Abstract
Artemisinins are widely used to treat Plasmodium infections due to their high clinical efficacy; however, the antimalarial mechanism of artemisinin remains unresolved. Mutations in P. falciparum ATPase6 (PfATP6), a sarcoplasmic endoplasmic reticulum Ca2+-transporting ATPase, are associated with increased tolerance to artemisinin. We utilized Saccharomyces cerevisiae as a model to examine the involvement of Pmr1p, a functional homolog of PfATP6, on the toxicity of artemisinin. Our analysis demonstrated that cells lacking Pmr1p are less susceptible to growth inhibition from artemisinin and its derivatives. No association between sensitivity to artemisinin and altered trafficking of the drug efflux pump Pdr5p, calcium homeostasis, or protein glycosylation was found in pmr1∆ yeast. Basal ROS levels are elevated in pmr1∆ yeast and artemisinin exposure does not enhance ROS accumulation. This is in contrast to WT cells that exhibit a significant increase in ROS production following treatment with artemisinin. Yeast deleted for PMR1 are known to accumulate excess manganese ions that can function as ROS-scavenging molecules, but no correlation between manganese content and artemisinin resistance was observed. We propose that loss of function mutations in Pmr1p in yeast cells and PfATP6 in P. falciparum are protective against artemisinin toxicity due to reduced intracellular oxidative damage.
1. Introduction
Artemisinin, a natural product isolated from the leaves of the traditional Chinese medicinal plant Artemisia annua, has been proven to be a potent antimalarial compound [1]. Since its discovery, several derivatives of artemisinin, collectively referred to as artemisinins, have been produced including the water-soluble artesunate, the lipid-soluble artemether, and dihydroartemisinin [2]. Key structural features of artemisinins are a sesquiterpene lactone backbone and an endoperoxide bridge (Figure 1), the latter being essential for antimalarial activity [3]. Artemisinins have been widely used to treat both Plasmodium falciparum and Plasmodium vivax infections due to their high clinical efficacy (immediate onset and rapid parasite clearance) and minimal side effects [4,5,6,7]. Moreover, artemisinins remain highly effective against malaria parasites that show resistance to other classes of antimalarial medicine [8]. Combinations of artemisinin and other antimalarial drugs are utilized clinically to enhance effectiveness and minimize the development of artemisinin resistance in Plasmodium species [9,10,11]. Despite this strategy, artemisinin-resistant Plasmodium isolates have already emerged [12,13,14,15,16].
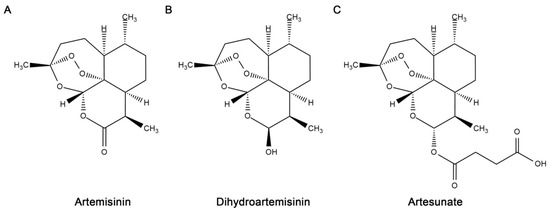
Figure 1.
Structures of artemisinin and major derivatives prepared with ChemDraw Ultra, version 12.
The mechanism of artemisinin action remains unresolved; however, several potential antimalarial effects have been proposed. One possible mechanism of artemisinin toxicity is by the generation of damaging reactive oxygen species (ROS) through iron-mediated endoperoxide cleavage [17,18]. However, the mechanism of artemisinin bioactivation and how ROS derived from artemisinins cause cellular damage is still a matter of debate [3,8]. Another potential mode of artemisinin action is through inhibition of P. falciparum ATPase6 (PfATP6), a sarcoplasmic endoplasmic reticulum Ca2+-transporting ATPase (SERCA). This mechanism was first proposed based on the structural similarity between artemisinins and thapsigargin, a known SERCA inhibitor [19]. PfATP6 is the only SERCA found in P. falciparum and limiting its activity could potentially cause growth inhibition by altering calcium homeostasis. When expressed in Xenopus laevis oocytes, PfATP6 activity can be inhibited by artemisinin [19]. In addition, artemisinins inhibit Ca2+-dependent ATPase activity in membrane fractions from Trypanosoma cruzi [20]. However, unlike the case for thapsigargin, artemisinin does not inhibit purified PfATP6 [21]. This finding suggests that artemisinins may not directly inhibit PfATP6 in vivo but instead exert their effect on PfATP6 through an indirect mechanism. Interestingly, a mutation in PfATP6 in parasite field isolates from French Guiana and Senegal was reported to associate with reduced susceptibility to artemisinin derivatives [13,22]. However, how PfATP6 inhibition participates in the antimalarial action of artemisinin remains unclear.
The yeast Saccharomyces cerevisiae is a powerful model system for identifying genes and biochemical pathways involved in the action of drugs [23,24,25] and artemisinins are active against this model organism [26]. The S. cerevisiae Ca2+-ATPases, vacuolar localized Pmc1p and Golgi localized Pmr1p [27], appear to be targets for artemisinin [28], providing an attractive cellular model to investigate the mechanism of PfATP6 inhibition by artemisinins. In this study, we examined the role of S. cerevisiae Pmr1p on the toxicity of artemisinin and its derivatives. Our analysis demonstrated that cells lacking Pmr1p are less susceptible to artemisinins. The investigation into the underlying cause of artemisinin resistance in pmr1Δ cells found no alteration in the localization or accumulation of the multi-drug transporter Pdr5p as well as no association between defects in calcium homeostasis or protein glycosylation and sensitivity to artemisinin. These findings suggest that the artemisinin resistance observed in pmr1∆ yeast is not due to defects in processes known to be associated with loss of Pmr1p function. Our analysis has revealed that pmr1∆ cells exhibit elevated basal levels of ROS, which are not further increased following artemisinin exposure. This is in sharp contrast to WT cells, where artemisinin treatment produced a significant increase in intracellular ROS. It is possible that Pmr1p may play a role in ROS generation or accumulation from artemisinin. We propose that the loss of function in Pmr1p or PfATP6 results in reduced artemisinin sensitivity due to insufficient ROS being generated to cause significant cellular damage.
2. Results
2.1. Yeast Cells Lacking Pmr1p Display Resistance to Artemisinin and Its Derivatives
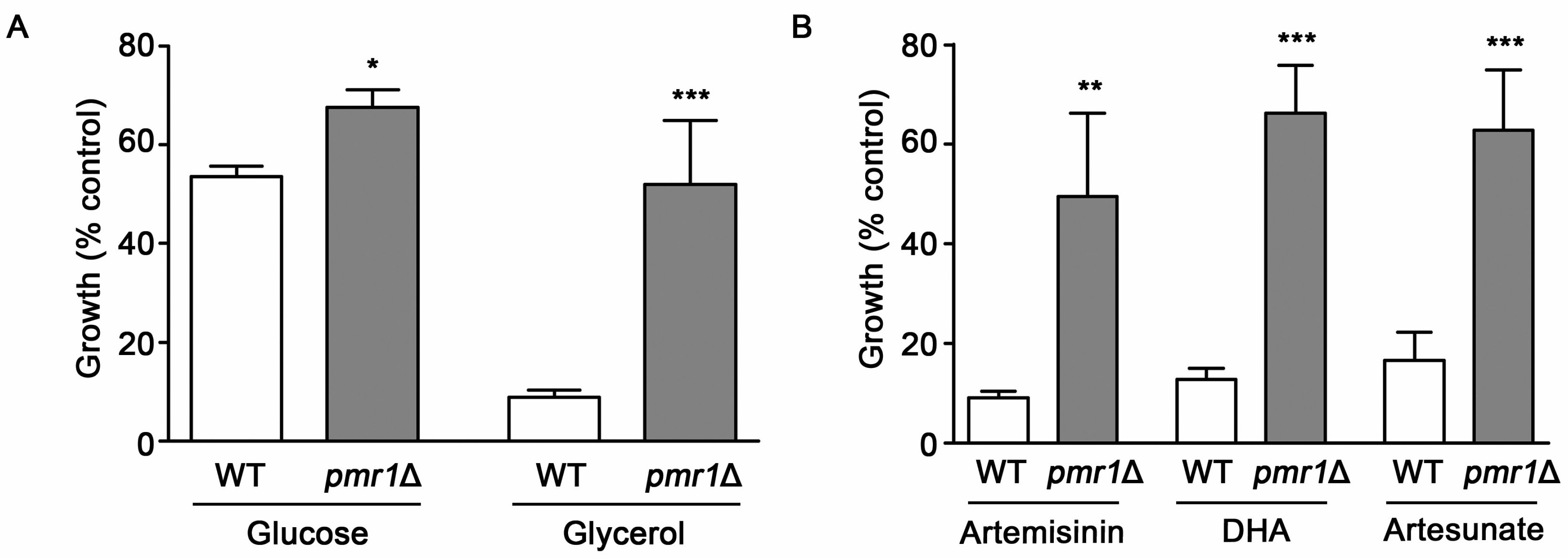
The P. falciparum sarcoendoplasmic reticulum Ca2+ ATPase6 (PfATP6) has been suggested as one of the key targets of the toxic actions of artemisinin [19]. Consistent with this proposal, mutations in PfATP6 have been shown to be associated with decreased sensitivity to artemisinin [13,29]. How PfATP6 is involved in modulating artemisinin toxicity has not been clearly elucidated. Analysis of yeast cells lacking the Ca2+/Mn2+ P-type ATPase Pmr1p, a homolog of Plasmodium PfATP6, has the potential to inform regarding possible mechanisms that limit artemisinin toxicity. In WT yeast, artemisinin toxicity is enhanced under growth conditions that require respiration [26]. However, it is not known if altered artemisinin sensitivity from PfATP6 mutations is linked to respiratory activity. Our analysis of artemisinin sensitivity for WT yeast in respiratory (glycerol) and fermentative (glucose) medium gave results consistent with previous reports, indicating increased artemisinin sensitivity under respiratory conditions [26]. The pmr1∆ strain exhibited reduced susceptibility to artemisinin, compared to WT yeast, in both respiratory and fermentative medium (Figure 2A). Due to the higher levels of artemisinin sensitivity seen in WT cells with respiratory medium, these conditions were used in subsequent experiments. Reduced toxicity to artemisinin derivatives dihydroartemisinin and artesunate were also observed in pmr1∆ yeast (Figure 2B), suggesting that artemisinin derivatives containing an endoperoxide bridge may require the presence of Pmr1p for their toxic actions.
