fluid
3.5.3. α-Glucosidase Inhibitory Assay In Vitro
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tseng, H.; Wu, W.; Huang, H.; Wu, M. Quantification of fractions from longan seeds and their antimicrobial activity. Int. J. Med. Sci. 2013, I, 9–17. [Google Scholar]
- Rangkadilok, N.; Sitthimonchai, S.; Worasuttayangkurn, L.; Mahidol, C.; Ruchirawat, M.; Satayavivad, J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract. Food Chem. Toxicol. 2007, 45, 328–336. [Google Scholar] [CrossRef]
- Li, J.; Miao, S.; Jiang, Y. Changes in quality attributes of longan juice during storage in relation to effects of thermal processing. J. Food Qual. 2009, 32, 48–57. [Google Scholar] [CrossRef]
- Liu, G.; Sun, J.; He, X.; Tang, Y.; Li, J.; Ling, D.; Li, C.; Li, L.; Zheng, F.; Sheng, J.; et al. Fermentation process optimization and chemical constituent analysis on longan (Dimocarpus longan Lour.) wine. Food Chem. 2018, 256, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Liao, S.; Zhang, M.; Shi, J.; Zhang, R.; Deng, Y.; Wei, Z. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Jiang, Y.; Shi, J.; Chen, F.; Ashraf, M. Extraction and pharmacological properties of bioactive compounds from longan (Dimocarpus longan Lour.) fruit—A review. Food Res. Int. 2011, 44, 1837–1842. [Google Scholar] [CrossRef]
- Huang, G.; Wang, B.; Lin, W.; Huang, S.; Lee, C.; Yen, M.; Huang, M. Antioxidant and anti-inflammatory properties of longan (Dimocarpus longan Lour.) pericarp. Evid-Based. Complement. Altern. Med. 2012, 2012, 1–10. [Google Scholar]
- Yang, B.; Zhao, M.; Shi, J.; Yang, N.; Jiang, Y. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem. 2008, 106, 685–690. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, K.; Huang, S.; Wang, H.; Mu, X.; He, C.; Ji, X.; Zhang, J.; Huang, F. Antioxidant activity of microwave-assisted extract of longan (Dimocarpus longan Lour.) peel. Food Chem. 2008, 106, 1264–1270. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Worasuttayangkurn, L.; Bennett, R.; Satayavivad, J. Identification and quantification of polyphenolic compounds in longan (Euphoria longana Lam.) fruit. J. Agric. Food Chem. 2005, 53, 1387–1392. [Google Scholar] [CrossRef]
- Chung, Y.; Lin, C.; Chou, C.; Hsu, C. The effect of longan seed polyphenols on colorectal carcinoma cells. Eur. J. Clin. Investig. 2010, 40, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Nagendra Prasada, K.; Hao, J.; Shi, J.; Liu, T.; Li, J.; Wei, X.; Qiu, S.; Xue, S.; Jiang, Y. Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Innov. Food Sci. Emerg. Technol. 2009, 10, 413–419. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Li, Y.; Chen, R.; Ouyang, S.; Sun, P.; Pan, L.; Ren, H.; Yang, B. Antitumor activity of a polysaccharide from longan seed on lung cancer cell line A549 in vitro and in vivo. Tumour Biol. 2014, 35, 7259–7266. [Google Scholar] [CrossRef]
- Lin, C.; Chung, Y.; Hsu, C. Potential roles of longan flower and seed extracts for anti-cancer. World J. Exp. Med. 2012, 2, 78–85. [Google Scholar] [CrossRef]
- Walter, M.; Marchesan, E. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Biol. Technol. 2011, 54, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Cai, W.; Xu, B. From rice bag to table: Fate of phenolic chemical compositions and antioxidant activities in waxy and non-waxy black rice during home cooking. Food Chem. 2016, 191, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yan, X.; Yang, Z.; Chen, Y.; Lu, F.; Guan, X.; Huang, Y.; Li, D. Antioxidant capacity and contents of polyphenols in pericarps and stones of Dimocarpus longan. Food Sci. Technol. 2014, 39, 203–211. [Google Scholar]
