Synthesis and Bio-Evaluation of Natural Butenolides-Acrylate Conjugates
Abstract
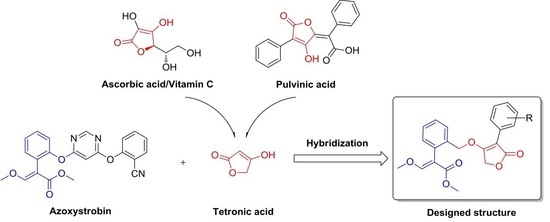
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Target Compounds
2.2. Speculated Synthetic Mechanism of the Key Intermediate II
2.3. Spectral Analysis of Target Compounds
2.4. Biological Activity
2.4.1. Primary Fungicidal Activities
2.4.2. Further Fungicidal Activities
2.5. Analysis of Structure-Activity Relationships
3. Conclusions
4. Experimental Section
4.1. Instruments and Reagents
4.2. Experimental Method
4.2.1. Synthesis of Methoxyacrylate Intermediates
4.2.2. General Procedure for Synthesis of the Key Lactone Intermediate II (6a–6k)
4.2.3. General Procedure for Synthesis of Target Compounds (7a–k)
4.3. Biological Activity Assay
4.3.1. Primary Screening Test for Inhibiting Mycelial Growth of Pathogenic Fungi
4.3.2. Further Screening Test for Inhibiting Mycelial Growth of Pathogenic Fungi
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef]
- Sauter, H.; Steglich, W.; Anke, T. Strobilurins: Evolution of a new class of active substances. Angew. Chem. Int. Ed. 1999, 38, 1328–1349. [Google Scholar] [CrossRef]
- Liu, W.J.; Li, Q.S.; Xu, F.B. New active ingredients of pesticides from 2011 to 2017 and application of natural products derivatization method in agrochemical molecular design. Nat. Prod. Res. Dev. 2019, 31, 363–371. [Google Scholar]
- Giuliani, M.M.; Carucci, F.; Nardella, E. Combined effects of deficit irrigation and strobilurin application on gas exchange, yield and water use efficiency in tomato (Solanum lycopersicum L.). Sci. Hortic. 2018, 233, 149–158. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Chen, M.G.; Wu, C.C.; Hua, X.W.; Zhou, S.; Wang, B.L.; Li, Z.M. Design, synthesis and bioactivities of novel strobilurin derivatives containing 1,3,4-oxadiazole moity. Chin. J. Org. Chem. 2017, 37, 403–410. [Google Scholar] [CrossRef]
- Balba, H. Review of strobilurin fungicide chemicals. J. Environ. Sci. Health B 2007, 42, 441–451. [Google Scholar] [CrossRef]
- Mallinger, A.; Gall, T.L.; Mioskowski, C. 3-Aryltetronic acids: Efficient preparation and use as precursors for vulpinic acids. J. Org. Chem. 2009, 74, 1124–1129. [Google Scholar] [CrossRef]
- Ma, S.M.; Wu, B.; Shi, Z.J. An efficient synthesis of 4-halo-5-hydroxyfuran-2(5H)-ones via the sequential halolactonization and γ-hydroxylation of 4-aryl-2,3-alkadienoic acids. J. Org. Chem. 2004, 69, 1429–1431. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Benet-Buchholz, J.; Fischer, R.; Nauen, R. Spirodiclofen and spiromesifen—Novel acaricidal and insecticidal tetronic acid derivatives with a new mode of action. Chimia 2003, 57, 697–701. [Google Scholar] [CrossRef]
- Wang, X.D.; Wei, W.; Wang, P.F.; Yi, L.C.; Shi, W.K.; Xie, Y.X.; Wu, L.Z.; Tang, N.; Zhu, L.S.; Peng, J.; et al. Synthesis, molecular docking and biological evaluation of 3-arylfuran-2(5H)-ones as anti-gastric ulcer agent. Bioorg. Med. Chem. 2015, 23, 4860–4865. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, J.; Zhang, H. Total synthesis of pulverolide: Revision of its structure. Tetrahedron Lett. 2010, 51, 4874–4876. [Google Scholar] [CrossRef]