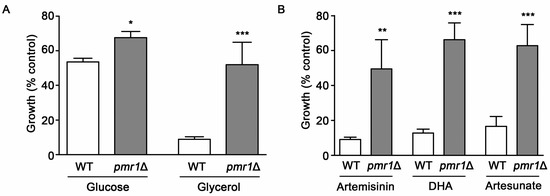
Figure 2.
Yeast cells lacking Pmr1p are less susceptible to artemisinin and its derivatives. (A) WT and pmr1∆ cells were cultured in synthetic complete media supplemented with either 2% glucose (SC) or 2% glycerol (SCG) as a carbon source with vehicle or 10 μM artemisinin and growth was monitored at 24 h after treatment. (B) WT and pmr1∆ cells were cultured in SCG medium as described in (A) and treated with vehicle, 10 μM artemisinin, 2 μM dihydroartimisinine (DHA), or 10 μM artesunate for 24 h. Percent growth compared to untreated controls for each strain is shown. Results are from three independent experiments; values are the mean + SD with differences between strains analyzed using two-tailed unpaired Student’s t-test; ***P < 0.001.
2.2. The Slower Growth Rate of pmr1∆ Cells Does Not Appear Sufficient to Reduce Artemisinin Sensitivity
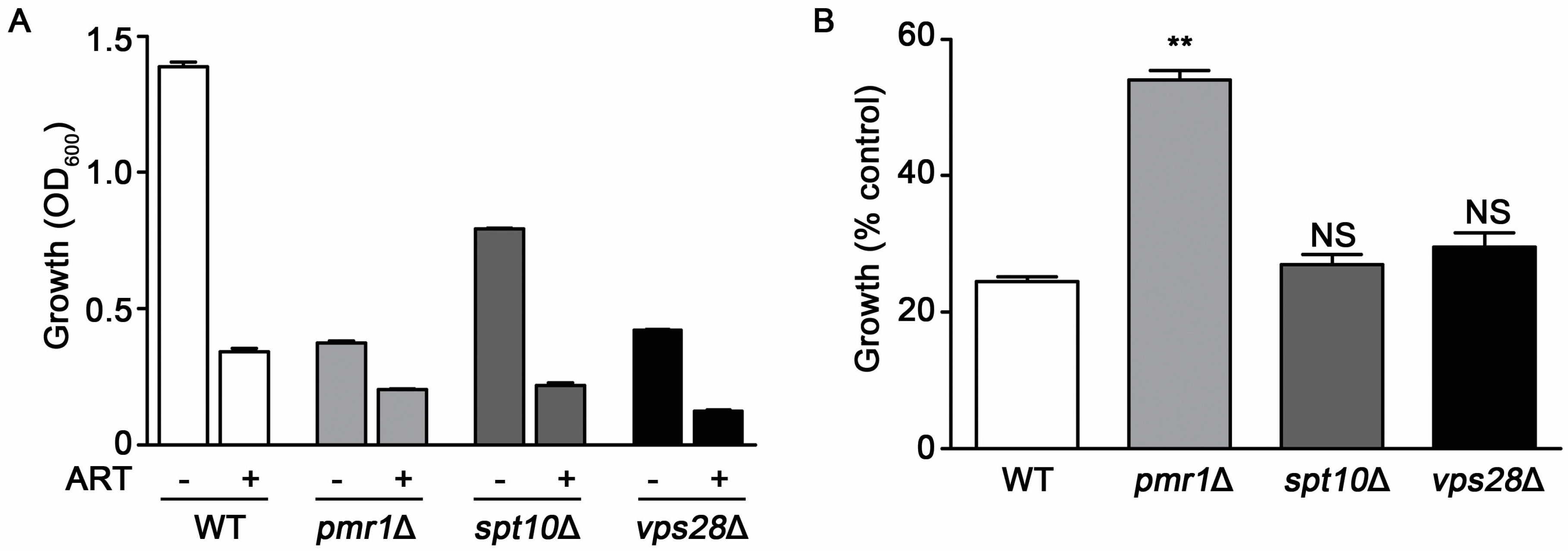
The growth rate of cells can alter susceptibility to several drugs. Slowly growing cells often survive exposure to drugs or other environmental stresses better than those replicating quickly [30]. Yeasts deleted for PMR1 exhibit substantial growth reduction compared to WT cells, which may contribute to their decreased sensitivity to artemisinin. To examine this possibility, we evaluated artemisinin sensitivity in two other deletion strains that exhibit reduced growth rates, spt10∆ and vma28∆. SPT10, encodes a histone H3 acetylase [31], while Vps28p is a component of the ESCRT-I complex [32]. Compared to the WT strain, pmr1∆ cells proliferate poorly with the vps28∆ and spt10∆ strains also showing reduced growth rates. Following treatment with artemisinin both spt10∆ and vps28∆ cells display a similar level of growth inhibition as the WT strain, indicating that these strains are sensitive to the effects of artemisinin toxicity (Figure 3A). To more easily observe the change in growth of strains when challenged with artemisinin, growth relative to vehicle-treated cells (% control growth) is also presented (Figure 3B).
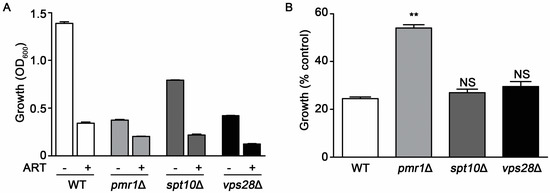
Figure 3.
Slow growth is not directly associated with artemisinin sensitivity. Growth profiles for WT, pmr1∆, spt10∆, and vps28∆ cells in SCG medium monitored at 24 h after treatment with vehicle or 10 μM artemisinin. (A) OD600 nm values and (B) percent growth compared to untreated controls for each strain. Results are from two independent experiments; values are the mean + SD with differences between WT and mutant strains analyzed using two-tailed unpaired Student’s t-test; **P < 0.01.