- Wen, J.; Xu, Y.; Xaio, G.; Li, J.; Liao, S.; Wu, J.; Li, S.; Tang, D. Comparative studies on processing characteristics of different longan cultivars. Food Sci. 2010, 31, 71–75. [Google Scholar]
- Xu, B.; Chang, S.K.C. Total phenolic, phenolic acid, anthocyanin, flavan-3-ol, and flavonol profiles and antioxidant properties of pinto and black beans (Pheaseolus vulgaris L.) as affected by thermal processing. J. Agric. Food Chem. 2009, 57, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Latté, K.; Kolodziej, H. Antifungal effects of hydrolysable tannins and related compounds on dermatophytes, mould fungi and yeasts. Z. Naturforsch C 2000, 55, 467–472. [Google Scholar] [CrossRef]
- Notka, F.; Meier, G.R.; Wagner, R. Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antivir. Res. 2003, 58, 175–186. [Google Scholar] [CrossRef]
- Okabe, S.; Suganuma, M.; Imayoshi, Y.; Taniguchi, S.; Yoshida, T.; Fujiki, H. New TNF-α releasing inhibitors, geraniin and corilagin, in leaves of Acer nikoense, Megusurino-ki. Biol. Pharm. Bull. 2001, 24, 1145–1148. [Google Scholar] [CrossRef]
- Dey, A.; Mukherjee, A. Chapter 6—Plant-derived alkaloids: A promising window for neuroprotective drug discovery. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 273–320. [Google Scholar]
- Watson, A.; Fleet, G.; Asano, N.; Molyneux, R.; Nash, R. Polyhydroxylated alkaloids—Natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265–295. [Google Scholar] [CrossRef]
- Arbo, M.; Larentis, E.; Linck, V.; Aboy, A.; Pimentel, A.; Henriques, A.; Dallegrave, E.; Garcia, S.; Leal, M.; Limberger, R. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem. Toxicol. 2008, 46, 2770–2775. [Google Scholar] [CrossRef]
- Xian, J.; Chen, C. Study on extraction technology of total alkaloid from the longan seed. Food Res. Dev. 2013, 34, 28–30. (in Chinese). [Google Scholar]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef]
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar]
- Yuan, Y.; Zhang, T.; Zhuang, H.; Wang, K.; Zheng, Y.; Zhang, H.; Zhou, B.; Liu, J. Survey of nitrite content in foods from north-east China. Food Addit. Contam. Part B Surveill. 2010, 3, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chung, M.; Lee, S.; Shin, J.; Sung, N. N-nitrosamine inhibition by strawberry, garlic, kale, and the effects of nitrite-scavenging and N-nitrosamine formation by functional compounds in strawberry and garlic. Food Control 2007, 18, 485–491. [Google Scholar] [CrossRef]
- Mirvish, S. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995, 93, 17–48. [Google Scholar] [CrossRef]
- Mirvish, S. Effects of vitamin C and E on N-nitroso compound formation, carcinogenesis, and cancer. Cancer 1986, 58, 1842–1850. [Google Scholar] [CrossRef]
- González-Mancebo, S.; García-Santos, M.; Hernández-Benito, J.; Calle, E.; Casado, J. Nitrosation of phenolic compounds: Inhibition and enhancement. J. Agric. Food. Chem. 1999, 47, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Liang, Y. Microwave-assisted extraction of flavonoids from longan pericarp and its antioxidant activity study. Nat. Prod. Res. Dev. 2015, 27, 438–441. (In Chinese) [Google Scholar]
- Samejima, K.; Kanazawa, K.; Ashida, H.; Danno, G. Luteolin: A strong antimutagen against dietary carcinogen, Trp-P-2, in peppermint, sage, and thyme. J. Agric. Food Chem. 1995, 43, 410–414. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.; Wang, Z.; Wang, C.; Wang, E.; Zhang, Y.; Liu, J. Supereritieal fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability. Food Bioprod. Process. 2011, 89, 333–339. [Google Scholar] [CrossRef]