- Manchoju, A.; Pansare, S.V. Catalytic undirected intermolecular C−H functionalization of arenes with 3-diazofuran-2,4-dione: Synthesis of 3-aryl tetronic acids, vulpinic acid, pinastric acid, and methyl isoxerocomate. Org. Lett. 2016, 18, 5952–5955. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.W.; Xu, C.; Fan, Z.Q.; Zhao, L.J.; Liu, H.M. A facile synthesis, antibacterial activity of pulvinone and its derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.N.; Matsumoto, H.; Adachi, J. Antifungal studies on drugs. I. antifungal activity of five-membered lactone derivatives. Yakugaku Zasshi 1968, 88, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Weber, V.; Rubat, C.; Duroux, E.; Lartigue, C.; Madesclaire, M.; Coudert, P. New 3- and 4-hydroxyfuranones as anti-oxidants and anti-inflammatory agents. Bioorg. Med. Chem. 2005, 13, 4552–4564. [Google Scholar] [CrossRef] [PubMed]
- Martinčič, R.; Kuzmanovski, I.; Wagner, A.; Novič, M. Development of models for prediction of the antioxidant activity of derivatives of natural compounds. Anal. Chim. Acta 2015, 868, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.P.; Ouyang, H.; Wang, X.D.; Lv, P.C.; Huang, Z.J.; Yu, S.R.; Yi, T.F.; Yang, Y.L.; Zhu, H.L. 4-Alkoxy-3-arylfuran-2(5H)-ones as inhibitors of tyrosyl-tRNA synthetase: Synthesis, molecular docking and antibacterial evaluation. Bioorg. Med. Chem. 2011, 19, 3884–3891. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.; Readshaw, S.A. Structural studies of MM46115, a novel tetronic acid containing macrolide with antiviral and antibacterial activity isolated from actinomadura pelletieri. J. Chem. Soc. Perkin Trans. 1991, 7, 1641–1644. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Lu, A.; Yang, C.L. Synthesis, characterization, antifungal evaluation and 3D-QSAR study of phenylhydrazine substituted tetronic acid derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 3772–3776. [Google Scholar] [CrossRef]
- Cray, J.A.; Houghton, J.D.R.; Cooke, L.R.; Hallsworth, J.E. A simple inhibition coefficient for quantifying potency of biocontrol agents against plant-pathogenic fungi. Biol. Control 2015, 81, 93–100. [Google Scholar] [CrossRef]
- Qi, R.; Wang, T.; Zhao, W.; Li, P.; Ding, J.; Gao, Z. Activity of ten fungicides against Phytophthora capsici isolates resistant to metalaxyl. J. Phytopathol. 2012, 160, 717–722. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.; Gao, Y.; Wang, H.; Guo, B.; Li, J. Synthesis and antifungal activities of new type β-methoxyacrylate-based strobilurin analogues. Chin. J. Chem. 2012, 30, 1517–1524. [Google Scholar] [CrossRef]
- Sridhara, A.M.; Venugopala Reddy, K.R.; Keshavayya, J.; Ambika, D.M.S.; Gopinath, V.S.; Bose, P.; Goud, S.K.; Peethambar, S.K. Synthesis, antimicrobial and cytotoxicity studies of some novel modified strobilurin derivatives. J. Braz. Chem. Soc. 2011, 22, 849–856. [Google Scholar] [CrossRef]
Sample Availability: Samples of the target compounds 7a–7k are available from the authors. |



| Antifungal Activities In Vitro (100 µg·mL−1, Inhibitory Rate %) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd. No. | Rhizoctonia solani | Phomopsis adianticola | Rap Sclerotinia stem rot | Pestallozzia theae | Gibberella zeae | Sclerotinia sclerotiorum | Altermaria alternata | Pestalotiopsis theae | Monilinia fructicola | Phytophthora capsici | Colletotrichum gloeosporioides | Magnapothe grisea |
| 6c | - a | 7.9 | 12.24 | 12.27 | / | 4.12 | 7.08 | 37.79 | 16.70 | / | - | 7.87 |