2.3. Localization and Accumulation of the Drug Efflux Transporter pdr5p Is Not Altered in pmr1∆ Cells
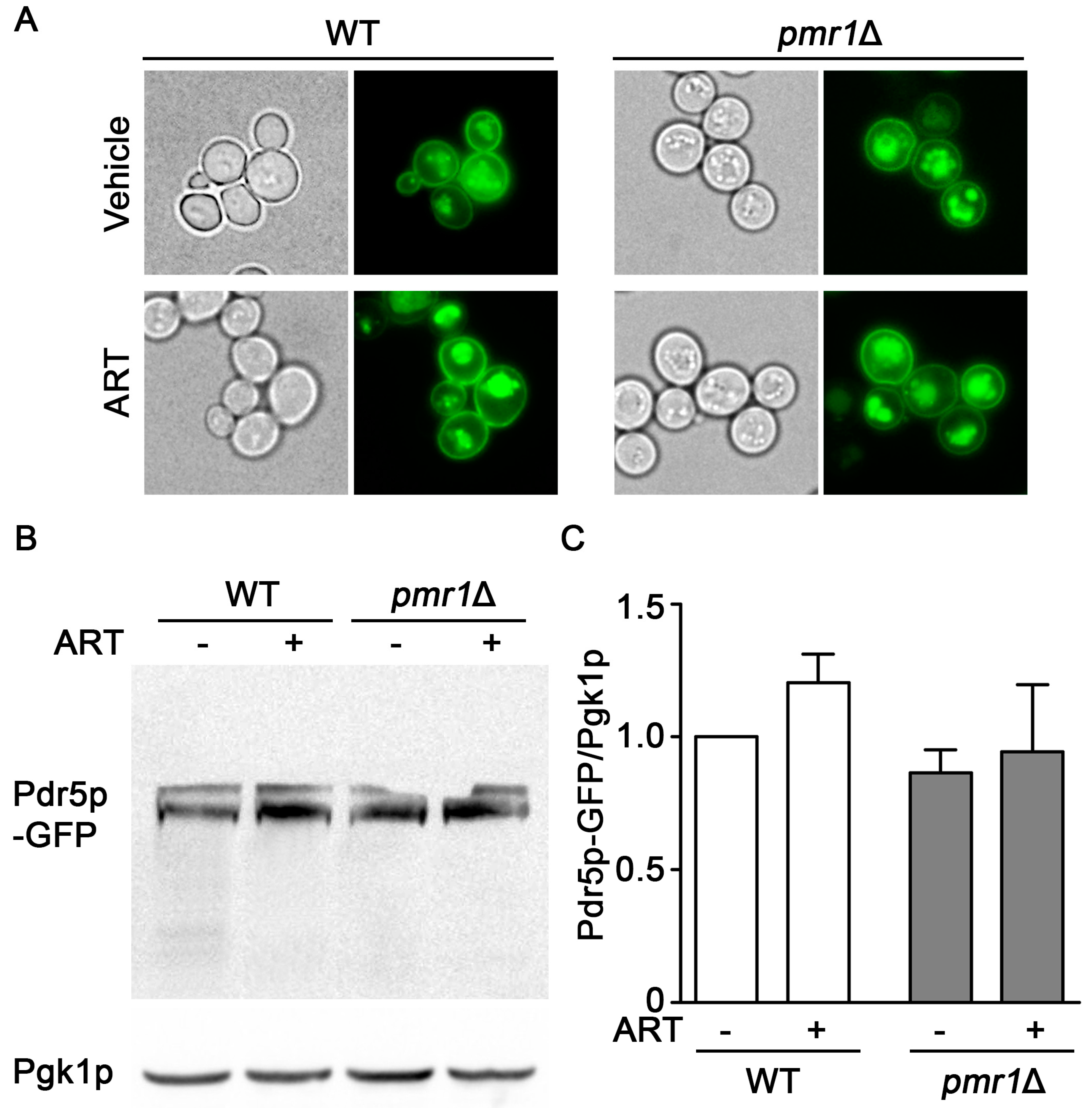
Increased expression of the plasma membrane multi-drug resistant transporter Pdr5p is associated with resistance to multiple drugs and functions by facilitating drug efflux from the cell [33]. Expression of Pdr5p following exposure to artemisinin appears critical for resistance to this drug; in addition, pdr5∆ cells are sensitized to artemisinin [34]. Pdr5p is transported to the plasma membrane through the secretory pathway and, when targeted for degradation, is delivered to the vacuole through transport vesicles [35]. PMR1 deletion mutants are defective in several functions in the secretory pathway, which can result in longer residency times for plasma membrane proteins [36,37]. This effect of PMR1 deletion on plasma membrane proteins raised the question of whether aberrant accumulation or localization of Pdr5p may contribute to increased artemisinin resistance. Examining Pdr5p localization using a GFP fusion indicated similar localization patterns in both WT and pmr1∆ yeast, regardless of artemisinin exposure (Figure 4A). The abundance of Pdr5p-GFP was also examined using immunoblots and no significant change in Pdr5p levels was observed between WT and pmr1∆ cells, regardless of artemisinin exposure (Figure 4B,C). It thus seems unlikely that artemisinin resistance in pmr1Δ cells is due to the increased Pdr5p-mediated cellular efflux of artemisinin.
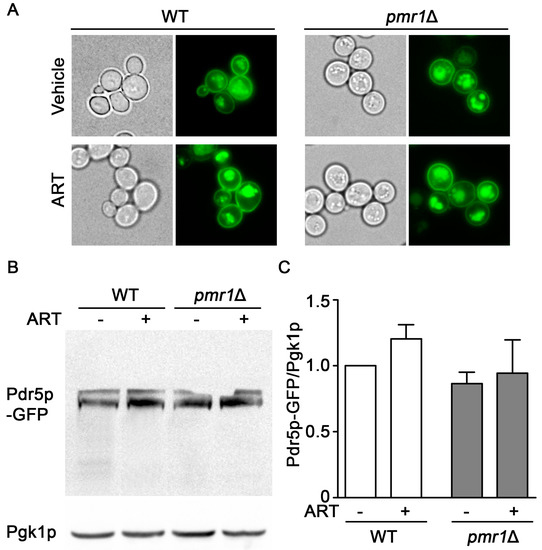
Figure 4.
Localization and accumulation of the multi-drug transporter Pdr5p are similar in pmr1Δ and WT cells. WT and pmr1Δ yeast transformed with a plasmid expressing PDR5-GFP fusion driven by the constitutive PGK1 promoter were grown overnight in SCG media with vehicle or 10 μM artemisinin. (A) Cellular localization of Pdr5p-GFP was observed by fluorescence microscopy at 60x magnification. (B) The abundance of Pdr5p-GFP was monitored with immunoblots using Pgk1p levels as the loading control. (C) Quantitation of Pdr5p-GFP results are normalized to the intensity of Pgk1p with levels normalized to WT = 1. Values are mean + SD (n = 2). No statistical difference between samples was observed using Student’s t-test.
2.4. Deletions of Genes Encoding Proteins Involved in Ca2+ homeostasis or Protein Glycosylation Does Not Promote Artemisinin Resistance
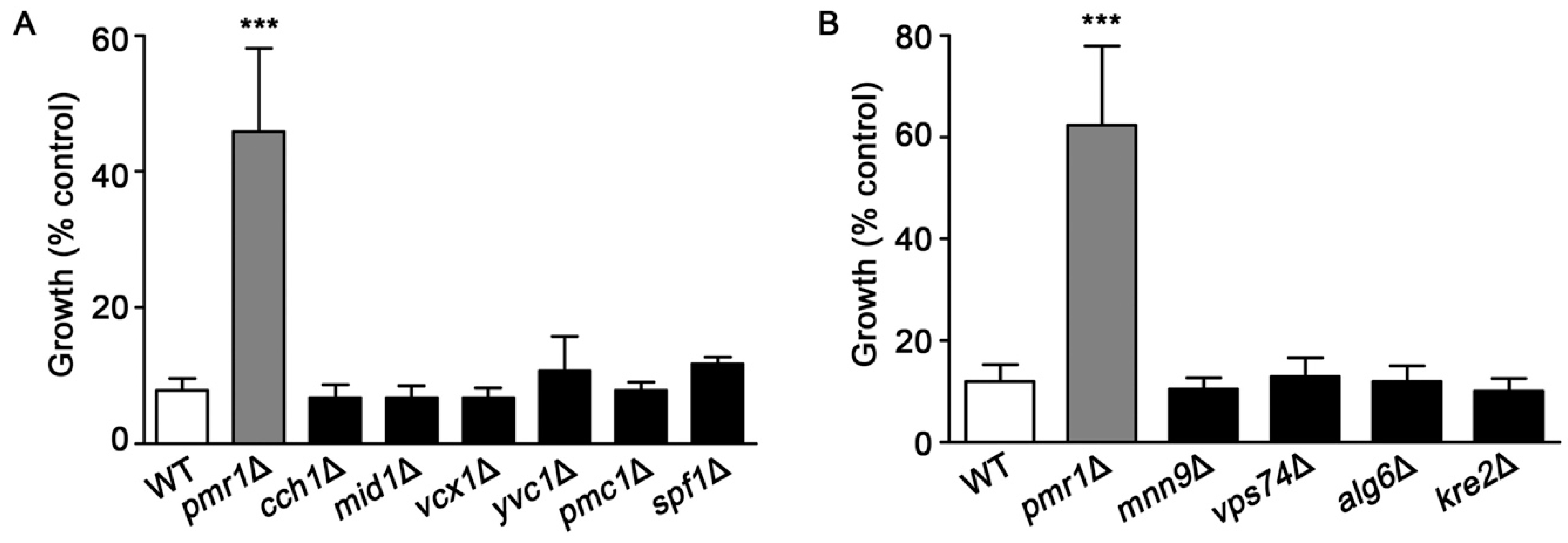
Pmr1p is a key transporter for uptake of Ca2+ into the Golgi, which is important for maintaining the Ca2+ homeostasis of yeast cells [38,39]. We investigated whether the loss of other proteins that participate in Ca2+ transport would alter sensitivity to artemisinin. Yeast strains examined were deleted for genes encoding the plasma membrane Cch1p/Mid1p channel complex [40,41]; vacuolar Ca2+ channels Vcx1p and Pmc1p, and Yvc1p [42,43,44,45]; and the ER-associated P-type ATPase Spf1p [46]. Among the yeast strains defective in Ca2+ homeostasis, only cells lacking Pmr1p showed resistance to artemisinin (Figure 5A).
