Multielemental Analysis of Various Kinds of Whisky
Abstract
1. Introduction
1.1. Whisky in the World
1.1.1. Scotch Whisky
- (1)
- Single Malt Scotch Whisky,
- (2)
- Single Grain Scotch Whisky,
- (3)
- Blended Malt Scotch Whisky,
- (4)
- Blended Grain Scotch Whisky,
- (5)
- Blended Scotch Whisky.
1.1.2. Irish Whiskey
- The alcohol must be produced in Ireland from grain malt with or without mixtures of unmalted and malted cereals,
- Irish whisky must be triple distilled (three pot stills for malt whiskey; continuously distilled with three-column systems for grain whiskey),
- The only addition to the distillate is water and dye E150a—caramel,
- The minimum alcohol volume content must be 40%,
- The maturation process, for a minimum of 3 years, takes place in Ireland,
- The term ‘single’ may refer only to a distillate from one distillery [8].
1.1.3. American Bourbon
- Has to be distilled in the United States,
- Has to consist of a mixture of grains (at least 51% corn),
- Has to be matured in new, charred casks,
- Has to contain at most 80% pure alcohol after distillation,
- Not be poured into barrels if it contains more than 62.5% pure alcohol,
1.1.4. Other Countries
1.2. Product Authentication
2. Results and Discussion
2.1. Mercury Analysis
2.2. Mass Spectra Profiles
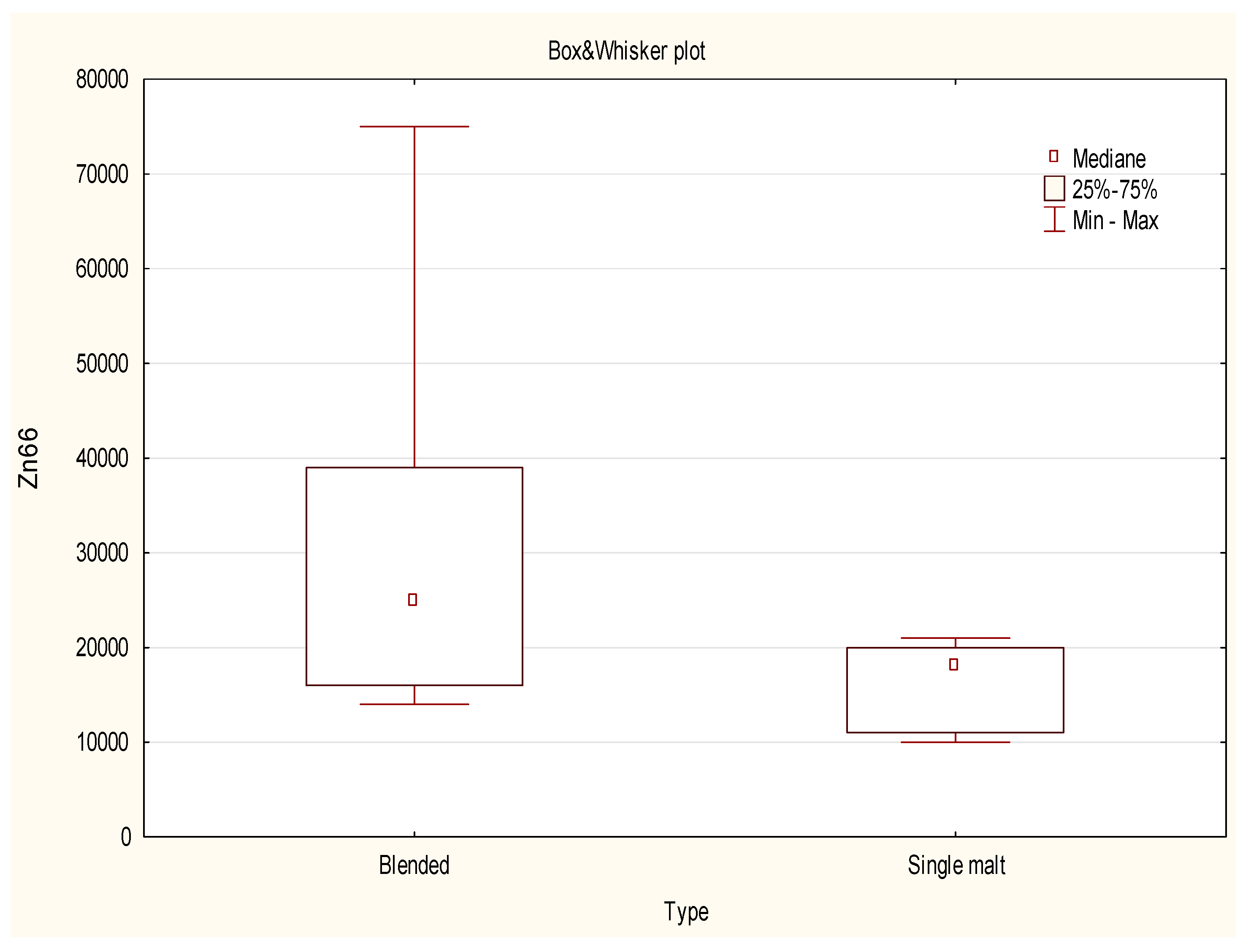
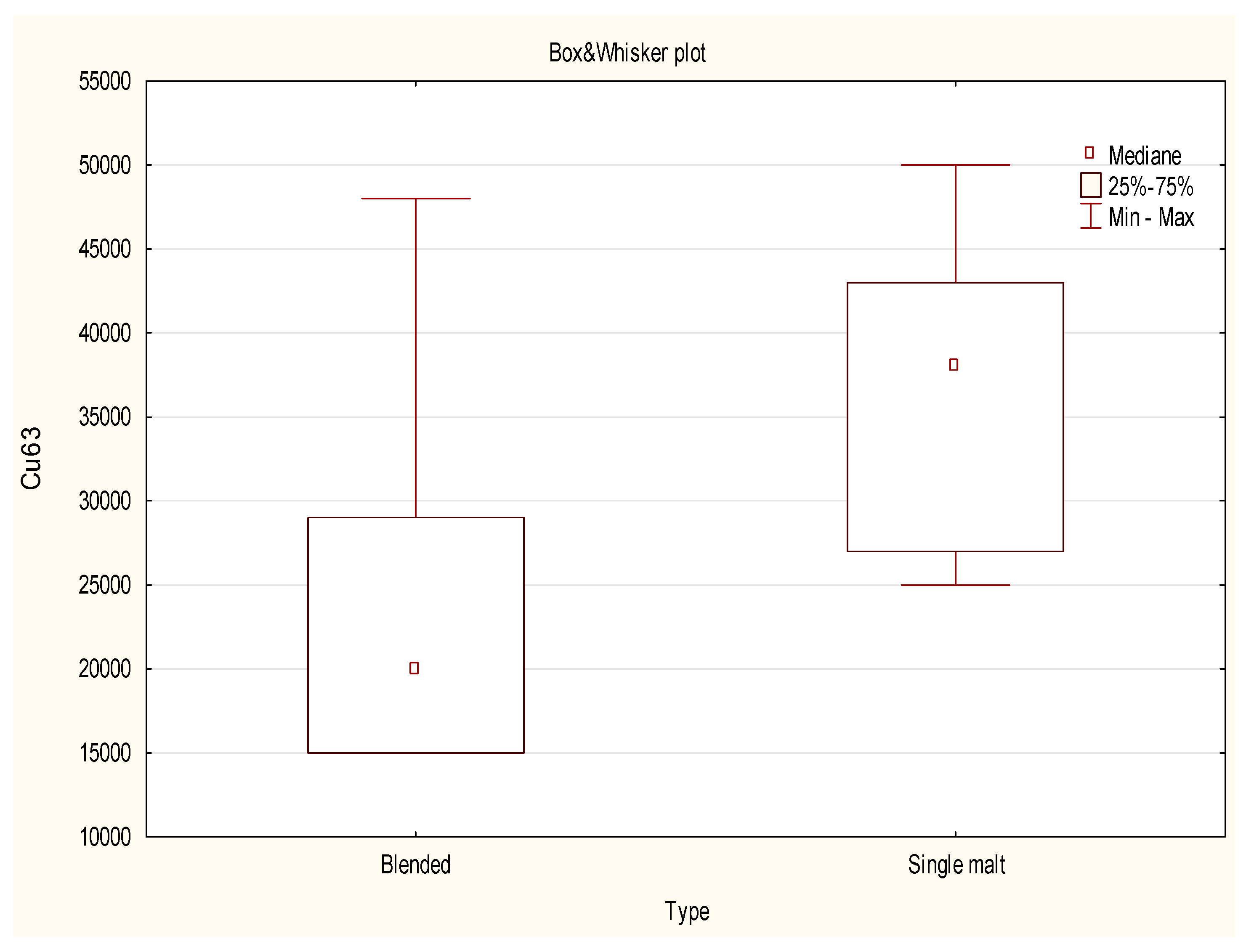
2.3. Data Analysis Using a Non-Parametric test
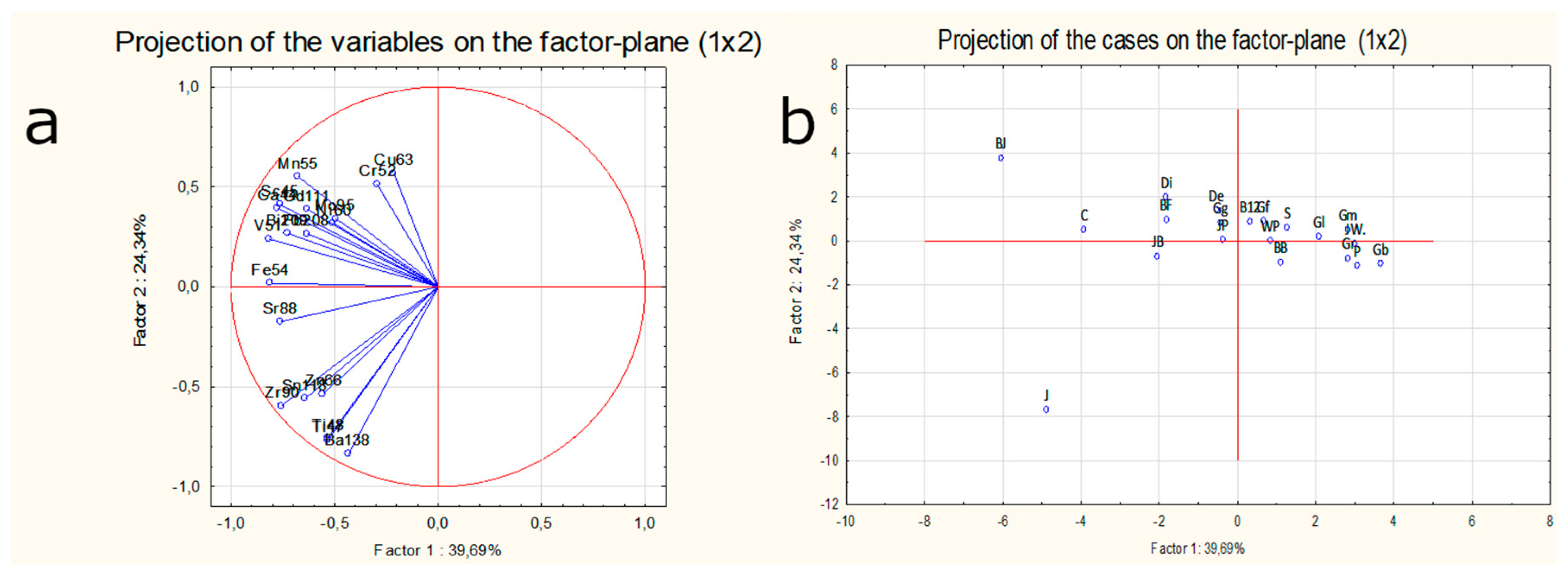
2.4. Semi-Quantitative Multivariate Data Analysis
3. Materials and Methods
3.1. Samples
3.2. Samples Preparation and Measurement
- stage I (20 min): max. pressure inside the reactor 130 bar, max. temperature inside the reactor 230 °C and outside temperature 60 °C with a max. microwave power of 1500 W;
- stage II (10 min): max. pressure inside the reactor 130 bar max. temperature inside the reactor 230 °C and outside 60 °C with a max. microwave power of 1500 W.
3.3. Equipment
- -
- an ultra-sensitive cell for small ranges of mercury (from 0.002 ng to 10 ng Hg),
- -
- a cell for larger ranges of mercury (from 10 ng to 25,000 ng). Depending on the amount of mercury in the studied samples, the calibration range can be chosen for the quantitative analysis [22].
3.4. Data Analysis
4. Conclusions
- (1)
- The highest average intensity of the signal among all analyzed isotopes for studied whisky samples was recorded for titanium (48Ti) and barium (138Ba). Therefore, these elements play a key role in distinguishing Irish whisky from the rest. Moreover, for this sample also the highest intensity of signal for isotopes like: 66Zn, 88Ti, 90Zr, 118Sn was observed. All these isotopes create a characteristic fingerprint of Irish—made whisky.
- (2)
- The use of multivariate analysis was crucial for the discrimination of samples based on their origin and type. The projection of cases on the factor plane for the first two components revealed that the sample of Irish whisky was an evident outlier. On the other hand the projection of the cases of the factor plane for the first two components proved that the blended type of whisky was more dispersed in this diagram, while the single malt type of samples was grouped within the same cluster. Within the studied population the scatter of results for most of the (with in exception of: 63Cu, 101Ru) analyzed isotopes for blended types of whisky was much more visible than for single malts. The dispersion of the results for both types of whisky can also be a consequence of the unequal number of valid cases.
