1H-NMR Profiling and Carbon Isotope Discrimination as Tools for the Comparative Assessment of Walnut (Juglans regia L.) Cultivars with Various Geographical and Genetic Origins—A Preliminary Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Geographical and Year of Harvest Discrimination
2.2. Stable Carbon Isotopic Composition
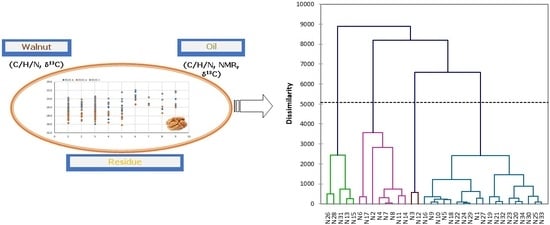
2.3. Composition Discrimination
3. Materials and Methods
3.1. Plant Material and Collection Site
3.2. Sample Preparation
3.3. C/H/N Composition
3.4. NMR Analysis
3.5. δ13C Measurement
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pereira, J.A.; Oliverira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevihno, L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Baciu, A.; Achim, G.; Botu, M.; Trandafir, I. Mineral Composition of fruits in different walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2009, 37, 156–160. [Google Scholar]
- Li, L.; Tsao, R.; Yang, R.; Kramer, J.K.G.; Hernandez, M. Fatty acids profiles, tocopherol contents, and antioxidant activities of heartnut (Juglans ailanthiofolia Var. cordiformis) and Persian walnut (Juglans regia L.). J. Agric. Food Chem. 2007, 55, 1164–1168. [Google Scholar] [CrossRef]
- Tapia, M.I.; Sárnchez-Morgado, J.R.; Garcia-Para, J.; Ramirez, R.; Hernández, T.; Gonzáles Gómez, D. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglas regia L.) cultivars. J. Food Compost. Anal. 2013, 31, 232–237. [Google Scholar] [CrossRef]
- Sabaté, J.; Fraser, G.E.; Burke, K.; Knutsen, S.F.; Bennett, H.; Lindsted, K.D. Effects of walnuts on serum lipid levels and blood pressure in normal men. N. Engl. J. Med. 1993, 328, 603–607. [Google Scholar] [CrossRef]
- Lavedrine, F.; Zmirou, D.; Ravel, A.; Balducci, F.; Alary, J. Blood cholesterol and walnut consumption: A cross-sectional survey in France. Prev. Med. 1999, 28, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglas regia L.) chemical composition and research in human health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef]
- Molnar, T.J.; Zaurov, D.E.; Capik, J.M.; Eisenman, S.W.; Ford, T.; Nikolyi, L.V.; Funk, C.R. Persian Walnut (Juglas regia L.) in central Asia. Annu. Rep. North. Nut Growers Assoc. 2011, 101, 56–69. [Google Scholar]
- Mitra, S.K.; Rathore, D.S.; Bose, T.K. Walnut. In Temperate Fruits; Horticulture and Allied Publishers: Calcutta, India, 1991; pp. 377–414. [Google Scholar]
- Food and Agriculture Organisation of United Nations. Available online: http://www.fao.org/faostat (accessed on 9 April 2018).