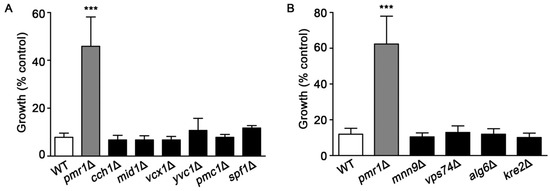
Figure 5.
Yeast mutants lacking proteins involved in calcium homeostasis or protein glycosylation are not resistant to artemisinin. WT, pmr1Δ, and strains deleted for genes encoding proteins functioning in (A) cellular calcium homeostasis; cch1Δ, mid1Δ, vcx1Δ, yvc1Δ, pmc1Δ, and cod1Δ or (B) protein glycosylation; mnn9Δ, vps74Δ, alg6Δ, and kre2Δ were cultured in SCG media with vehicle or 10 μM artemisinin. Growth was assessed at 24 h by measuring OD600. Results are from three independent experiments; values are the mean + SD. ***P < 0.001 were determined using one-way ANOVA.
Yeast pmr1∆ mutants also exhibit defects in protein glycosylation [36,37], which could be associated with enhanced resistance to artemisinin. Several yeast mutants, in addition to pmr1∆, have significant protein glycosylation defects, these include mnn9Δ, vps74Δ, alg6Δ, and kre2Δ [47,48,49,50,51]. As shown in Figure 5B, deletion strains with protein glycosylation defects did not exhibit the increased resistance to artemisinin seen in cells lacking Pmr1p. Together, these results suggest that altered calcium trafficking or defects in protein glycosylation are not sufficient to reduce toxicity from artemisinin.
2.5. Increased Intracellular Oxidation Is Not Observed in pmr1∆ Yeast Following Artemisinin Exposure
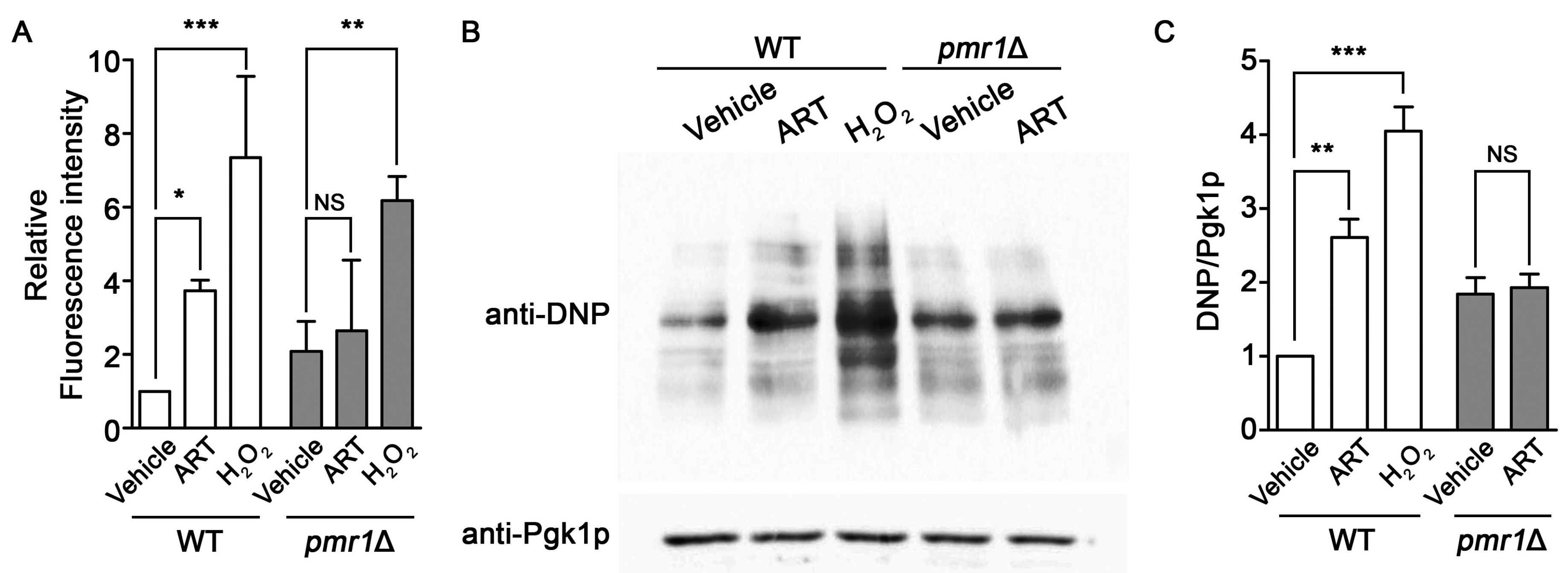
Artemisinin can promote the production of intracellular ROS and oxidative damage, resulting in cellular injury following drug exposure [26,52]. PMR1 deletions are capable of protecting against oxidative damage in yeast lacking Cu/Zn superoxide dismutase [53], suggesting the possibility of a similar effect of this mutant on artemisinin generated ROS. Intracellular ROS levels, determined using DCFH-DA, clearly show increased ROS accumulation in WT cells exposed to artemisinin or hydrogen peroxide. ROS production in pmr1∆ yeast was not elevated following artemisinin treatment; although, ROS levels in the untreated pmr1∆ strain was elevated compared to the WT control. Treatment with hydrogen peroxide was capable of increasing ROS levels in the pmr1∆ mutant to levels greater than that seen in untreated cells or following artemisinin exposure (Figure 6A). This indicates that the lack of ROS production in pmr1∆ yeast exposed to artemisinin is not due to saturation of the detection system. In addition, the ability of hydrogen peroxide to increase ROS levels suggests that deletion of PMR1 is not protective against ROS generation from all sources. Analysis of oxidative damage to proteins, through monitoring of protein carbonylation, revealed similar results as obtained with ROS determination. Protein carbonylation in WT cells was substantially increased following exposure to artemisinin or hydrogen peroxide. Consistent with ROS levels, similar quantities of carbonylated proteins were detected in untreated pmr1∆ yeast and cells exposed to artemisinin; protein carbonyl levels were elevated relative to untreated WT cells (Figure 6B,C). It appears that the production of damaging intracellular ROS following artemisinin exposure may require the activity of Pmr1p.
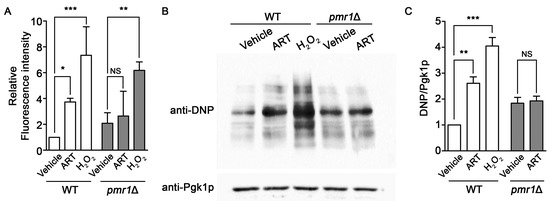
Figure 6.
Yeast pmr1∆ mutants exhibit increased basal intracellular ROS and protein oxidation, which is not elevated following artemisinin exposure. WT and pmr1∆ cells were cultured in SCG media with vehicle or 10 μM artemisinin for 24 h. (A) ROS levels were monitored using DCFH-DA. The fluorescence intensity of the samples was normalized to protein content with levels set to WT = 1. Results are from two independent experiments; values are the mean + SD. Differences between strains were analyzed using the two-tailed unpaired Student’s t-test; ***P < 0.001. (B) Protein carbonyls from the oxidation of protein side chains were monitored in whole cell lysates following derivatization with DNPH (2,4-dinitrophenylhydrazine). DNP-adducts were detected by immunoblotting with an anti-DNP antibody (upper panel) and the cytosolic protein Pgk1p (lower panel) was monitored as a loading control. (C) Quantitation of protein carbonylation results are normalized to the intensity of Pgk1p with levels normalized to WT = 1. Values are mean + SD (n = 2). * P > 0.05, ** P > 0.01, ***P < 0.001, determined using Student’s t-test.