- (3)
- In any case the mercury content in analyzed whisky samples does not exceed the allowed level of 20 μg/L (according to the Polish Regulation of the Minister of Health concerning of the maximum level of mercury in spirit products containing more than 20% of alcohol).
- (4)
- Future studies are needed in which quantitative data will be presented for a bigger number of objects originating from different regions of Scotlad and from the same distillery in order to evaluate if the analyzed bottle is representative for a particular brand.
Author Contributions
Conflicts of Interest
References
- Aylott, R. Whisky Analysis, in Whisky-Technology, Production and Marketing, 2nd ed.; Russell, I., Stewart, G., Eds.; International Centre for Brewing and Distilling, Heriot-Watt University: Edinburgh, UK, 2014; Volume 14, pp. 243–270. [Google Scholar]
- Dominé, A. Alkohole Świata Uniwersalny Podręcznik Barmana, 1st ed.; Wydawnictwo Olesiejuk: Ożarów Mazowiecki, Polska, 2008; pp. 106–107, 198, 305–363. [Google Scholar]
- Arthur, H. Whisky–Woda Życia, 1st ed.; Wydawnictwo Bellona: Warszawa, Polska, 2008; pp. 15–70, 95–97. [Google Scholar]
- Waishart, D. Whisky-Leksykon Smakosza, 2nd ed.; Wydawnictwo RM: Warszawa, Polska, 2013; pp. 108–149. [Google Scholar]
- Lyons, T.P. Production of Scotch and Irish whiskies: Their history and evolution. In The Alcohol Textbook, 4th ed.; Jacques, K.A., Lyons, T.P., Kelsall, D.R., Eds.; Nottingham University Press: Nottingham, UK, 2003; pp. 193–222. [Google Scholar]
- The Scotch Whisky Regulations 2009. Available online: http://www.scotch-whisky.org.uk/media/12744/ scotchwhiskyreg guidance2009.pdf (accessed on 1 February 2019).
- Whisky Regions & Tours. Available online: http://www.scotch-whisky.org.uk/understanding-scotch/ whisky-regions-tours/ (accessed on 1 February 2019).
- Available online: http://www.irishstatutebook.ie/eli/1980/act/33/section/1/enacted/en/html#sec1 (accessed on 1 February 2019).
- Charles, K. Who Invented Bourbon? Malt Advocate Mag. 2002, 11, 72–75. [Google Scholar]
- Kim, M. Determination of lead and cadmium in wines by graphite furnace atomic absorption spectrometry. Food Addit. Contam. 2004, 21, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Determination of 23 Trace Elements in Wines by ICP-AES. Available online: https://www.ncbi.nlm.nih.gov/pubmed/?term= Determination+of+23+trace+elements+in+wines+by+ICP-AES%2C (accessed on 1 February 2019).
- Płotka-Wasylka, J.; Rutkowska, M.; Cieślik, B.; Tyburcy, A.; Namieśnik, J. Determination of selected metals in fruit wines by spectroscopic techniques. J. Anal. Meth. Chem. 2017, 2017, 5283917. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Frankwski, M.; Simeonov, V.; Polkowska, Ż.; Namieśnik, J. Determination of Metals Content in Wine Samples by Inductively Coupled Plasma-Mass Spectrometry. Molecules 2018, 23, 2886. [Google Scholar] [CrossRef] [PubMed]
- Brilli, C.; Bronzi, B. Wine traceability: “Vigneto Italia” project. In Proceedings of the IMSC 2018, Florence, Italy, 26–31 August 2018. WP-75. [Google Scholar]
- Wiśniewska, P.; Dymerski, T.; Wardecki, W.; Namieśnik, J. Chemical composition analysis and authentication of whisky. J. Sci. Food Agric. 2015, 95, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Meier-Augenstein, W.; Kemp, H.F.; Hardie, S.M.L. Detection of counterfeit Scotch whisky by 2H and 18O stable isotope analysis. Food Chem. 2012, 133, 1070–1074. [Google Scholar] [CrossRef]
- Parker, I.G.; Kelly, S.D.; Sharman, M.; Dennis, M.J.; Howie, D. Investigations into the use of carbon isotope ratios (13C/12C) of Scotch whisky congeners to establish brand authenticity using gas chromatography – combustion–isotope ratio mass spectrometry. Food Chem. 1998, 63, 423–428. [Google Scholar] [CrossRef]
- Adam, T.; Duthie, E.; Feldmann, J. Investigations into the use of copper and other metals as indicators for the authenticity of Scotch whiskies. J. Inst. Brew. 2002, 108, 459–464. [Google Scholar] [CrossRef]
- Shand, C.A.; Wendler, R.; Dawson, L.; Yates, K.; Stephenson, H. Multivariate analysis of Scotch whisky by total reflection x-ray fluorescence and chemometric methods: A potential tool in the identification of counterfeits. Anal. Chim. Acta 2017, 976, 14–24. [Google Scholar] [CrossRef] [PubMed]
- No, K.M.; Kang, K.M.; Baek, S.L.; Choi, H.; Park, S.K.; Kim, D.S. Monitoring of heavy metal content in alcoholic beverages. JFSH 2010, 25, 24–29. [Google Scholar]
- Mineralizator Mikrofalowy UltraWAVE. Available online: http://www.spectro-lab.pl/produkt/mineralizator-ultrawave/ (accessed on 1 February 2019).