- Marcone, M.F.; Wang, S.; Albabish, W.; Nie, S.; Somnarain, D.; Hill, A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res. Int. 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Popescu, R.; Costinel, D.; Dinca, O.R.; Marinescu, A.; Stefanescu, I.; Ionete, R.E. Discrimination of vegetable oils using NMR spectroscopy and chemometrics. Food Control 2015, 48, 84–90. [Google Scholar] [CrossRef]
- Spangenberg, J.E.; Ogrinc, N. Authentication of vegetable oils by bulk and molecular carbon isotope analyses with emphasis on olive oil and pumpkin seed oil. J. Agric. Food Chem. 2001, 49, 1534–1540. [Google Scholar] [CrossRef]
- Paolini, M.; Bontempo, L.; Camin, F. Compund-specific δ13C and δ2H analysis of olive oil fatty acids. Talanta 2017, 174, 38–43. [Google Scholar] [CrossRef]
- Guyader, S.; Thomas, F.; Portaluri, V.; Jamin, E.; Akoka, S.; Silvestre, V.; Remaud, G. Authentication of edible fats and oils by non-targeted 13C INEPT NMR spectroscopy. Food Control 2018, 91, 216–224. [Google Scholar] [CrossRef]
- Esteki, M.; Vander Heyden, Y.; Farajmand, B.; Kolahderazi, Y. Qualitative and quantitative analysis of peanut adulteration in almond powder samples using multi-elemental fingerprinting combined with multivariate data analysis methods. Food Control 2017, 82, 31–41. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P.P. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef] [PubMed]
- Batun, P.; Bakkalbasi, E.; Kazankaya, A.; Cavioglu, I. Fatty acid profiles and mineral contents of walnuts from different provinces of Van Lake. GIDA 2017, 42, 155–162. [Google Scholar] [CrossRef]
- Arranz, S.; Cert, R.; Perez-Jimenez, J.; Cert, A.; Saura-Calixto, F. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008, 110, 985–990. [Google Scholar] [CrossRef]
- Bujdoso, G.; Konya, E.; Berki, M.; Nagy-Gasztonyi, M.; Bartha-Szughi, K.; Marton, B.; Izsepi, F.; Adanyi, N. Fatty acid composition, oxidative stability, and antioxidant properties of some Hungarian and other Persian walnut cultivars. Turk. J. Agric. For. 2016, 40, 160–168. [Google Scholar] [CrossRef]
- Al-Bachir, M. Effect of gamma irradiation on fungal load, chemical and sensory characteristics of walnuts (Juglans regia L.). J. Stored Prod. Res. 2004, 40, 355–362. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lee, M.; Guiet, S.; Winkelmann, W. Study of the main constituents of some authentic walnut oils. J. Agric. Food Chem. 2005, 53, 4853–4860. [Google Scholar] [CrossRef]
- Christopoulos, M.; Tsantili, E. Oil composition in stored walnut cultivars–quality and nutritional value. Eur. J. Lipid Sci. Technol. 2014, 116, 1–11. [Google Scholar] [CrossRef]
- Spangenberg, J.E.; Macko, S.A.; Hunziker, J. Characterization of olive oil by carbon isotope analysis of individual fatty acids: implications for authentication. J. Agric. Food Chem. 1998, 46, 4179–4184. [Google Scholar] [CrossRef]
- Morrison, D.J.; Dodson, B.; Slater, C.; Preston, T. 13C natural abundance in the British diet: implications for 13C breath tests. Rapid Commun. Mass Spectrom. 2000, 14, 1321–1324. [Google Scholar] [CrossRef]
- Guo, L.X.; Xu, X.M.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Characterization and authentication of significant Chinese edible oilseed oils by Stable Carbon Isotope Analysis. J. Am. Oil Chem. Soc. 2010, 87, 839–848. [Google Scholar] [CrossRef]
- Martinez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- National Meteorological Administration-Climate Monitoring. Available online: http://www.meteoromania.ro/anm2/clima/monitorizare-climatica/ (accessed on 9 April 2018).