2.6. Elevated Intracellular Mn Is Not Protective against Artemisinin Toxicity
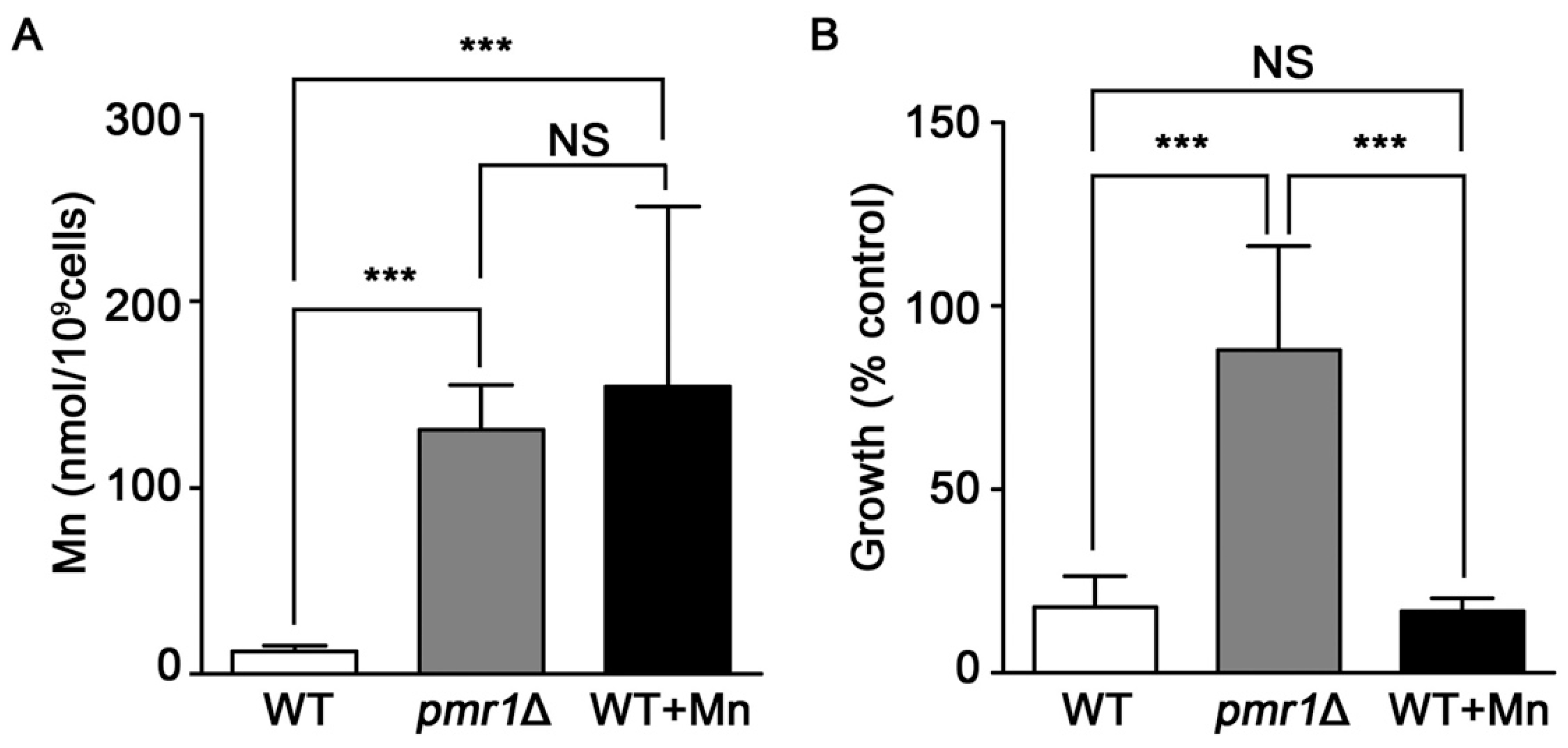
Yeast deleted for PMR1 exhibit high levels of intracellular Mn ions [53], which can provide protection against oxidative stress by functioning as ROS scavengers when complexed with phosphate or other small molecules [53,54,55,56]. It is possible that the elevated accumulation of Mn in pmr1Δ cells is protective against oxidative injuries caused by artemisinin, limiting the toxicity of the drug. We addressed this possibility by growing WT yeast in medium supplemented with sufficient Mn to produce similar intracellular Mn levels to that found in pmr1Δ cells (Figure 7A). However, WT yeast with elevated levels of intracellular Mn do not display an increase in artemisinin resistance (Figure 7B). Thus it appears that elevated intracellular Mn is not sufficient to protect against artemisinin toxicity, indicating that another mechanism is responsible for limiting artemisinin sensitivity in pmr1∆ cells.
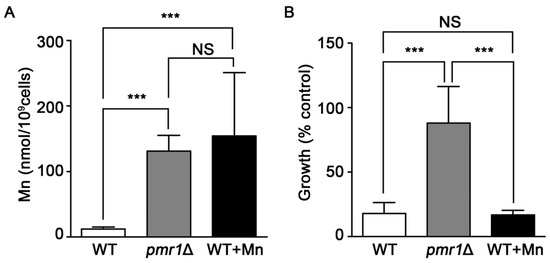
Figure 7.
Elevated levels of intracellular Mn do not appear to result in reduced artemisinin susceptibility. (A) WT, pmr1Δ, and WT treated with 12 mM Mn were grown in SCG media for 24 h and the intracellular Mn levels were measured with atomic absorption spectroscopy. Results are from duplicate measurements (B) WT, pmr1Δ, and WT pre-treated with 12 mM Mn for 24 h were cultured in SCG media with vehicle or 10 μM artemisinin and growth was measured by OD600 at 24 h. Percent of growth compared to vehicle is shown. Results are from three independent experiments; values are the mean + SD. ***P < 0.001 determined using one-way ANOVA.
3. Discussion
Artemisinins have been used for the treatment of malaria for many decades; however, the precise mechanism for its antimalarial action is still in question. One of the proposed targets of artemisinin is PfATP6, the sarcoendoplasmic reticulum Ca2+ ATPase from Plasmodium falciparum [19]. However, the limited genetic tools available for Plasmodium species makes the use of model systems a valuable alternative for the investigation into the causes of artemisinin toxicity. In this study, we utilized S. cerevisiae to examine the contribution of Pmr1p, a functional homolog to PfATP6, in artemisinin toxicity. Consistent with a previous report on the reduced susceptibility of yeast lacking both PfATP6 homologs Pmr1p and Pmc1p to artemisinin [28], we observed that yeast lacking Pmr1p are much less sensitive to artemisinin than WT cells. Additionally, our analysis demonstrated that the pmr1∆ strain was also less sensitive to artemisinin derivatives, dihydroartemisinin, and artesunate, which contain the endoperoxide bridge. This suggests that loss of Pmr1p is likely protective against artemisinin toxicity exerted through the endoperoxide structure essential for the antimalarial activity of artemisinins.
To better understand the molecular processes that lead to reduced artemisinin sensitivity in pmr1∆ cells, several possible protective mechanisms were examined. One of the major effects from loss of Pmr1p is impaired N- and O-linked glycosylation, resulting in protein sorting and processing defects in the secretory pathway [37]. Increased sorting and stabilization of Gap1p at the plasma membrane is observed in pmr1∆ cells [57] and accumulation of other transporters may be similarly enhanced. Expression of Pdr5p, a major ATP-binding cassette (ABC) drug efflux pump, contributes to artemisinin resistance by facilitating the export of this drug [34]. Plasma membrane localization of Pdr5p is required to facilitate artemisinin tolerance. Yeast with impaired ER to Golgi trafficking exhibit mislocalization and decreased abundance of Pdr5p, leading to artemisinin sensitivity [58]. However, the localization of the Pdr5p transporter was not altered either by deletion of PMR1 or exposure to artemisinin. Enhanced plasma membrane localization of this drug efflux transporter does not appear to be a major factor in artemisinin resistance in pmr1∆ yeast. Consistent with this finding, deletion strains with disturbances in glycosylation or protein sorting (mnn9Δ, vps74Δ, alg6Δ, and kre2Δ) do not show enhanced artemisinin resistance compared to WT yeast. Over-expression of PDR5 can also promote artemisinin resistance [34,58]; however, the accumulation of Pdr5p-GFP was not significantly different between WT and pmr1∆ cells treated with either vehicle or artemisinin. Together, these results indicate that defects in Pdr5p sorting, processing, or accumulation are not the major underlying cause promoting artemisinin resistance in cells deficient for Pmr1p.
Calcium is involved in many cellular processes and sustained increases in intracellular calcium have been associated with drug resistance [59]. In the absence of Pmr1p, cytosolic Ca2+ concentrations are elevated due to decreased transport of this cation into the Golgi [60]. The contribution of Pmr1p to calcium homeostasis could be a factor in the decreased artemisinin sensitivity seen in pmr1∆ yeast. However, artemisinin toxicity in yeast with deletion of genes encoding calcium transporters localized to the plasma membrane (cch1Δ and mid1Δ) [40,41], vacuole (vcx1Δ, yvc1Δ, and pmc1Δ) [43,45,61], or ER (spf1Δ) [46] did not produce enhanced resistance to artemisinin. It appears that more than altered calcium homeostasis is required to facilitate resistance to artemisinin.