| 6i | - | 13.75 | 41.49 | 34.33 | 17.65 | 51.03 | 21.77 | 47.16 | 64.09 | 10.25 | - | 28.32 |
| 7a | / b | 64.43 | 72.84 | 33.17 | / | 14.71 | 9.56 | 0.33 | 35.91 | / | / | 25.52 |
| 7b | 32.31 | 58.25 | 74.18 | 48.92 | 16.34 | 43.82 | 20.18 | 33.61 | 37.88 | / | 5.61 | 38.46 |
| 7c | - | 43.64 | 73.28 | 37.81 | 29.20 | 20.29 | 20.00 | 19.73 | 36.09 | 2.65 | - | 29.37 |
| 7d | 1.15 | 43.64 | 73.28 | 45.94 | 23.20 | 12.94 | 20.00 | 30.77 | 41.47 | 24.91 | 2.46 | 29.72 |
| 7e | 46.73 | 66.15 | 72.99 | 49.25 | 45.10 | 24.26 | 21.59 | 23.41 | 52.42 | 13.60 | 7.72 | 27.62 |
| 7f | 23.85 | 38.14 | 35.07 | 33.00 | 29.90 | 13.53 | 19.82 | / | 24.06 | 17.31 | 3.86 | 29.55 |
| 7g | / | 86.94 | 20.45 | 22.06 | 28.76 | 19.12 | 17.52 | / | 9.16 | / | / | 38.81 |
| 7h | / | 29.55 | 13.43 | 37.31 | 14.87 | 8.53 | 21.42 | 38.13 | 30.52 | / | 4.21 | 28.67 |
| 7i | - | 36.08 | 31.94 | 36.65 | 32.68 | 10.88 | 27.08 | 8.36 | 36.80 | / | - | 26.05 |
| 7j | / | 55.50 | 27.31 | 39.64 | 5.07 | 6.32 | 17.88 | 34.28 | 33.57 | 19.08 | 5.96 | 24.48 |
| 7k | 1.92 | 51.03 | 73.58 | 45.11 | 44.28 | 17.94 | 23.19 | 32.27 | 33.93 | 10.60 | 10.18 | 38.11 |
| Intermediate I | 33.08 | 73.88 | 60.60 | 63.18 | 66.99 | 97.65 | 39.82 | 60.03 | 77.74 | 0.00 | 21.93 | 29.02 |
| Azoxystrobin | 88.65 | 95.19 | 93.43 | 100.00 | 68.63 | 100.00 | 59.65 | 100.00 | 93.00 | 24.03 | 58.95 | 100.00 |
| Carbendazim | 100.00 | 100.00 | 99.40 | 100.00 | 100.00 | 100.00 | 17.70 | 100.00 | 100.00 | 6.36 | 100.00 | 100.00 |
| Compd. No. | Concentration | Phomopsis adianticola | Pestallozzia theae | Gibberella zeae | Sclerotinia sclerotiorum |
|---|---|---|---|---|---|
| 7k | 100 | 36.91 | 39.14 | 46.62 | 33.14 |
| 50 | 36.76 | 38.97 | 10.44 | 27.44 | |
| 25 | 29.71 | 37.93 | 12.66 | 23.32 | |
| 12.5 | 30.15 | 31.03 | 7.47 | 12.07 | |
| 6.25 | 4.12 | 20.00 | 3.91 | 8.90 | |
| 7e | 100 | 35.44 | 42.59 | / a | 43.28 |
| 50 | 35.00 | 40.17 | 22.60 | 44.07 | |
| 25 | 28.38 | 22.59 | 5.99 | 50.10 | |
| 12.5 | 13.53 | 24.31 | / | 11.28 | |
| 6.25 | 10.59 | 14.66 | 1.54 | 19.04 | |
| Carbendazim | 100 | 100.00 | 100.00 | 100.00 | 100.00 |
| 50 | 50.00 | 100.00 | 87.69 | 100.00 | |
| 25 | 100.00 | 100.00 | 89.77 | 100.00 | |
| 12.5 | 100.00 | 100.00 | 89.92 | 100.00 | |
| 6.25 | 100.00 | 100.00 | 82.21 | 100.00 | |
| Intermediate I | 100 | 69.26 | 32.76 | 1.84 | 26.96 |
| 50 | 41.76 | 23.62 | / | 14.29 | |
| 25 | 30.29 | 17.07 | / | / | |
| 12.5 | 12.94 | / | / | / | |
| 6.25 | 21.18 | 6.38 | / | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, L.; Wang, S.; Song, D.; Wang, J.; Cao, X.; Ke, S. Synthesis and Bio-Evaluation of Natural Butenolides-Acrylate Conjugates. Molecules 2019, 24, 1304. https://doi.org/10.3390/molecules24071304
Bao L, Wang S, Song D, Wang J, Cao X, Ke S. Synthesis and Bio-Evaluation of Natural Butenolides-Acrylate Conjugates. Molecules. 2019; 24(7):1304. https://doi.org/10.3390/molecules24071304
Chicago/Turabian StyleBao, Longzhu, Shuangshuang Wang, Di Song, Jingjing Wang, Xiufang Cao, and Shaoyong Ke. 2019. "Synthesis and Bio-Evaluation of Natural Butenolides-Acrylate Conjugates" Molecules 24, no. 7: 1304. https://doi.org/10.3390/molecules24071304
APA StyleBao, L., Wang, S., Song, D., Wang, J., Cao, X., & Ke, S. (2019). Synthesis and Bio-Evaluation of Natural Butenolides-Acrylate Conjugates. Molecules, 24(7), 1304. https://doi.org/10.3390/molecules24071304