- Iacumin, P.; Bernini, L.; Boschetti, T. Climatic factors influencing the isotope composition of Italian olive oils and geographic characterization. Rapid Commun. Mass Spectrom. 2009, 23, 448–454. [Google Scholar] [CrossRef]
- Costinel, D.; Tudorache, A.; Ionete, R.E.; Vremera, R. The impact of grape varieties to wine isotopic characterization. Anal. Lett. 2011, 44, 2856–2864. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin, Germany, 1980; Volume 9. [Google Scholar]
- Rubel, F.; Kottek, M. Observed and projected climate shifts 1901-2100 depicted by world maps of the Köppen-Geiger climate classification. Meteorol. Z. 2010, 19, 135–141. [Google Scholar] [CrossRef]
- Constantinescu, M.; Oancea, S.; Bucura, F.; Ciucure, C.; Ionete, R.E. Evaluation of the fuel potential of sewage sludge mixtures with beech sawdust and lignite. J. Renew. Sustain. Ener. 2018, 10, 053106. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Geographical Origin/Composition | All Samples (n = 64) | Germplasm Collection of Fruit Growing Research Station (SCDP) Vâlcea, Grown to RO-VL1 | RO-VL2 Local Selections (n = 3) | RO-DB Red Kernel (n = 1) | GR (n = 2) | PRC (n = 2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA Cultivars (n = 18) | FR Cultivars (n = 7) | RO Cultivars (n = 20) | RO Selections (n = 5) | RO Hybrids (n = 6) | Samples Collected in Both 2015 and 2016 (n = 30) | |||||||||
| 2015 | 2016 | GR-1 | GR-2 | PRC-1 | PRC-2 | |||||||||
| kernel | ||||||||||||||
| Total fat-k (%) | 69.0 ± 7.6 | 66.6 ± 7.1 | 73.6 ± 6.1 | 71.0 ± 9.3 | 66.3 ± 2.3 | 66.5 ± 3.9 | 66.4 ± 4.0 | 73.4 ± 10.7 | 66.7 ± 4.0 | 79.6 | 72.0 | 60.6 | 79.4 | 61.1 |
| Protein-k (%) | 20.3 ± 6.4 | 19.8 ± 7.9 | 19.7 ± 5.9 | 20.1 ± 6.3 | 22.8 ± 4.9 | 26.0 ± 2.2 | 26.2 ± 4.6 | 13.3 ± 2.2 | 18.1 ± 4.5 | 16.7 | 12.9 | 17.4 | 14.6 | 15.6 |
| Carbohydrates-k (%) | 10.7 ± 8.0 | 13.5 ± 9.8 | 6.7 ± 1.8 | 8.9 ± 8.3 | 10.8 ± 5.9 | 7.5 ± 5.5 | 7.3 ± 5.7 | 10.2 ± 0.9 | 15.2 ± 0.9 | 3.7 | 15.1 | 22.1 | 6.0 | 23.2 |
| Energy-k (kcal/100 g) | 700 ± 22 | 703 ± 24 | 699 ± 19 | 699 ± 22 | 693 ± 9 | 702 ± 33 | 696 ± 21 | 709 ± 21 | 693 ± 18 | 713 | 730 | 698 | 704 | 711 |
| oil | ||||||||||||||
| SFA-o (%molar) | 10.6 ± 0.9 | 10.2 ± 0.8 | 10.9 ± 0.7 | 10.7 ± 1.0 | 10.5 ± 0.7 | 10.8 ± 0.8 | 10.7 ± 0.7 | 10.2 ± 0.9 | 11.1 ± 0.7 | 13.4 | 11.0 | 12.3 | 8.9 | 9.3 |
| Oleic-o (%molar) | 22.7 ± 5.2 | 23.6 ± 5.5 | 21.3 ± 3.4 | 23.1 ± 4.2 | 24.7 ± 5.6 | 18.8 ± 3.5 | 19.9 ± 3.2 | 26.1 ± 5.2 | 16.1 ± 2.4 | 20.9 | 19.9 | 20.2 | 38.5 | 31.2 |
