Metal-Based Scaffolds of Schiff Bases Derived from Naproxen: Synthesis, Antibacterial Activities, and Molecular Docking Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure of the Schiff Base (5)
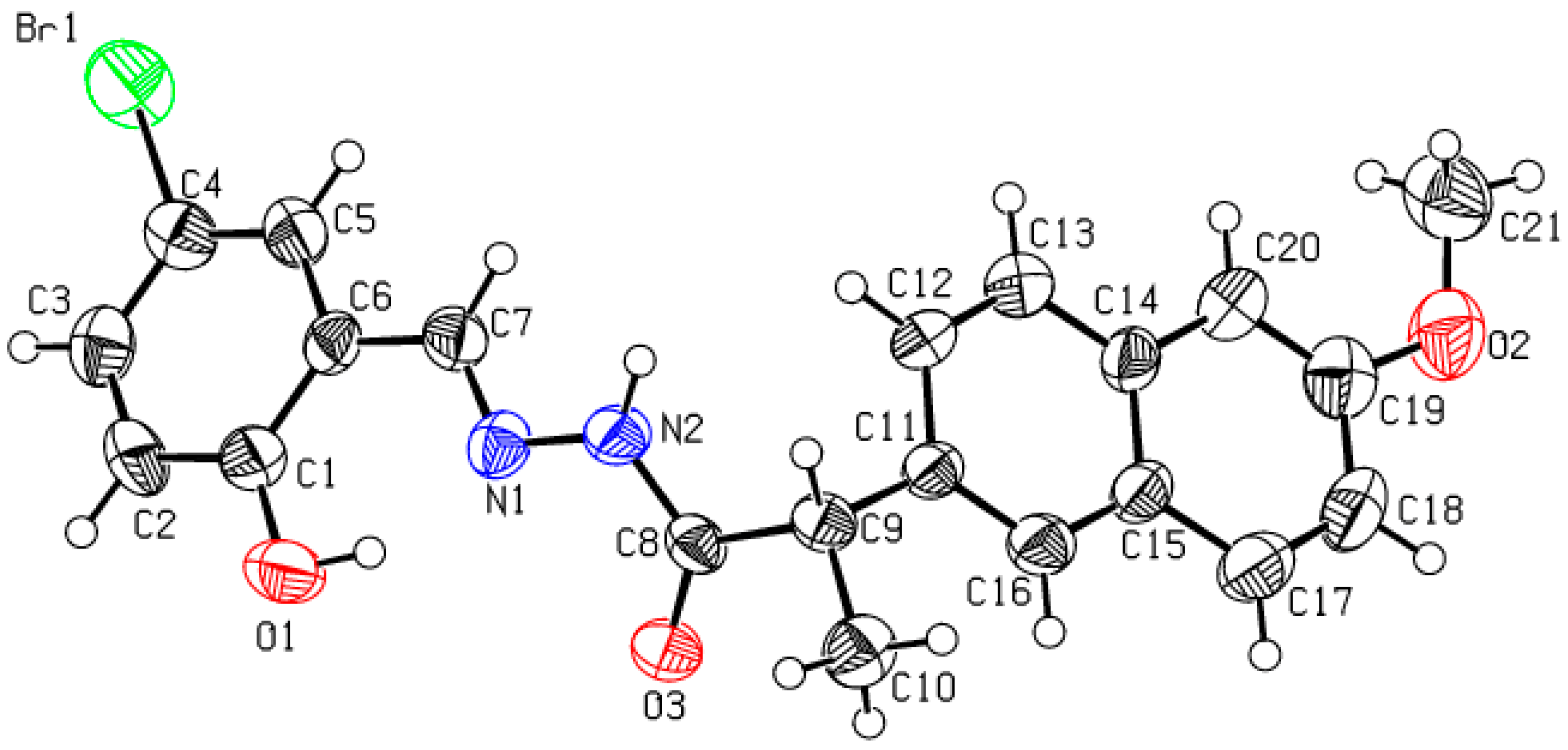
2.2. Single Crystal XRD Analysis for the Schiff Base (5)
2.3. Synthesis of Target Molecules 6a–f
2.4. Anti-Bacterial Activity
2.5. Computational Analysis of Synthesized Compounds Against COX1
2.5.1. Molecular Docking Analyses
2.5.2. Structure Activity Relationship (SAR) Analyses of Synthesized Compounds and Target Protein
3. Materials and Methods
3.1. Synthesis of 2-(6-Methoxynaphthalen-2-yl)propanoicacid (2)
3.2. Synthesis of 2−(6−Methoxynaphthalen−2−yl)propanehydrazide (4)
3.3. Synthesis of (N’-[(E)-(5-bromo-2-hydroxyphenyl) methylidene]-2-(6-methoxynaphthalen-2-yl)propanehydrazide) (5)
3.4. Synthesis of Metal Complexes 6a-f from Schiff Base Ligand (5)
3.4.1. Synthesis of Ni Complex (6a)
3.4.2. Synthesis of Mn (II) Complex (6b)
3.4.3. Synthesis of Cu Complex (6c)
3.4.4. Synthesis of Zn Complex (6d)
3.4.5. Synthesis of Co Complex (6e)
3.4.6. Synthesis of Cd Complex (6f)
3.5. Anti-Bacterial Testing
3.6. Molecular Docking
3.6.1. Retrieval of COX1 Structure from the Protein Data Bank (PDB)
3.6.2. Designing of Ligands and Molecular Docking
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ute, J.; Kowol, C.R.; Keppler, B.K.; Hartinger, C.G.; Walter, B.; Petra, H. Anticancer activity of metal complexes: Involvement of redox processes. Antioxid. Redox Signal. 2011, 15, 1085–1127. [Google Scholar]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Hartinger, C.; Nazarov, A.S.; Dyson, P.; Keppler, B. Carbohydrate-metal complexes and their potential as anticancer agents. Curr. Med. Chem. 2008, 15, 2574–2591. [Google Scholar] [CrossRef]
- Lodeiro, C.; Bastida, R.; Bértolo, E.; Macías, A.; Rodríguez, A. Synthesis and characterisation of four novel NxOy -Schiff-base macrocyclic ligands and their metal complexes. Transit. Met. Chem. 2003, 28, 388–394. [Google Scholar] [CrossRef]
- Yamada, S. Advancement in stereochemical aspects of Schiff base metal complexes. Coord. Chem. Rev. 1999, 190, 537–555. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The challenge of cyclic and acyclic schiff bases and related derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Christofis, P.; Katsarou, M.; Papakyriakou, A.; Sanakis, Y.; Katsaros, N.; Psomas, G. Mononuclear metal complexes with Piroxicam: Synthesis, structure and biological activity. J. Inorg. Biochem. 2005, 99, 2197–2210. [Google Scholar] [CrossRef]