Production of ROS following artemisinin exposure has been well documented and cleavage of the endoperoxide bridge appears to be a key event that facilitates cellular oxidative damage [62,63,64]. Evaluation of ROS production and oxidative damage to proteins revealed a substantial increase in both of these parameters in WT yeast following artemisinin exposure. In contrast, pmr1∆ cells exhibit an elevation in ROS production and protein oxidative damage compared to untreated WT cells, but no apparent increase when challenged with artemisinin. Although an upward trend for ROS levels in pmr1∆ yeast exposed to artemisinin was seen, this difference was not statistically significant. Even though pmr1∆ yeast do not exhibit a statistically significant increase in ROS following artemisinin exposure, these cells are experiencing elevated basal levels of ROS. The cause of the elevated basal ROS levels in pmr1∆ cells is not clear but may be related to impaired calcium trafficking [65].
Yeast deleted for PMR1 can bypass the oxygen sensitivity of cells lacking Cu/Zn superoxide dismutase (sod1∆), an important enzyme in the detoxification of oxygen radicals [53]. The ability of PMR1 deletion to protect sod1∆ cells from oxygen toxicity is linked to the over-accumulation of intracellular manganese ions. Supplementation of WT yeast with manganese can also provide enhanced protection against oxidative insults through the formation of Mn-dependent ROS scavenging complexes [56]. However, providing WT yeast with manganese did not protect against artemisinin toxicity, suggesting that artemisinin resistance in cells lacking Pmr1p does not simply arise as a result of an elevated intracellular concentration of manganese that may act as antioxidant molecules. Examination of a PMR1 knockdown in Caenorhabditis elegans has also suggested a role for Mn-based antioxidant complexes in protecting against oxidative stress. In addition, it was proposed that cytosolic Mn may alter transcriptional activity, increasing expression of antioxidant enzymes, such as mitochondrial Mn-superoxide dismutase [66]. Currently, it is not known how the transcription pattern of yeast is altered by the deletion of PMR1. Although, the activity of yeast Mn-Sod2p in not elevated in pmr1∆ cells [67]. It is possible that the protection against artemisinin toxicity provided by deletion of PMR1 in yeast is mediated through altered expression or activity of antioxidant enzymes.
The elevated ROS accumulation in pmr1∆ cells may be functioning to promote an adaptive response that is protective against the effects of artemisinin exposure. Pretreatment with sub-lethal stress can in many cases lead to greater resistance to the lethal effects of subsequent treatment of higher concentrations of pro-oxidant molecules [68,69,70]. Alternatively, it is possible that a direct interaction between artemisinin and Pmr1p may promote ROS production. While the cause of elevated ROS levels in the pmr1∆ strain is not clear, Pmr1p activity is required to prevent higher than normal basal levels of ROS. Based on this observation, inhibition of Pmr1p by artemisinin would lead to enhanced oxidative stress similar to that seen in pmr1∆ cells. Deletion of PMR1 may prevent additional ROS formation, above the already elevated basal levels, by removing a target of artemisinin action. An indirect mechanism in which Pmr1p facilitates ROS generation is also possible, although how Pmr1p could modulate ROS production from artemisinin remains unknown. Overall, our results herein suggest a possible role for Pmr1p in the generation of damaging ROS from artemisinin. If this proposed role for Pmr1p is proven to be the case, this may explain the reduced artemisinin of P. falciparum containing mutations or polymorphisms in PfTAP6.
4. Materials and Methods
4.1. Yeast Strains, Plasmid, and Culture Conditions
The strains used in this study are isogenic to the haploid strain BY4742 (Mat α, leu2∆0, lys2∆0, ura3∆0, his3∆1). Single gene deletion strains, pmr1Δ, cch1Δ, mid1Δ, vcx1Δ, yvc1Δ, pmc1Δ, spf1Δ, mnn9Δ, vps74Δ, alg6Δ, kre2Δ, spt10∆, and vps28∆ were purchased from Open Biosystems, Inc. (Layafette, CO, USA). Plasmids pWC018 expressing PDR5-GFP driven by the PGK1 promoter has been described previously [58]. Cells were maintained at 30 °C on a synthetic complete medium supplemented with either 2% glucose (SC) or 2% glycerol (SCG). Yeast transformations were performed using the lithium acetate procedure [71]. Transformants were selected using an SC medium lacking uracil. Artemisinin and derivatives (AdooQ Biosciences, Irvine, CA, USA) were dissolved in 100% ethanol.
4.2. Fluorescence Microscopy
The localization of Pdr5p was examined by employing a plasmid overexpressing GFP-tagged Pdr5p (PWC018). Cells containing GFP-Pdr5p were cultured overnight with or without artemisinin treatment at 30 °C to achieve an OD600 value of 1 in the SCG liquid medium lacking uracil. The intracellular localization of GFP-tagged Pdr5p was visualized in live cells and imaged as previously described [72]. Brightfield images were also captured to visualize cell area. Fluorescence was viewed using an Olympus BX53 fluorescent microscope at a magnification of 60× (Olympus Bioimaging Center, Mahidol University).
4.3. Measurement of Intracellular ROS Levels
2,7-Dichlorofluorescein diacetate (DCFH-DA) (Sigma, St. Louis, MO, USA) was used to measure intracellular ROS levels in response to artemisinin treatment. Yeast were treated with vehicle, artemisinin, or H2O2 for 24 h, followed by incubation with 10 μM DCFH-DA for one hour. Cell lysates, prepared in PBS buffer with glass bead extraction, were utilized for the measurement of fluorescence intensity as this technique has been shown to give higher sensitivity compared to using intact yeast [73,74]. The fluorescence signal was measured with an excitation at 490 nm and emission at 535 nm using a Spark 10M multimode microplate reader. Fluorescence intensity was normalized to the protein concentration of each sample. Results are from three independent experiments.
4.4. Immunoblots and Protein Carbonylation Analysis
Cultures for determination of Pdr5p-GFP abundance were grown in SCG medium. Extracts were generated using NaOH lysis with TCA precipitation as previously described [35]. Immunoblots were probed with anti-GFP (Santa Cruz Biotechnology, Dallas, TX, USA) or anti-Pgk1 (Abcam, Cambridge, MA, USA) antibody at a dilution of 1:5000. Carbonylated proteins were detected following derivatization with 2,4-dinitrophenylhydrazine (DNPH) as previously described [75]. Lysates were prepared from cells grown in SCG medium treated with vehicle, 10 μM artemisinin, or 5 mM H2O2 for 24 h. Twenty-five micrograms of protein from each sample were reacted with DNPH for 15 min at 25 °C. DNP-derivatized proteins were detected using immunoblots as described previously using an anti-DNP antibody (Merck Millipore, Burlington, MA, USA) at a dilution of 1:5000 [76]. Visualization of immunoblots utilized an HRP-conjugated secondary antibody and ECL detection (Merck Ltd.) with a G:Box Chemi XL1.4 chemiluminescence imaging system (Syngene, Frederick, MD, USA). Quantitation of protein intensity utilized ImageJ 1.45S software (National Institute of Health, Bethesda, MD, USA) [77].
4.5. Measurement of Intracellular Manganese
WT yeast, WT supplemented with 12 mM manganese (Mn), and pmr1Δ cells were cultured in SCG media for 24 h. Cells were then collected and washed with 1ml Tris HCl with EDTA pH 6.5 and Type 1 water. Mn levels were measured using graphite furnace atomic absorption spectroscopy (PinAAcle 900T; PerkinElmer, Waltham, MA, USA) and reported as nmol Mn/109 cells.
4.6. Statistical Analysis
Experimental data are reported as the mean + the standard deviation (SD). Significant differences between or among groups are indicated with, **P < 0.01 and ***P < 0.001. Data were analyzed by one-way ANOVA with post hoc Tukey test or Student’s t-test as appropriate.
5. Conclusions
Overall, our analysis has revealed an interesting role for S. cerevisiae Pmr1p, a functional homolog of PfATP6, in modulating toxicity from artemisinins. Deletion of PMR1 appears to prevent the induction of oxidative stress from artemisinin exposure through a mechanism unrelated to enhanced accumulation of manganese or altered calcium homeostasis. Further analysis into Pmr1p/PfATP6-mediated artemisinin toxicity may allow for better understanding into the development of drug resistance in Plasmodium species.