- Nowoczesne Metody Oznaczania Rtęci. Available online: http://docplayer.pl/2532327-Nowoczesne-metody-oznaczania-rteci.html (accessed on 1 February 2019).
Sample Availability: Samples of the compounds are available from the authors. |



| Sample Code | Type | Age | Origin |
|---|---|---|---|
| C | Blended (B) | 12 | Scotland-Speyside (SCT S) |
| BJ | Blended (B) | 3 | Scotland-Highlands (SCT H) |
| WP | Blended (B) | 3 | Scotland-Speyside (SCT S) |
| J | Blended (B) | 5 | Ireland (IRL) |
| BF | Blended (B) | 3 | Scotland-Highlands (SCT H) |
| B12 | Blended (B) | 12 | Scotland-Highlands (SCT H) |
| BB | Blended (B) | 3 | Scotland-Highlands (SCT H) |
| Gg | Blended (B) | 3 | Scotland-Speyside (SCT S) |
| Gb | Blended (B) | 3 | Scotland-Speyside (SCT S) |
| Gr | Blended (B) | 3 | Scotland-Speyside (SCT S) |
| JP | Blended (B) | 3 | Scotland-Highlands (SCT H) |
| JB | Blended (B) | 6 | United States of America (USA) |
| P | Blended (B) | 3 | Scotland-Speyside (SCT S) |
| JW. | Blended (B) | 3 | Scotland-Lowlands (SCT L) |
| S | Single malt (Sm) | 15 | Scotland-Speyside (SCT S) |
| Gf | Single malt (Sm) | 18 | Scotland-Speyside (SCT S) |
| Gl | Single malt (Sm) | 12 | Scotland-Speyside (SCT S) |
| Gm | Single malt (Sm) | 10 | Scotland-Speyside (SCT S) |
| Di | Blended (B) | 15 | Scotland-Lowlands (SCT L) |
| De | Single malt (Sm) | 12 | Scotland-Highlands (SCT H) |
| ICP–ToF–MS | |
|---|---|
| Radio frequency power generator [W] | 1350 |
| Torch | Quartz |
| Nebuliser | Concentric quartz |
| Carrier gas | Argon |
| Spray chamber | Cyclonic |
| Plasma gas flow rate [L·min−1] | 10 |
| Auxiliary gas flow rate [L·min−1] | 0.9 |
| Nebulization gas flow rate [L·min−1] | 0.76 |
| Number of replicates | 3 |
| Acquisition time [s] Sampler Cones Sampler and Skimmer Cones | 3 Ni Ni/Cu |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlaczyk, A.; Gajek, M.; Jozwik, K.; Szynkowska, M.I. Multielemental Analysis of Various Kinds of Whisky. Molecules 2019, 24, 1193. https://doi.org/10.3390/molecules24071193
Pawlaczyk A, Gajek M, Jozwik K, Szynkowska MI. Multielemental Analysis of Various Kinds of Whisky. Molecules. 2019; 24(7):1193. https://doi.org/10.3390/molecules24071193
Chicago/Turabian StylePawlaczyk, Aleksandra, Magdalena Gajek, Krzysztof Jozwik, and Malgorzata Iwona Szynkowska. 2019. "Multielemental Analysis of Various Kinds of Whisky" Molecules 24, no. 7: 1193. https://doi.org/10.3390/molecules24071193
APA StylePawlaczyk, A., Gajek, M., Jozwik, K., & Szynkowska, M. I. (2019). Multielemental Analysis of Various Kinds of Whisky. Molecules, 24(7), 1193. https://doi.org/10.3390/molecules24071193