- Cini, R. Anti-Inflammatory Compounds as Ligands in Metal Complexes as Revealed in X-Ray Structural Studies. Comments Inorg. Chem. 2000, 22, 151–186. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D. Recent advances on non-steroidal anti-inflammatory drugs, NSAIDs: Organotin complexes of NSAIDs. J. Organomet. Chem. 2006, 691, 1767–1774. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, R.; Sarkar, M. Direct binding of Cu(II)-complexes of oxicam NSAIDs with DNA backbone. J. Inorg. Biochem. 2006, 100, 1320–1331. [Google Scholar] [CrossRef]
- Warden, S.J. Prophylactic use of NSAIDs by athletes: A risk/benefit assessment. Phys. Sportsmed. 2010, 38, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Galani, A.; Kovala-Demertzi, D.; Kourkoumelis, N.; Koutsodimou, A.; Dokorou, V.; Ciunik, Z.; Russo, U.; Demertzis, M.A. Organotin adducts of indomethacin: Synthesis, crystal structures and spectral characterization of the first organotin complexes of indomethacin. Polyhedron 2004, 23, 2021–2030. [Google Scholar] [CrossRef]
- Filitsa, D.; Papadopoulos, A.N.; Vassilis, T.; Vassilis, P.; Raptopoulou, C.P.; Kessissoglou, D.P.; George, P.J.D.T. Biological evaluation of non-steroidal anti-inflammatory drugs-cobalt(II) complexes. Dalton Trans. 2010, 39, 4517–4528. [Google Scholar]
- Dimiza, F.; Papadopoulos, A.N.; Tangoulis, V.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Biological evaluation of cobalt(II) complexes with non-steroidal anti-inflammatory drug naproxen. J. Inorg. Biochem. 2012, 107, 54–64. [Google Scholar] [CrossRef]
- Tarushi, A.; Karaflou, Z.; Kljun, J.; Turel, I.; Psomas, G.; Papadopoulos, A.N.; Kessissoglou, D.P. Antioxidant capacity and DNA-interaction studies of zinc complexes with a non-steroidal anti-inflammatory drug, mefenamic acid. J. Inorg. Biochem. 2013, 128, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Tsiliou, S.; Kefala, L.A.; Perdih, F.; Turel, I.; Kessissoglou, D.P.; Psomasa, G. Cobalt(II) complexes with non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological evaluation. Eur. J. Med. Chem. 2012, 48, 132–142. [Google Scholar] [CrossRef]
- Marianthi, Z.; Natalia, R.; Vassilis, T.; Papadopoulos, A.N.; Franc, P.; Iztok, T.; George, P. Manganese(II) complexes with the non-steroidal anti-inflammatory drug tolfenamic acid: Structure and biological perspectives. Inorg. Chem. 2014, 53, 2040–2052. [Google Scholar]
- Dimiza, F.; Papadopoulos, A.N.; Tangoulis, V.; Psycharis, V.; Raptopoulou, C.; Kessissoglou, D.; Psomas, G. Biological Evaluation of Non-Steroidal Anti-Inflammatory Drugs-Cobalt(II) Complexes. Dalton Trans. 2010, 39, 4517–4528. [Google Scholar] [CrossRef] [PubMed]
- Tolia, C.; Papadopoulos, A.N.; Raptopoulou, C.P.; Psycharis, V.; Garino, C.; Salassa, L.; Psomas, G. Copper(II) interacting with the non-steroidal antiinflammatory drug flufenamic acid: Structure, antioxidant activity and binding to DNA and albumins. J. Inorg. Biochem. 2013, 123, 53–65. [Google Scholar] [CrossRef]