Author Contributions
Conceptualization, A.N.J. and T.P.; Methodology, A.N.J., T.P.; Formal Analysis, A.N.J., O.P.; Investigation, A.N.J., T.P., O.P., S.S.; Writing—Original Draft Preparation, A.N.J., T.P.; Writing—Review & Editing, A.N.J., L.T.J.; Visualization, O.P., S.S.; Supervision, A.N.J.; Project Administration, A.N.J.; Funding Acquisition, A.N.J.
Funding
This research was funded by the Thailand Research Fund (TRG5880158 (ANJ) and IRG5980008 (LTJ)), the Faculty of Science, Mahidol University (ANJ), and the Central Instrument Facility, Faculty of Science, Mahidol University (ANJ).
Acknowledgments
We thank the Olympus Bioimaging Center, Mahidol University for providing equipment for imaging studies.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Klayman, D.L. Qinghaosu (artemisinin): An antimalarial drug from China. Science 1985, 228, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, H.; Olliaro, P. Artemisinin derivatives for treating uncomplicated malaria. Cochrane Database Syst. Rev. 1999. [Google Scholar] [CrossRef]
- Cui, L.; Su, X.Z. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev. Anti Infect. Ther. 2009, 7, 999–1013. [Google Scholar] [CrossRef]
- White, N.J. Artemisinin: Current status. Trans. R. Soc. Trop. Med. Hyg. 1994, 88 (Suppl. 1), S3–S4. [Google Scholar] [CrossRef]
- Hien, T.T.; White, N.J. Qinghaosu. Lancet 1993, 341, 603–608. [Google Scholar] [CrossRef]
- De Vries, P.J.; Dien, T.K. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 1996, 52, 818–836. [Google Scholar] [CrossRef]
- Li, G.Q.; Guo, X.B.; Fu, L.C.; Jian, H.X.; Wang, X.H. Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans. R. Soc. Trop. Med. Hyg. 1994, 88 (Suppl. 1), S5–S6. [Google Scholar] [CrossRef]
- Krishna, S.; Uhlemann, A.C.; Haynes, R.K. Artemisinins: Mechanisms of action and potential for resistance. Drug Resist. Updat. 2004, 7, 233–244. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Olliaro, P.L. Strategies for the prevention of antimalarial drug resistance: Rationale for combination chemotherapy for malaria. Parasitol. Today 1996, 12, 399–401. [Google Scholar] [CrossRef]
- Nosten, F.; White, N.J. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 2007, 77 (Suppl. 6), 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.G.; Krishna, S. Antimalarial combinations. Lancet 2004, 364, 285–294. [Google Scholar] [CrossRef]
- Yang, H.; Liu, D.; Yang, Y.; Fan, B.; Yang, P.; Li, X.; Li, C.; Dong, Y.; Yang, C. Changes in susceptibility of Plasmodium falciparum to artesunate in vitro in Yunnan Province, China. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 226–228. [Google Scholar] [PubMed]
- Jambou, R.; Legrand, E.; Niang, M.; Khim, N.; Lim, P.; Volney, B.; Ekala, M.T.; Bouchier, C.; Esterre, P.; Fandeur, T.; et al. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 2005, 366, 1960–1963. [Google Scholar] [CrossRef]
- Huong, N.M.; Hewitt, S.; Davis, T.M.; Dao, L.D.; Toan, T.Q.; Kim, T.B.; Hanh, N.T.; Phuong, V.N.; Nhan, D.H.; Cong, L.D. Resistance of Plasmodium falciparum to antimalarial drugs in a highly endemic area of southern Viet Nam: A study in vivo and in vitro. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 325–329. [Google Scholar] [CrossRef]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M.; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef] [PubMed]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- O’Neill, P.M.; Posner, G.H. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 2004, 47, 2945–2964. [Google Scholar] [CrossRef]
- Mercer, A.E. The role of bioactivation in the pharmacology and toxicology of the artemisinin-based antimalarials. Curr. Opin. Drug Discov. Dev. 2009, 12, 125–132. [Google Scholar]
- Eckstein-Ludwig, U.; Webb, R.J.; Van Goethem, I.D.; East, J.M.; Lee, A.G.; Kimura, M.; O’Neill, P.M.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar] [CrossRef]
- Mishina, Y.V.; Krishna, S.; Haynes, R.K.; Meade, J.C. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrob. Agents Chemother. 2007, 51, 1852–1854. [Google Scholar] [CrossRef]
- Cardi, D.; Pozza, A.; Arnou, B.; Marchal, E.; Clausen, J.D.; Andersen, J.P.; Krishna, S.; Moller, J.V.; le Maire, M.; Jaxel, C. Purified E255L mutant SERCA1a and purified PfATP6 are sensitive to SERCA-type inhibitors but insensitive to artemisinins. J. Biol. Chem. 2010, 285, 26406–26416. [Google Scholar] [CrossRef]
- Jambou, R.; Martinelli, A.; Pinto, J.; Gribaldo, S.; Legrand, E.; Niang, M.; Kim, N.; Pharath, L.; Volnay, B.; Ekala, M.T.; et al. Geographic Structuring of the Plasmodium falciparum Sarco(endo)plasmic Reticulum Ca2+ ATPase (PfSERCA) Gene Diversity. PLoS ONE 2010, 5, e9424. [Google Scholar] [CrossRef]
- Menacho-Marquez, M.; Murguia, J.R. Yeast on drugs: Saccharomyces cerevisiae as a tool for anticancer drug research. Clin. Transl. Oncol. 2007, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.M.; Kemmer, D.; Anderson, H.J.; Roberge, M. Yeast as a tool to uncover the cellular targets of drugs. Biotechnol. J. 2006, 1, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.R. Yeast and drug discovery. Funct. Integr. Genom. 2002, 2, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mo, W.; Shen, D.; Sun, L.; Wang, J.; Lu, S.; Gitschier, J.M.; Zhou, B. Yeast model uncovers dual roles of mitochondria in action of artemisinin. PLoS Genet. 2005, 1, e36. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.W.; Fink, G.R. Ca2+ transport in Saccharomyces cerevisiae. J. Exp. Biol. 1994, 196, 157. [Google Scholar]
- Moore, C.M.; Hoey, E.M.; Trudgett, A.; Timson, D.J. Artemisinins act through at least two targets in a yeast model. Fems Yeast Res. 2011, 11, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, S.; Staines, H.M.; Pittman, J.K.; Slavic, K.; Doerig, C.; Halbert, J.; Tewari, R.; Shah, F.; Avery, M.A.; Haynes, R.K.; et al. Expression in yeast links field polymorphisms in PfATP6 to in vitro artemisinin resistance and identifies new inhibitor classes. J. Infect. Dis. 2013, 208, 468–478. [Google Scholar] [CrossRef]
- Brown, M.R.W.; Williams, P. Influence of substrate limitation and growth phase on sensitivity to antimicrobial agents. J. Antimicrob. Chemother. 1985, 15 (Suppl. A), 7–14. [Google Scholar] [CrossRef]
- Natsoulis, G.; Dollard, C.; Winston, F.; Boeke, J.D. The products of the SPT10 and SPT21 genes of Saccharomyces cerevisiae increase the amplitude of transcriptional regulation at a large number of unlinked loci. New Biol. 1991, 3, 1249–1259. [Google Scholar] [PubMed]
- Rothman, J.H.; Howald, I.; Stevens, T.H. Characterization of genes required for protein sorting and vacuolar function in the yeast Saccharomyces cerevisiae. Embo J. 1989, 8, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowski, M.; van der Rest, M.; Cybularz-Kolaczkowska, A.; Soumillion, J.P.; Konings, W.N.; Goffeau, A. Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5p. J. Biol. Chem. 1996, 271, 31543–31548. [Google Scholar] [CrossRef]
- Alenquer, M.; Tenreiro, S.; Sa-Correia, I. Adaptive response to the antimalarial drug artesunate in yeast involves Pdr1p/Pdr3p-mediated transcriptional activation of the resistance determinants TPO1 and PDR5. Fems Yeast Res. 2006, 6, 1130–1139. [Google Scholar] [CrossRef]
- Egner, R.; Mahe, Y.; Pandjaitan, R.; Kuchler, K. Endocytosis and vacuolar degradation of the plasma membrane-localized Pdr5 ATP-binding cassette multidrug transporter in Saccharomyces cerevisiae. Mol. Cell Biol. 1995, 15, 5879–5887. [Google Scholar] [CrossRef]
- Rudolph, H.K.; Antebi, A.; Fink, G.R.; Buckley, C.M.; Dorman, T.E.; LeVitre, J.; Davidow, L.S.; Mao, J.I.; Moir, D.T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 1989, 58, 133–145. [Google Scholar] [CrossRef]