| Linoleic-o (%molar) | 55.6 ± 4.6 | 55.2 ± 5.0 | 56.3 ± 2.1 | 55.1 ± 4.0 | 54.8 ± 3.9 | 59.2 ± 5.3 | 58.0 ± 3.3 | 52.7 ± 4.4 | 60.5 ± 1.5 | 54.1 | 55.2 | 55.7 | 43.4 | 50.6 |
| Linolenic-o (%molar) | 11.1 ± 1.6 | 11.1 ± 1.6 | 11.5 ± 1.7 | 11.0 ± 1.4 | 10.1 ± 2.0 | 11.2 ± 2.1 | 11.4 ± 2.0 | 11.0 ± 1.0 | 12.4 ± 0.4 | 11.6 | 14.0 | 11.8 | 9.3 | 8.9 |
| IV-o | 139 ± 4 | 138 ± 4 | 140 ± 4 | 138 ± 4 | 137 ± 6 | 142 ± 2 | 141 ± 4 | 136 ± 4 | 144 ± 1.3 | 134 | 141 | 138 | 126 | 131 |
| PUFA (%molar) | 66.7 ± 4.8 | 66.2 ± 5.0 | 67.8 ± 3.3 | 66.2 ± 4.0 | 64.9 ± 5.2 | 70.4 ± 3.4 | 69.4 ± 3.3 | 63.7 ± 4.7 | 72.9 ± 1.7 | 65.7 | 69.2 | 67.5 | 52.6 | 59.5 |
| UFA/SFA | 8.49 ± 0.83 | 8.9 ± 0.8 | 8.2 ± 0.6 | 8.38 ± 0.78 | 8.58 ± 0.62 | 8.30 ± 0.66 | 8.43 ± 0.63 | 8.87 ± 0.96 | 8.07 ± 0.6 | 6.46 | 8.13 | 7.12 | 10.2 | 9.8 |
| PUFA/SFA | 6.32 ± 0.55 | 6.5 ± 0.5 | 6.3 ± 0.5 | 6.21 ± 0.62 | 6.20 ± 0.39 | 6.55 ± 0.57 | 6.55 ± 0.57 | 6.26 ± 0.51 | 6.61 ± 0.3 | 4.90 | 6.31 | 5.48 | 5.92 | 6.43 |
| Energy-o (kcal/100 g) | 874 ± 69 | 900 ± 56 | 848 ± 60 | 854 ± 71 | 892 ± 11 | 886 ± 11 | 886 ± 18 | 857 ± 104 | 887 ± 9 | 816 | 903 | 903 | 744 | 902 |
| residue | ||||||||||||||
| Protein-r (%) | 41.3 ± 6.7 | 39.8 ± 6.8 | 41.7 ± 2.6 | 40.2 ± 7.6 | 43.7 ± 5.3 | 40.7 ± 6.0 | 42.5 ± 7.9 | 38.7 ± 6.0 | 52.3 ± 6.2 | 34.7 | 39.2 | 44.1 | 45.3 | 46.2 |
| Energy-r (kcal/100 g) | 303 ± 17 | 307 ± 23 | 293 ± 6 | 303 ± 18 | 300 ± 8 | 310 ± 16 | 312 ± 23 | 295 ± 11 | 304 ± 7 | 312 | 286 | 289 | 297 | 300 |
| δ13C-Kernel (‰) | δ13C-Oil (‰) | δ13C-Residue (‰) | ||
|---|---|---|---|---|
| All samples (n = 64) | −26.8 ± 1.5 | −27.4 ± 1.2 | −24.9 ± 1.4 | |
| Samples collected in both years | 2015 (n = 15) | −27.3 ± 1.4 | −27.6 ± 0.8 | −25.6 ± 1.3 |
| 2016 (n = 15) | −26.9 ± 1.4 | −27.1 ± 0.7 | −24.5 ± 0.7 | |
| Samples growth to SCDP | USA cultivars (n = 18) | −27.4 ± 1.2 | −27.5 ± 0.8 | −25.3 ± 1.1 |
| RO selections (n = 5) | −27.1 ± 1.3 | −28.3 ± 1.2 | −25.2 ± 1.3 | |
| RO cultivars (n = 20) | −27.0 ± 1.2 | −27.4 ± 0.9 | −25.2 ± 1.4 | |
| RO hybrids (n = 6) | −26.9 ± 0.9 | −28.0 ± 1.3 | −24.5 ± 1.4 | |
| FR cultivars (n = 7) | −26.8 ± 1.4 | −27.8 ± 0.8 | −25.0 ± 0.7 | |
| Samples from Greece | GR-2 2016 | −26.3 | −27.3 | −24.1 |
| GR-1 2015 | −26.3 | −25.8 | −22.7 | |
| Samples from China | PRC-2 (Zhong Lin no.1) | −25.6 | −25.2 | −22.1 |
| PRC-1 (Jin Bo Feng no.1) | −24.0 | −26.0 | −22.4 | |
| