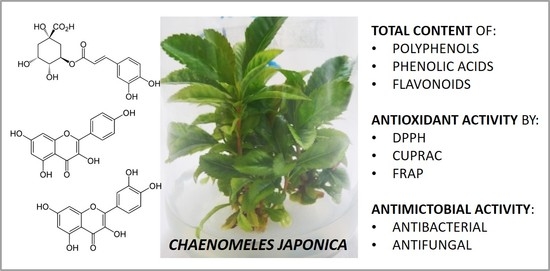
Micropropagation of Chaenomeles japonica: A Step towards Production of Polyphenol-rich Extracts Showing Antioxidant and Antimicrobial Activities
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
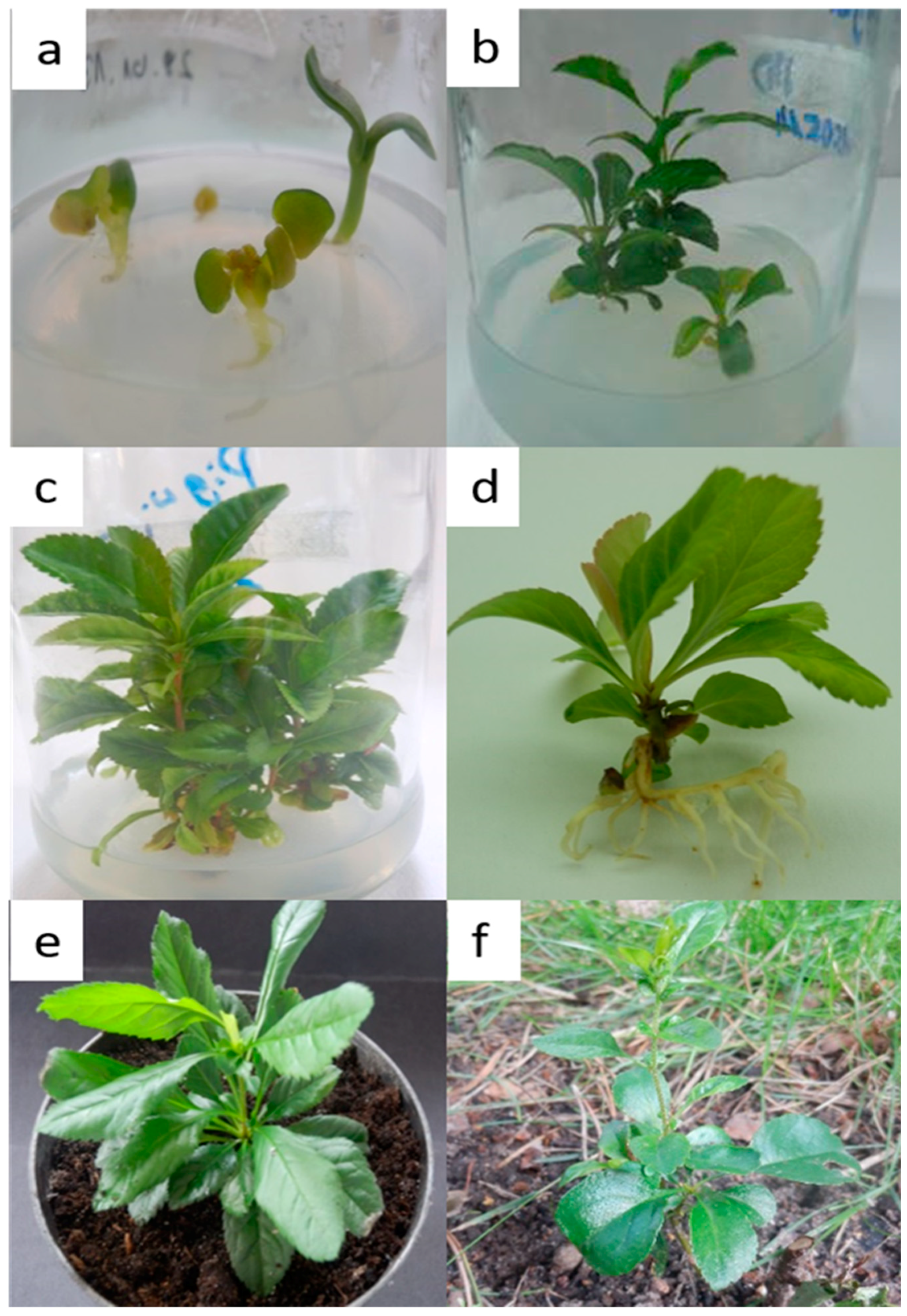
3.1. Plant Material and Surface Disinfection
3.2. Establishment of Shoot Cultures and Effect of PGRs on Shoot Multiplication
3.3. Rooting of Shoots and Plantlet Acclimatization
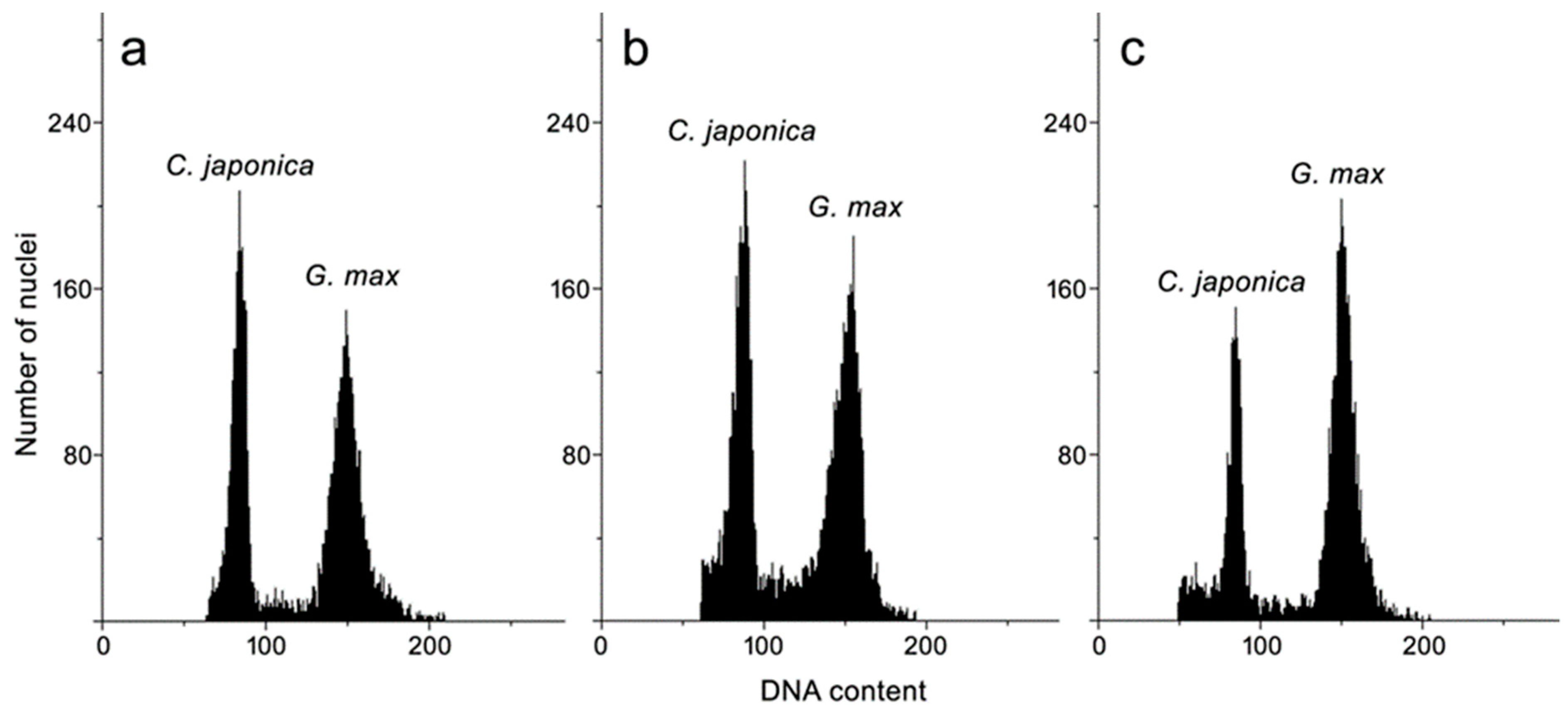
3.4. Genome Size Estimation
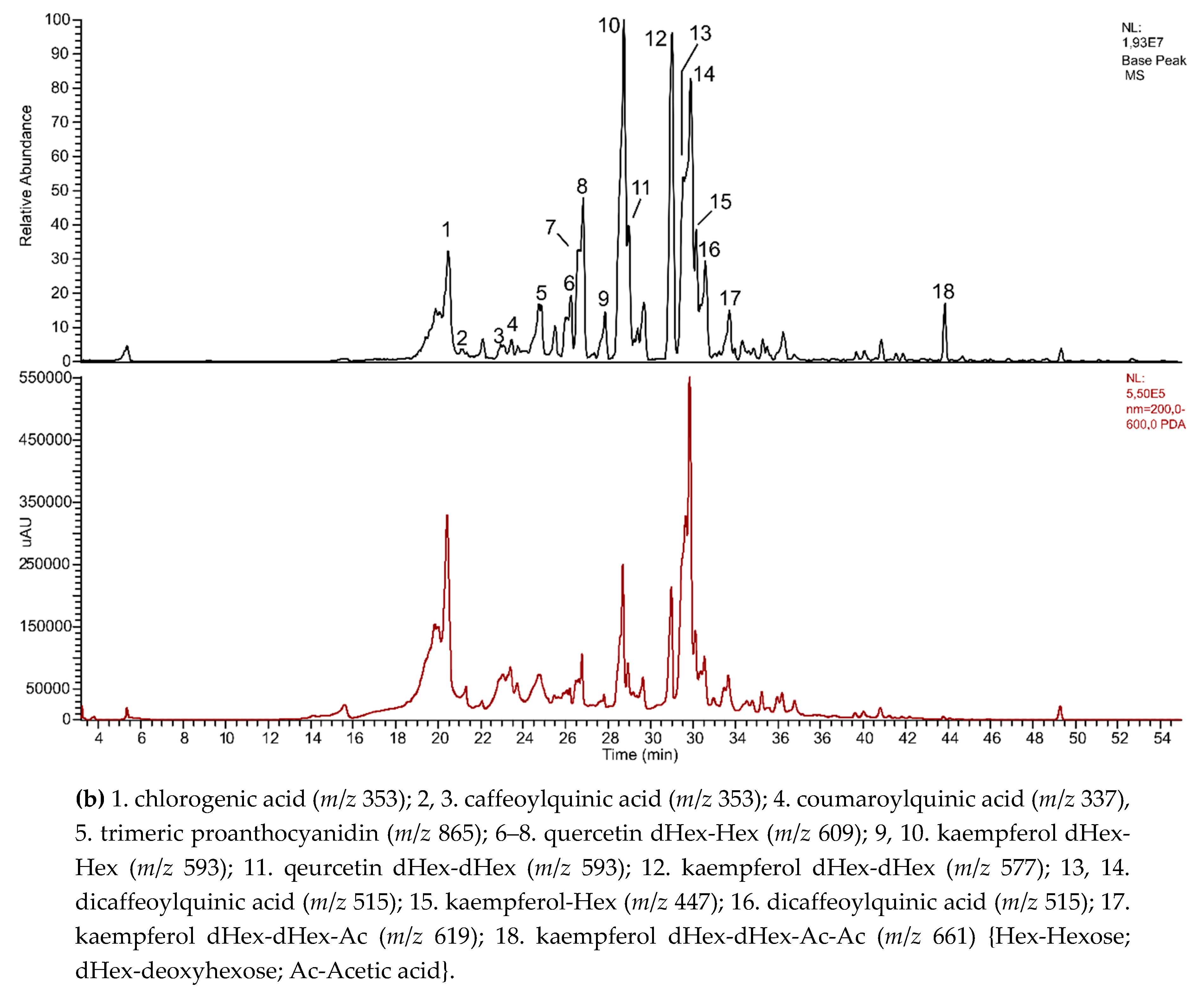
3.5. Phytochemical Screening of Polyphenols
3.6. Determination of Total Phenolic, Phenolic Acid, and Flavonoid Contents
3.7. Determination of Antioxidant Activity Using DPPH, CUPRAC, and FRAP Assays
3.8. Antimicrobial Assay
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weber, C. The genus Chaenomeles (Rosaceae). J. Arnold Arbor. 1964, 45, 161–205, 302–345. [Google Scholar]
- Phipps, J.B.; Robertson, K.R.; Smith, P.G.; Rohrer, J.R. A checklist of the subfamily Maloideae (Rosaceae). Canad. J. Bot. 1990, 68, 2209–2269. [Google Scholar] [CrossRef]
- Rumpunen, K. Chaenomeles: Potential new fruit crop for northern Europe. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHA Press: Alexandria, VA, USA, 2002; pp. 385–392. [Google Scholar]
- Lesińska, E.; Kraus, D. Charakterystyka morfologiczna owoców pigwowca. Zesz. Nauk. Akad. Rol. im. H. Kołłątaja w Krakowie. Ogrodnictwo Z. 1887, 17, 47–59, (article in Polish with an abstract in English). [Google Scholar]
- Lesińska, E.; Przybylski, R.; Eskin, N.A.M. Some volatile and nonvolatile flavour components of the Dwarf Quince (Chaenomeles japonica). J. Food Sci. 1988, 53, 854–856. [Google Scholar] [CrossRef]
- Rumpunen, K.; Kviklys, D. Combining ability and patterns of inheritance for plant and fruit traits in Japanese quince (Chaenomeles japonica). Euphytica 2003, 132, 139–149. [Google Scholar] [CrossRef]