- Feelisch, M.; Fernandez, B.; Bryan, N.; Garcia-Saura, M.; Bauer, S.; Whitlock, D.; Ford, P.; Janero, D.; Rodriguez, J.; Ashrafian, H. Tissue processing of nitrite in hypoxia: An intricate interplay of nitric oxide-generating and-scavenging systems. J. Biol. Chem. 2008, 283, 33927–33934. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; He, X.; Zhao, M.; Li, L.; Li, C.; Dong, Y. Antioxidant and nitrite-scavenging capacities of phenolic compounds from sugarcane (Saccharum officinarum L.) tops. Molecules 2014, 19, 13147–13160. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Chen, L. α-Glucosidase and α-amylase inhibitors from seed oil: A review of liposoluble substance to treat diabetes. Crit. Rev. Food. Sci. Nutr. 2017, 57, 3438–3448. [Google Scholar] [CrossRef]
- Chawla, R.; Thakur, P.; Chowdhry, A.; Jaiswal, S.; Sharma, A.; Goel, R.; Sharma, J.; Priyadarshi, S.S.; Kumar, V.; Sharma, R.K.; et al. Evidence based herbal drug standardization approach in coping with challenges of holistic management of diabetes: A dreadful lifestyle disorder of 21st century. J. Diabetes Metab. Disord. 2013, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Mu, Y.; Han, L.; Liu, R.; Cai, Y.; Huang, X. Chemical characterization and anti-hyperglycaemic effects of polyphenol enriched longan (Dimocarpus longan Lour.) pericarp extracts. J. Funct. Foods 2015, 13, 314–322. [Google Scholar] [CrossRef]
- Borkataky, M.; Kakoty, B.; Saikia, L. Influence of total phenolic content and total flavonoid content of the DPPH radical scavenging activity of Eclipta Alba (L.) Hassk. Int. J. Pharm. Pharm. Sci. 2013, 5, 224–327. [Google Scholar]
- Ganga Rao, B.; Umamaheswara Rao, P.; Sambasiva Rao, E.; Mallikarjuna Rao, T.; Praneeth, D. Studies on phytochemical constituents, quantification of total phenolic, alkaloid content and in-vitro anti-oxidant activity of Thespesia populnea seeds. Free Radic. Antioxid. 2011, 1, 56–61. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, W.B.; Zhou, Y.X.; Guo, T. Nitrite scavenging activity of apple polyphenols in simulated gastric condition. Adv. Mater. Res. 2013, 773, 327–330. [Google Scholar] [CrossRef]
- Kang, W.; Li, Y.; Gu, X.; Xu, Q.; Huang, X. Antioxidant activities, α-glucosidase inhibitory effect in vitro and antihyperglycemic of Trapa acornis shell in alloxan-induced diabetic rats. J. Med. Plants Res. 2011, 5, 6805–6812. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |




| Sample | TPC (mg GAE/g) | TFC (mg RE/g) | TAC (mg HE/g) |
|---|---|---|---|
| Pulp (control) | 0.08 ± 0.00 c | 0.10 ± 0.01 c | 1.67 ± 0.13 c |
| Pericarp | 4.59 ± 0.25 a | 2.08 ± 0.04 a | 6.44 ± 0.23 b |
| Seed | 3.77 ± 0.08 b | 1.94 ± 0.01 b | 7.40 ± 1.04 a |
| Phenolic Compounds | Phenolic Content in Longan Pericarp (mg/g) | Phenolic Content in Longan Seed (mg/g) | ||
|---|---|---|---|---|
| 50% Ethanol Extract | 70% Ethanol Extract | 50% Ethanol Extract | 70% Ethanol Extract | |
| Gallic acid | 0.07 ± 0.00 k | 0.08 ± 0.00 k | 0.47 ± 0.01 g | 0.49 ± 0.02 g |
| Ethyl gallate | 0.27 ± 0.01 h | 0.28 ± 0.03 h | 0.88 ± 0.02 f | 1.84 ± 0.06 d |
| Ellagic acid | 0.15 ± 0.00 j | 0.18 ± 0.00 i,j | 0.21 ± 0.01 i | 0.83 ± 0.00 f |
| Corilagin | 1.75 ± 0.05 e | 2.15 ± 0.08 c | 5.03 ± 0.03 b | 5.53 ± 0.05 a |
| Sample | Phenolic Extracts (μg Extract/g) | Flavonoid Extracts (μg Extract/g) | Alkaloid Extracts (μg Extract/g) |
|---|---|---|---|
| Pericarp | 1827.11 ± 16.64 a | 451.96 ± 3.62 c | 29.17 ± 0.10 e |
| Seed | 1007.89 ± 23.22 b | 427.66 ± 3.01 d | 22.90 ± 1.20 f |
| Sample | Reaction Time | Nitrite Scavenging Activity (%) | ||||
|---|---|---|---|---|---|---|
| 0.2 mL Extracts | 0.4 mL Extracts | 0.6 mL Extracts | 0.8 mL Extracts | 1.0 mL Extracts | ||
| Pericarp | 10 min | 47.85 ± 1.09 p | 55.69 ± 1.31 n | 67.28 ± 1.80 k | 73.03 ± 2.05 i | 80.46 ± 1.96 g,h |
| 30 min | 48.31 ± 0.53 p | 70.15 ± 1.41 j | 80.00 ± 0.53 g,h | 85.74 ± 1.39 e | 88.15 ± 0.22 d | |
| 60 min | 60.62 ± 1.07 m | 79.18 ± 1.08 h | 87.90 ± 1.08 d | 90.97 ± 1.69 b,c | 94.26 ± 0.89 a | |
| Seed | 10 min | 42.70 ± 0.94 q | 59.67 ± 1.08 m | 70.37 ± 0.53 j | 78.55 ± 0.22 h | 83.13 ± 0.64 f |
| 30 min | 52.62 ± 0.22 o | 71.60 ± 1.35 i,j | 83.85 ± 0.78 e,f | 89.30 ± 1.28 c,d | 91.98 ± 1.93 b | |
| 60 min | 64.30 ± 1.08 l | 82.00 ± 0.78 f,g | 90.74 ± 1.41 b,c | 94.75 ± 1.07 a | 95.99 ± 1.11 a | |
| Sample | Reaction Time | Nitrite Scavenging Activity (%) | ||||
|---|---|---|---|---|---|---|
| 0.2 mL Extracts | 0.4 mL Extracts | 0.6 mL Extracts | 0.8 mL Extracts | 1.0 mL Extracts | ||
| Pericarp | 10 min | 50.27 ± 1.92 n | 58.22 ± 1.62 l | 68.37 ± 0.55 i,j | 73.37 ± 0.56 h | 75.97 ± 0.67 h |
| 30 min | 55.84 ± 2.08 m | 68.79 ± 2.91 i,j | 75.86 ± 0.29 h | 84.25 ± 0.62 f | 85.28 ± 1.28 d,e | |
| 60 min | 60.73 ± 0.43 k | 79.46 ± 0.60 g | 86.20 ± 0.92 c,d | 91.14 ± 0.76 b | 92.04 ± 0.46 a,b | |
| Seed | 10 min | 42.76 ± 0.33 o | 55.69 ± 0.41 m | 60.81 ± 0.88 k | 66.55 ± 1.02 j | 69.69 ± 0.84 i |
| 30 min | 51.21 ± 0.20 n | 67.13 ± 2.25 j | 75.77 ± 1.10 h | 81.66 ± 0.17 f,g | 83.30 ± 1.21 e,f | |
| 60 min | 60.80 ± 0.69 k | 76.48 ± 0.91 h | 83.89 ± 1.18 d,e,f | 88.07 ± 1.44 c | 93.64 ± 1.06 a | |
| Sample | Reaction Time | Nitrite Scavenging Activity (%) | ||||
|---|---|---|---|---|---|---|
| 0.2 mL Extracts | 0.4 mL Extracts | 0.6 mL Extracts | 0.8 mL Extracts | 1.0 mL Extracts | ||
| Pericarp | 10 min | 38.14 ± 1.51 r | 51.83 ± 1.38 o | 59.78 ± 1.74 m | 62.53 ± 1.38l | 68.53 ± 0.86 j |
| 30 min | 56.89 ± 2.92 n | 72.96 ± 0.45 h,i | 80.12 ± 1.12 g | 84.57 ± 1.12 e,f | 87.59 ± 1.35 d | |
| 60 min | 70.26 ± 0.47 i,j | 85.85 ± 0.47 d,e | 91.96 ± 1.74 a,b,c | 92.26 ± 1.84 a,b | 93.08 ± 2.84 a | |
| Seed | 10 min | 29.18 ± 1.86 s | 41.98 ± 1.65 q | 48.22 ± 0.33 p | 56.35 ± 1.26 n | 61.58 ± 1.46 l,m |
| 30 min | 50.33 ± 1.35 o,p | 63.92 ± 1.42 l | 74.50 ± 1.07 h | 79.18 ± 1.02 g | 83.18 ± 0.77 f | |
| 60 min | 62.58 ± 0.22 l | 79.47 ± 0.99 g | 85.93 ± 0.18 d,e | 89.53 ± 0.92 c | 90.45 ± 0.31 b,c | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.-Y.; He, X.-M.; Sun, J.; Li, C.-B.; Li, L.; Sheng, J.-F.; Xin, M.; Li, Z.-C.; Zheng, F.-J.; Liu, G.-M.; et al. Polyphenols and Alkaloids in Byproducts of Longan Fruits (Dimocarpus Longan Lour.) and Their Bioactivities. Molecules 2019, 24, 1186. https://doi.org/10.3390/molecules24061186
Tang Y-Y, He X-M, Sun J, Li C-B, Li L, Sheng J-F, Xin M, Li Z-C, Zheng F-J, Liu G-M, et al. Polyphenols and Alkaloids in Byproducts of Longan Fruits (Dimocarpus Longan Lour.) and Their Bioactivities. Molecules. 2019; 24(6):1186. https://doi.org/10.3390/molecules24061186
Chicago/Turabian StyleTang, Ya-Yuan, Xue-Mei He, Jian Sun, Chang-Bao Li, Li Li, Jin-Feng Sheng, Ming Xin, Zhi-Chun Li, Feng-Jin Zheng, Guo-Ming Liu, and et al. 2019. "Polyphenols and Alkaloids in Byproducts of Longan Fruits (Dimocarpus Longan Lour.) and Their Bioactivities" Molecules 24, no. 6: 1186. https://doi.org/10.3390/molecules24061186
APA StyleTang, Y.-Y., He, X.-M., Sun, J., Li, C.-B., Li, L., Sheng, J.-F., Xin, M., Li, Z.-C., Zheng, F.-J., Liu, G.-M., Li, J.-M., & Ling, D.-N. (2019). Polyphenols and Alkaloids in Byproducts of Longan Fruits (Dimocarpus Longan Lour.) and Their Bioactivities. Molecules, 24(6), 1186. https://doi.org/10.3390/molecules24061186