- El-Husseiny, W.M.; El-Sayed, M.A.; Abdel-Aziz, N.I.; El-Azab, A.S.; Asiri, Y.A.; Abdel-Aziz, A.A. Structural alterations based on naproxen scaffold: Synthesis, evaluation of antitumor activity and COX-2 inhibition, and molecular docking. Eur. J. Med. Chem. 2018, 158, 134–143. [Google Scholar] [CrossRef]
- Dendrinou-Samara, C.; Tsotsou, G.; Ekateriniadou, L.V.; Kortsaris, A.H.; Raptopoulou, C.P.; Terzis, A.; Kyriakidis, D.A.; Kessissoglou, D.P. Anti-inflammatory drugs interacting with Zn(II), Cd(II) and Pt(II) metal ions. J. Inorg. Biochem. 1998, 71, 171. [Google Scholar] [CrossRef]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and its complexes in medicine: A biochemical approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef]
- Szymański, P.; Frączek, T.; Markowicz, M.; Mikiciuk-Olasik, E. Development of copper based drugs, radiopharmaceuticals and medical materials. Biometals 2012, 25, 1089–1112. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.J.; Yan, Y.K.; Lee, P.P.F.; Lim, K.H. Copper, gold and silver compounds as potential new anti-tumor metallodrugs. Future Med. Chem. 2010, 2, 1591–1608. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological activities of hydrazone derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chauhan, K.; Srivastava, R.K.; Singh, S.V.; Srivastava, K.; Saxena, J.K.; Puri, S.K.; Chauhan, P.M. Design and synthesis of a new class of 4-aminoquinolinyl- and 9-anilinoacridinyl Schiff base hydrazones as potent antimalarial agents. Chem. Biol. Drug Des. 2014, 84, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, S.; Perumal, Y.; Ruth Vandana, D. Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg. Med. Chem. 2006, 14, 3113–3118. [Google Scholar]
- Asif, M.; Husain, A. Analgesic, Anti-Inflammatory, and Antiplatelet Profile of Hydrazones Containing Synthetic Molecules. J. Appl. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Wu, L.M.; Teng, H.B.; Ke, X.B.; Xu, W.J.; Su, J.T.; Liang, S.C.; Hu, X.M. Copper(II) complexes of salicylaldehyde hydrazones: Synthesis, structure, and DNA interaction. Chem. Biodivers. 2010, 4, 2198–2209. [Google Scholar] [CrossRef]
- Wang, H.; Ren, S.X.; He, Z.Y.; Wang, D.L.; Yan, X.N.; Feng, J.T.; Zhang, X. Synthesis, antifungal activities and qualitative structure activity relationship of carabrone hydrazone derivatives as potential antifungal agents. Int. J. Mol. Sci. 2014, 15, 4257–4272. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Altintop, M.D.; Özdemir, A.; Turan-Zitouni, G.; Khan, S.I.; Tabanca, N. Synthesis and Biological Evaluation of Some Hydrazone Derivatives as Anti-inflammatory Agents. Lett. Drug Des. Discov. 2012, 9. [Google Scholar] [CrossRef]
- Nassiri, K.M.; Assarzadeh, M.J.; Almasirad, A.; Ghasemi-Niri, S.F.; Amini, M.; Kebriaeezadeh, A.; Nassiri, K.N.; Ghadimi, M.; Tabei, A. Synthesis and analgesic activity of novel hydrazide and hydrazine derivatives. Iran. J. Pharm. Res. 2013, 12, 721–727. [Google Scholar]