- Hellin, P.; Jordan, M.J.; Vila, R.; Gustafsson, M.; Göransson, E.; Akesson, B.; Gröön, I.; Laencina, J.; Ros, J.M. Processing and products of Japanese Quince (Chaenomeles japonica) fruits. In Japanise Quince—Potential Fruit Crop for Northern Europe; Swedish University of Agricultural Sciences: Lomma Municipality, Sweden, 2003; pp. 169–175. [Google Scholar]
- Thomas, M.; Guillemin, F.; Guillon, F.; Thibault, J.-F. Pectins in the Japanese quince (Chaenomeles japonica). Carbohydr. Polym. 2003, 53, 361–372. [Google Scholar] [CrossRef]
- Hallmann, E.; Orpel, E.; Rembiałkowska, E. The content of biologically active compounds in some fruits from natural state. Veg. Crop. Res. Bull. 2011, 75, 81–90. [Google Scholar] [CrossRef]
- Biednasz, M.; Dziedzic, E.; Kaczmarczyk, E. The effect of storage and processing on vitamin C content in Japanese quince fruit. Folia Hort. 2017, 29, 83–93. [Google Scholar] [Green Version]
- Du, H.; Wu, J.; Li, H. Polyphenols and triterpenes from Chaenomeles fruits: Chemical analysis and antioxidant activities assessment. Food Chem. 2013, 141, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Han, L.-Y.; Zhang, H.; Xin, H.-L. Chaenomeles speciosa: A review of chemistry and pharmacology. Biomed. Rep. 2014, 2, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H. The Medicinal Plants of Korea; Kyo-Hak Publishing Co. Ltd.: Seul, South Kora, 2000. [Google Scholar]
- Xu, Y.N.; Kim, J.S.; Kang, S.S.; Son, K.H.; Kim, H.P.; Chang, H.W.; Bae, K. A new acylated triterpene from the roots of Chaenomeles japonica. Chem. Pharm. Bull. 2002, 50, 1124–1125. [Google Scholar] [CrossRef]
- Andersone, D.; Kaufmane, E. Flowering and fruit set in Japanese Quince (Chaenomeles japonica). In Japanese Quince—Potential Fruit Crop for Northern Europe; Rumpunen, K., Ed.; Final report of FAIR-CT97-3894; Swedish University of Agricultural Sciences: Alnarp, Sweden, 2003; pp. 29–36. [Google Scholar]
- Kviklys, D.; Rumpunen, K. Preliminary investigations on propagation of Chaenomeles spp. by softwood cuttings. In Rpt. 1992–1994, Balsgard-Dept. Hort. Plant Breeding; Swedish University of Agricultural Sciences: Alnarp, Sweden, 1996; pp. 183–185. [Google Scholar]
- Panavas, T. Optimization of the growth medium for the micropropagation of Japanese quince (Chaenomeles japonica Thunb.). Biologija 1994, 3, 44–49. [Google Scholar]
- Bach, A.; Kraus, D.; Grabarczyk, D. Mikrorozmnażanie pigwowca japońskiego [Chaenomeles japonica Lindl.]. Zesz. Nauk. Akad. Tech.-Rol. w Bydgoszczy 1996, 39, 115–121, (article in Polish with an abstract in English). [Google Scholar]
- Kauppinen, S.; Kviklys, D.; Rumpunem, K.; Stanys, V.; Svensson, M. Propagation of Japanese Quince (Chaenomeles japonica) plants. In Japanese Quince – Potential Fruit Crop for Nothern Europe; Rumpunen, K., Ed.; Final report of FAIR-CT97-3894; Swedish University of Agricultural Sciences: Alnarp, Sweden, 2003; pp. 81–92. [Google Scholar]
- Ghanbari, A. Impacts of plant growth regulators and culture media on in vitro propagation of three apple (Malus domestica Borkh.) rootstocks. Iranian J. Genet. Plant Breed. 2014, 3, 11–20. [Google Scholar]
- Kauppinen, S. Optimizing shoot proliferation and rooting of micropropagated Japanese Quince (Chaenomeles japonica (Thunb.) Lindl. Ex. Spach). Acta Horticult. 2001, 560, 433–436. [Google Scholar] [CrossRef]
- Stanys, V.; Weckman, A.; Staniene, G.; Duchovskis, P. In vitro induction of polyploidy in Japanese quince (Chaenomeles japonica). Plant Cell Tiss. Organ Cult. 2006, 84, 263–268. [Google Scholar] [CrossRef]
- Boudabous, M.; Mars, M.; Marzougui, N.; Ferchichi, A. Micropropagation of apple (Malus domestica L. cultivar Douce de Djerba) though in vitro culture of axillary buds. Acta Bot. Gall. 2010, 157, 513–524. [Google Scholar] [CrossRef]
- Norton, M.E.; Boe, A.A. In vitro propagation of ornamental Rosaceous plants. Hort. Sci. 1982, 17, 190–191. [Google Scholar]
- Norton, M.E.; Norton, C.R. Change in shoot proliferation with repeated in vitro subculture of shoots of woody species of Rosaceae. Plant Cell Tiss. Organ Cult. 1986, 5, 187–197. [Google Scholar] [CrossRef]
- Grimaldi, F.; Grohskopf, M.A.; Muniz, A.W.; Guidolin, A.F. In vitro rooting of fruit trees of the Rosaceae family. Rev. Cienc. Agrovet. 2008, 7, 160–168. [Google Scholar]
- Thiem, B.; Sliwinska, E. Flow cytometric analysis of nuclear DNA content in cloudberry (Rubus chamaemorus L.) in vitro cultures. Plant Sci. 2003, 164, 129–134. [Google Scholar] [CrossRef]
- Makowczyńska, J.; Andrzejewska-Golec, E.; Sliwinska, E. Nuclear DNA content in different plant material of Plantago asiatica L. cultured in vitro. Plant Cell Tiss. Organ Cult. 2008, 94, 65–71. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Krawczyk, A.; Więckowska, B.; Sliwinska, E. Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tiss. Organ Cult. 2013, 114, 197–206. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B.; Sliwinska, E.; Rewers, M.; Kowalczyk, M.; Stochmal, A.; Oleszek, W. The effect of nutritional factors and plant growth regulators on micropropagation and production of phenolic acids and saponins from plantlets and adventitious root cultures of Eryngium maritimum L. J. Plant Growth Regul. 2014, 33, 809–819. [Google Scholar] [CrossRef]
- Kikowska, M.; Thiem, B.; Sliwinska, E.; Rewers, M.; Kowalczyk, M.; Stochmal, A.; Długaszewska, J. Micropropagation of Eryngium campestre L. via shoot culture provides valuable uniform plant material with enhanced content of phenolic acids and antimicrobial activity. Acta Biol. Cracov. Bot. 2016, 58, 43–56. [Google Scholar] [CrossRef]
- Dickson, E.E.; Arumuganathan, K.; Kresovich, S.; Doyle, J.J. Nuclear DNA content variation within the Rosaceae. Am. J. Bot. 1992, 79, 1081–1086. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 302–372. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Nowak, S.; Michel, P.; Banaszczak, P.; Kicel, A. Assessment of the content of phenolics and antioxidant action in inflorescences and leaves of selected species from the genus Sorbus sensu stricto. Molecules 2010, 15, 8769–8783. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Skrzycki, M.; Podsiad, M.; Czeczot, H. Evaluation of antioxidant potential of flavonoids: An in vitro study. Acta Pol. Pharm. 2011, 68, 611–615. [Google Scholar]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J. Agric. Food Chem. 2007, 7, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Intern. Plant Soc. 1981, 30, 421–427. [Google Scholar]
- Sliwinska, E.; Thiem, B. Genome size stability in six medicinal plant species propagated in vitro. Biol. Plant. 2007, 51, 556–558. [Google Scholar] [CrossRef]
- Marie, D.; Brown, S.C. A cytometric exercise in plant histograms, with 2C values for 70 species. Biol. Cell. 1993, 78, 41–51. [Google Scholar] [CrossRef]
- Doležel, J.; Doleželova, M.; Novak, F.J. Flow cytometric estimation of nuclear DNA amount in diploid bananas (Musa acuminata and M. balbisiana). Biol. Plant. 1994, 36, 351–357. [Google Scholar] [CrossRef]
- Dóka, O.; Bicanic, D. Determination of total polyphenolic content in red wines by means of the combined He-Ne laser optothermal window and Folin-Ciocalteu colorimetry assay. Anal. Chem. 2002, 74, 2157–2161. [Google Scholar] [CrossRef]
- Polish Pharmacopeia VI. Polish Pharmaceutical Society: Warsaw, Polish, 2002. Available online: http://www.urpl.gov.pl/en/polish-pharmacopoeia (accessed on 2 April 2019).
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoids and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Annegowda, H.V.; Anwar, L.N.; Mordi, M.N.; Ramanathans, S.; Mansor, S.M. Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacogn. Res. 2010, 2, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güdü, K.; Özyürek, M.; Celik, E.S. Mechanism of antioxidant capacity assays and CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 2008, 160, 413–419. [Google Scholar] [CrossRef]
- Tiveron, A.P.; Melo, P.S.; Bergamaschi, K.B.; Viera, T.M.F.S.; Regitano d’Arce, M.A.B.; Alencar, S.M. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int. J. Mol. Sci. 2012, 13, 8943–8957. [Google Scholar] [CrossRef]
Sample Availability: Plant biomass is available from the authors. |

| Medium | Cytokinin [mg L−1] | Auxin [mg L−1] | Shoot Induction [%] | Shoot No./Explant ± SE | Shoot Length [cm] ± SE |
|---|---|---|---|---|---|
| N6-benzyladenine (BA) | Indole-3-acetic acid (IAA) | ||||
| WPM | - | - | 100 | 4.17 ± 0.10 ab | 1.55 ± 0.06 bd |
| WPM | 1.0 | 0.1 | 100 | 4.14 ± 0.12 ab | 1.30 ± 0.07 bd |
| ½ MS | - | - | 100 | 1.03 ± 0.04 c | 1.25 ± 0.01 bd |
| MS | - | - | 100 | 4.62 ± 0.21 ab | 2.40 ± 0.17 a |
| MS | 2.0 | - | 100 | 4.38 ± 0.12 ab | 0.82 ± 0.03 c |
| MS | 1.0 | - | 100 | 4,37 ± 0.20 ab | 0.92 ± 0.04 cd |
| MS | 1.0 | 1.0 | 100 | 4.70 ± 0.05 ab | 1.00 ± 0.04 cd |
| MS | 1.0 | 0.1 | 100 | 5.22 ± 0.12 a | 2.38 ± 0.14 a |
| BA | α-naphthaleneacetic acid (NAA) | ||||
| MS | 1.0 | 1.0 | 100 | 4.33 ± 0.13 ab | 0.95 ± 0.04 cd |
| MS | 1.0 | 0.1 | 100 | 4.01 ± 0.16 b | 1.16 ± 0.10 bcd |
| Medium | Root Induction [%] | Root No./Explant ± SE | Root Length (cm) ± SE | ||
|---|---|---|---|---|---|
| Basal Nutrition | Sucrose [g L−1] | Auxin [mg L−1] | |||
| ½ MS | 30 | - | 95 | 2.42 ± 0.19 ab | 6.71 ± 0.58 ab |
| ½ MS | 30 | IAA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| ½ MS | 30 | IBA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| ½ MS | 30 | NAA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| ¾ MS | 30 | - | 85 | 2.29 ± 0.22 abc | 8.35 ± 1.24 a |
| ¾ MS | 30 | IAA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| ¾ MS | 30 | IBA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| ¾ MS | 30 | NAA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| MS | 15 | - | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| MS | 30 | - | 30 | 1.33 ± 0.21 c | 2.75 ± 0.44 c |
| MS | 60 | - | 20 | 1.25 ± 0.25 c | 1.90 ± 0.33 c |
| MS | 15 | IAA 1.0 | 50 | 3.00 ± 0.30 a | 2.09 ± 0.32 c |
| MS | 30 | IAA 1.0 | 45 | 1.33 ± 0.16 c | 2.12 ± 0.25 c |
| MS | 60 | IAA 1.0 | 70 | 2.50 ± 0.48 ab | 3.95 ± 0.54 bc |
| MS | 15 | IBA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| MS | 30 | IBA 1.0 | 81 | 2.05 ± 0.20 abc | 3.17 ± 0.57 c |
| MS | 60 | IBA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| MS | 15 | NAA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| MS | 30 | NAA 1.0 | 40 | 1.50 ± 0.19 bc | 1.88 ± 0.33 c |
| MS | 60 | NAA 1.0 | 0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Plant Material | DNA Content (pg/2C ± SD) |
|---|---|
| Leaf from field-grown plant | 1.43 ± 0.02 ns |
| Leaf of seedling | 1.41 ± 0.02 |
| Leaf of shoot culture | 1.41 ± 0.02 |
| Content of Selected Groups of Compounds in Extracts [± SD] | |||
|---|---|---|---|
| Total | Leaves from Shoot Culture | Leaves from Field-Grown Plant | Fruits |
| POLYPHENOLS [mg GAE g−1] | 57.42 ± 1.5 | 130.83 ± 2.5 | 26.16 ± 0.80 |
| PHENOLIC ACIDS [mg CA g−1] | 23.62 ± 0.23 | 64.81 ± 0.76 | 18.01 ± 0.37 |
| FLAVONOIDS [mg QE g−1] | 30.51 ± 0.45 | 77.45 ± 2.5 | 0.33 ± 0.00 |
| Method | Extracts IC50 (mg mL−1) | Standard | IC50 (µg mL−1) | ||
|---|---|---|---|---|---|
| Leaves from Shoot Culture | Leaves from Field-Grown Plant | Fruits | |||
| DPPH | 0.09 | 0.04 | 0.14 | - | - |
| CUPRAC | 1.25 | 1.25 | 0.99 | BHA | 1.35 |
| FRAP | 0.31 | 0.33 | 0.27 | BHA | 1.60 |
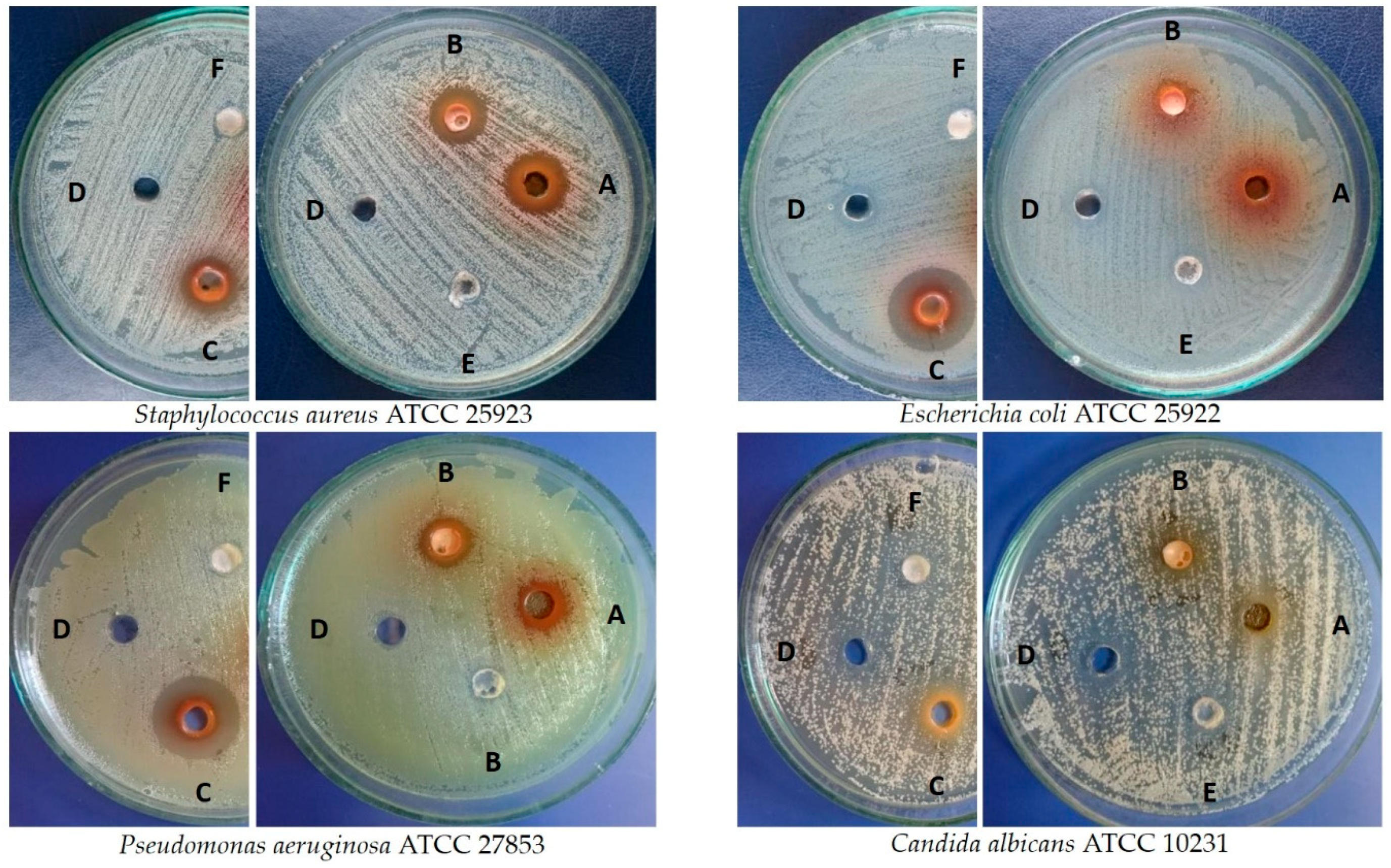
| Microbes | Zone of Inhibition (mm) ± SD against Microbial Strains | |||||||
|---|---|---|---|---|---|---|---|---|
| Leaves from Shoot Culture [5 mg] | Leaves from Field-Grown Plant [5 mg] | Fruits [5 mg] | DMSO | AN 1 [25 µg] | FZ 2 [50 µg] | UA 3 [50 µg] | OA 4 [50 µg] | |
| Staphylococcus aureus ATCC 25923 | 14.3 ± 0.6 | 14.0 ± 0.0 | 14.0 ± 0.0 | 6.0 ± 0.0 | 24.7 ± 0.6 | NT | 6.0 ± 0.0 | 6.0 ± 0.0 |
| Escherichia coli ATCC 25922 | 7.0 ± 1.0 | 7.3 ± 0.6 | 20.0 ± 0.0 | 6.0 ± 0.0 | 26.7 ± 0.6 | NT | 6.0 ± 0.0 | 6.0 ± 0.0 |
| Pseudomonas aeruginosa ATCC 27853 | 11.0 ± 0.0 | 10.7 ± 0.6 | 19.3 ± 0.6 | 6.3 ± 0.6 | 25.3 ± 0.6 | NT | 6.0 ± 0.0 | 6.0 ± 0.0 |
| Candida albicans ATCC 10231 | 8.3 ± 1.2 | 7.3 ± 0.6 | 11.3 ± 0.6 | 6.7 ± 0.6 | NT | 25.0 ± 0.0 | 6.0 ± 0.0 | 6.0 ± 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikowska, M.; Włodarczyk, A.; Rewers, M.; Sliwinska, E.; Studzińska-Sroka, E.; Witkowska-Banaszczak, E.; Stochmal, A.; Żuchowski, J.; Dlugaszewska, J.; Thiem, B. Micropropagation of Chaenomeles japonica: A Step towards Production of Polyphenol-rich Extracts Showing Antioxidant and Antimicrobial Activities. Molecules 2019, 24, 1314. https://doi.org/10.3390/molecules24071314
Kikowska M, Włodarczyk A, Rewers M, Sliwinska E, Studzińska-Sroka E, Witkowska-Banaszczak E, Stochmal A, Żuchowski J, Dlugaszewska J, Thiem B. Micropropagation of Chaenomeles japonica: A Step towards Production of Polyphenol-rich Extracts Showing Antioxidant and Antimicrobial Activities. Molecules. 2019; 24(7):1314. https://doi.org/10.3390/molecules24071314
Chicago/Turabian StyleKikowska, Małgorzata, Agata Włodarczyk, Monika Rewers, Elwira Sliwinska, Elżbieta Studzińska-Sroka, Ewa Witkowska-Banaszczak, Anna Stochmal, Jerzy Żuchowski, Jolanta Dlugaszewska, and Barbara Thiem. 2019. "Micropropagation of Chaenomeles japonica: A Step towards Production of Polyphenol-rich Extracts Showing Antioxidant and Antimicrobial Activities" Molecules 24, no. 7: 1314. https://doi.org/10.3390/molecules24071314