- Dürr, G.; Strayle, J.; Plemper, R.; Elbs, S.; Klee, S.K.; Catty, P.; Wolf, D.H.; Rudolph, H.K. The medial-Golgi Ion Pump Pmr1 Supplies the Yeast Secretory Pathway with Ca(2+) and Mn(2+) Required for Glycosylation, Sorting, and Endoplasmic Reticulum-Associated Protein Degradation. Mol. Biol. Cell 1998, 9, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Antebi, A.; Fink, G.R. The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell 1992, 3, 633–654. [Google Scholar] [CrossRef]
- Sorin, A.; Rosas, G.; Rao, R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 1997, 272, 9895–9901. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Nakamura, H.; Ono, T.; Okumura, M.S.; Anraku, Y. MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol. Cell Biol. 1994, 14, 8259–8271. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Schnell, N.; Chattaway, J.; Davies, P.; Dixon, G.; Sanders, D. The Saccharomyces cerevisiae CCH1 gene is involved in calcium influx and mating. Febs Lett. 1997, 419, 259–262. [Google Scholar] [CrossRef]
- Pozos, T.C.; Sekler, I.; Cyert, M.S. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol. Cell. Biol. 1996, 16, 3730–3741. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 1996, 16, 2226–2237. [Google Scholar] [CrossRef] [PubMed]
- Denis, V.; Cyert, M.S. Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 2002, 156, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.P.; Zhou, X.L.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef] [PubMed]
- Cronin, S.R.; Rao, R.; Hampton, R.Y. Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J. Cell Biol. 2002, 157, 1017–1028. [Google Scholar] [CrossRef]
- Häusler, A.; Ballou, L.; Ballou, C.E.; Robbins, P.W. Yeast glycoprotein biosynthesis: MNT1 encodes an alpha-1,2-mannosyltransferase involved in O-glycosylation. Proc. Natl. Acad. Sci. USA 1992, 89, 6846–6850. [Google Scholar] [CrossRef] [PubMed]
- Corbacho, I.; Olivero, I.; Hernández, L.M. Identification of the MNN3 gene of Saccharomyces cerevisiae. Glycobiology 2010, 20, 1336–1340. [Google Scholar] [CrossRef]
- Huffaker, T.C.; Robbins, P.W. Yeast mutants deficient in protein glycosylation. Proc. Natl. Acad. Sci. USA 1983, 80, 7466–7470. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.; Boone, C.; Goebl, M.; Puccia, R.; Sdicu, A.M.; Bussey, H. Yeast Kre2 Defines a New Gene Family Encoding Probable Secretory Proteins, and Is Required for the Correct N-Glycosylation of Proteins. Genetics 1992, 130, 273–283. [Google Scholar] [PubMed]
- Reiss, G.; Heesen, S. t.; Zimmerman, J.; Robbins, P.; Aebi, M. Isolation of the ALG6 locus of Saccharomyces cerevisiae required for glucosylation in the N-linked glycosylation pathway. Glycobiology 1996, 6, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.K.; Chan, W.C.; Lung, C.M.; Uhlemann, A.C.; Eckstein, U.; Taramelli, D.; Parapini, S.; Monti, D.; Krishna, S. The Fe2+-mediated decomposition, PfATP6 binding, and antimalarial activities of artemisone and other artemisinins: The unlikelihood of C-centered radicals as bioactive intermediates. Chem. Med. Chem. 2007, 2, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Lapinskas, P.J.; Cunningham, K.W.; Liu, X.F.; Fink, G.R.; Culotta, V.C. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 1995, 15, 1382–1388. [Google Scholar] [CrossRef]
- Archibald, F.S.; Fridovich, I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J. Bacteriol. 1981, 145, 442–451. [Google Scholar]
- Archibald, F.S.; Fridovich, I. The scavenging of superoxide radical by manganous complexes: In vitro. Arch. Biochem. Biophys. 1982, 214, 452–463. [Google Scholar] [CrossRef]
- Reddi, A.R.; Jensen, L.T.; Naranuntarat, A.; Rosenfeld, L.; Leung, E.; Shah, R.; Culotta, V.C. The overlapping roles of manganese and Cu/Zn SOD in oxidative stress protection. Free Radic. Biol. Med. 2009, 46, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Devasahayam, G.; Ritz, D.; Helliwell, S.B.; Burke, D.J.; Sturgill, T.W. Pmr1, a Golgi Ca2+/Mn2+-ATPase, is a regulator of the target of rapamycin (TOR) signaling pathway in yeast. Proc. Natl. Acad. Sci. USA 2006, 103, 17840–17845. [Google Scholar] [CrossRef]
- Jensen, A.N.; Chindaudomsate, W.; Thitiananpakorn, K.; Mongkolsuk, S.; Jensen, L.T. Improper protein trafficking contributes to artemisinin sensitivity in cells lacking the KDAC Rpd3p. Febs Lett. 2014, 588, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Liang, C.; Chen, E.; Chen, W.; Liang, F.; Zhi, X.; Wei, T.; Xue, F.; Li, G.; Yang, Q.; et al. Regulation of Multi-drug Resistance in hepatocellular carcinoma cells is TRPC6/Calcium Dependent. Sci. Rep. 2016, 6, 23269. [Google Scholar] [CrossRef] [PubMed]
- Locke, E.G.; Bonilla, M.; Liang, L.; Takita, Y.; Cunningham, K.W. A homolog of voltage-gated Ca(2+) channels stimulated by depletion of secretory Ca(2+) in yeast. Mol. Cell Biol. 2000, 20, 6686–6694. [Google Scholar] [CrossRef]
- Cunningham, K.W.; Fink, G.R. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J. Cell Biol. 1994, 124, 351–363. [Google Scholar] [CrossRef]
- Meshnick, S.R.; Yang, Y.Z.; Lima, V.; Kuypers, F.; Kamchonwongpaisan, S.; Yuthavong, Y. Iron-dependent free radical generation from the antimalarial agent artemisinin (qinghaosu). Antimicrob. Agents Chemother. 1993, 37, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Asawamahasakda, W.; Ittarat, I.; Pu, Y.M.; Ziffer, H.; Meshnick, S.R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother. 1994, 38, 1854–1858. [Google Scholar] [CrossRef]
- Berman, P.A.; Adams, P.A. Artemisinin enhances heme-catalysed oxidation of lipid membranes. Free Radic. Biol. Med. 1997, 22, 1283–1288. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kwon, H.J. Identification and validation of bioactive small molecule target through phenotypic screening. Bioorg. Med. Chem. 2012, 20, 1922–1928. [Google Scholar] [CrossRef]
- Luk, E.E.-C.; Culotta, V.C. Manganese Superoxide Dismutase in Saccharomyces cerevisiae Acquires Its Metal Co-factor through a Pathway Involving the Nramp Metal Transporter, Smf2p. J. Biol. Chem. 2001, 276, 47556–47562. [Google Scholar] [CrossRef]
- Jamieson, D.J. Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J. Bacteriol. 1992, 174, 6678–6681. [Google Scholar] [CrossRef] [PubMed]
- Collinson, L.P.; Dawes, I.W. Inducibility of the response of yeast cells to peroxide stress. J. Gen. Microbiol. 1992, 138, 329–335. [Google Scholar] [CrossRef]
- Marini, M.; Frabetti, F.; Musiani, D.; Franceschi, C. Oxygen radicals induce stress proteins and tolerance to oxidative stress in human lymphocytes. Int. J. Radiat. Biol. 1996, 70, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast 1991, 7, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.T.; Carroll, M.C.; Hall, M.D.; Harvey, C.J.; Beese, S.E.; Culotta, V.C. Down-regulation of a manganese transporter in the face of metal toxicity. Mol. Biol. Cell 2009, 20, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Bird, A.J.; Winge, D.R.; Eide, D.J. Regulation of the Yeast TSA1 Peroxiredoxin by ZAP1 Is an Adaptive Response to the Oxidative Stress of Zinc Deficiency. J. Biol. Chem. 2006, 282, 2184–2195. [Google Scholar] [CrossRef]
- James, J.; Fiji, N.; Roy, D.; Andrew MG, D.; Shihabudeen, M.S.; Chattopadhyay, D.; Thirumurugan, K. A rapid method to assess reactive oxygen species in yeast using H2DCF-DA. Anal. Methods 2015, 7, 8572–8575. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.; Ahn, B.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. In Methods in Enzymology; Packer, L., Glazer, A.N., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 1990; Volume 186, pp. 464–478. [Google Scholar]
- Kanprasoet, W.; Jensen, L.T.; Sriprach, S.; Thitiananpakorn, K.; Rattanapornsompong, K.; Jensen, A.N. Deletion of Mitochondrial Porin Alleviates Stress Sensitivity in the Yeast Model of Shwachman-Diamond Syndrome. J. Genet. Genom. 2015, 42, 671–684. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of artemisinin and derivatives are available from commercial chemical suppliers. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).