- Aboul-Fadl, T.; Faragany Abdel-Hamid, M.; Hassan, A.S. Synthesis, antitubercular activity and pharmacokinetic studies of some schiff bases derived from 1- alkylisatin and isonicotinic acid hydrazide (inh). Arch. Pharm. Res. 2003, 26, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Golcu, A.; Turner, M.; Demirelli, H.; Alan Wheatley, R. Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Hania, M.M. Synthesis of Some Imines and Investigation of their Biological Activity. J. Chem. 2012, 6, 629–632. [Google Scholar] [CrossRef]
- Pouralimardan, O.; Chamayou, A.C.; Janiak, C.; Hosseini-Monfared, H. Hydrazone Schiff base-manganese(II) complexes: Synthesis, crystal structure and catalytic reactivity. Inorg. Chim. Acta 2007, 360, 1599–1608. [Google Scholar] [CrossRef]
- Silva, C.M.; Silva, D.L.; Modolo, L.V.; Alves, R.B.; Resende, M.A.; Martins, V.B.; Fatima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, R.S.; Lee, J.Y.; Chong, Y.; Smith, W.L. Comparison of cyclooxygenase-1 crystal structures: Cross-talk between monomers comprising cyclooxygenase-1 homodimers. Biochemistry 2010, 49, 7069–7079. [Google Scholar] [CrossRef]
- Marques, M.; Carvalho, M.L.; Cabrita, E.J.; Fernandes, E.; Silva, A.M.S.; Seixas, R.S.G.R.; Erhardt, S.; Ribeiro, D.; Martins, A.C.; Nave, M. Synthesis and evaluation of new benzimidazole-based COX inhibitors: A naproxen-like interaction detected by STD-NMR. RSC Adv. 2015, 5, 49098–49109. [Google Scholar]
- Oniga, S.D.; Pacureanu, L.; Stoica, C.I.; Palage, M.D.; Crăciun, A.; Rusu, L.R.; Crisan, E.L.; Araniciu, C. COX Inhibition Profile and Molecular Docking Studies of Some 2-(Trimethoxyphenyl)-Thiazoles. Molecules 2017, 22, 1507. [Google Scholar] [CrossRef]
- Paola, V.; Stefania, T.; Maria Grazia, P.; Paola, M.; Laura, S.; Antonio, S.; Antonio, L.; Melania, D.; Emanuela, M.; Annalisa, B. Synthesis, pharmacological characterization, and docking analysis of a novel family of diarylisoxazoles as highly selective cyclooxygenase-1 (COX-1) inhibitors. J. Med. Chem. 2013, 56, 4277–4299. [Google Scholar]
- Melo, A.D.; Amaral, A.F.; Schaefer, G.; Luciano, F.B.; De, A.C.; Costa, L.B.; Rostagno, M.H. Antimicrobial effect against different bacterial strains and bacterial adaptation to essential oils used as feed additives. Can. J. Vet. Res. 2015, 79, 285–289. [Google Scholar]
- El-Sehemi, A.G.; Bondock, S.; Ammar, Y.A. Transformations of naproxen into pyrazolecarboxamides: Search for potent anti-inflammatory, analgesic and ulcerogenic agents. Med. Chem. Res. 2013, 23, 827–838. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar]
Sample Availability: Samples of the compounds are not available at the moment but can be easily prepared and provided by the authors on demand. |



| SHELX | |
|---|---|
| Crystal data | |
| Chemical formula | C21H19BrN2O3 |
| Mr | 427.29 |
| Crystal system, space group | Orthorhombic, P212121 |
| Temperature (K) | 296 |
| a, b, c (Å) | 4.9129 (8), 16.732 (3), 22.570 (3) |
| V (Å3) | 1855.3 (5) |
| Z | 4 |
| Radiation type | Mo Kα |
| µμ (mm−1) | 2.24 |
| Crystal size (mm) | 0.44 × 0.22 × 0.20 |
| Data collection | |
| Diffractometer | Bruker Kappa APEXII CCD Diffractometer |
| Absorption correction | – |
| No. of measured, independent and observed [I> 2σ(I)] reflections | 13779, 4040, 1985 |
| Rint | 0.091 |
| (sin θ/λ)max (Å−1) | 0.651 |
| Refinement | |
| R[F2> 2σ(F2)], wR(F2), S | 0.052, 0.105, 0.94 |
| No. of reflections | 4040 |
| No. of parameters | 249 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.28, −0.28 |
| Absolute structure | Flack x determined using 563 quotients [(I+) − (I−)]/[(I+) + (I−)] (Parsons, Flack and Wagner, ActaCryst. B69 (2013) 249-259). |
| Absolute structure parameter | 0.026 (12) |
| Sr. No. | Samples | Escherichia Coli | Streptococcus Aureous | Salmonella Typhae | Bacillus Subtilus |
|---|---|---|---|---|---|
| 1. | Schiff Base (5) | 12.7 | 11.8 | 12.1 | 12.2 |
| 2. | Ni Complex (6a) | 14.5 | 13.0 | 16.0 | 16.8 |
| 3 | Mn Complex (6b) | 11.9 | 12.3 | 14.2 | 13.4 |
| 4. | Cu Complex (6c) | 15.3 | 15.3 | 12.3 | 14.3 |
| 5. | Zn Complex (6d) | 11.5 | 11.3 | 13.3 | 16.5 |
| 6. | Co Complex (6e) | 11.3 | 16.3 | 14.1 | 13.1 |
| 7. | Cd Complex (6f) | 14.1 | 13.2 | 15.3 | 14.1 |
| 8. | Reference | 14.2 | 13.9 | 13.4 | 13.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, M.A.; Feng, S.; Anthony, M.; Tahir, M.N.; Hassan, M.; Seo, S.-Y.; Ahmad, S.; Iqbal, M.; Saleem, M.; Lu, C. Metal-Based Scaffolds of Schiff Bases Derived from Naproxen: Synthesis, Antibacterial Activities, and Molecular Docking Studies. Molecules 2019, 24, 1237. https://doi.org/10.3390/molecules24071237
Shaheen MA, Feng S, Anthony M, Tahir MN, Hassan M, Seo S-Y, Ahmad S, Iqbal M, Saleem M, Lu C. Metal-Based Scaffolds of Schiff Bases Derived from Naproxen: Synthesis, Antibacterial Activities, and Molecular Docking Studies. Molecules. 2019; 24(7):1237. https://doi.org/10.3390/molecules24071237
Chicago/Turabian StyleShaheen, Muhammad Ashraf, Shanshan Feng, Mehwish Anthony, Muhammad Nawaz Tahir, Mubashir Hassan, Sung-Yum Seo, Saeed Ahmad, Mudassir Iqbal, Muhammad Saleem, and Changrui Lu. 2019. "Metal-Based Scaffolds of Schiff Bases Derived from Naproxen: Synthesis, Antibacterial Activities, and Molecular Docking Studies" Molecules 24, no. 7: 1237. https://doi.org/10.3390/molecules24071237
APA StyleShaheen, M. A., Feng, S., Anthony, M., Tahir, M. N., Hassan, M., Seo, S.-Y., Ahmad, S., Iqbal, M., Saleem, M., & Lu, C. (2019). Metal-Based Scaffolds of Schiff Bases Derived from Naproxen: Synthesis, Antibacterial Activities, and Molecular Docking Studies. Molecules, 24(7), 1237. https://doi.org/10.3390/molecules24071237






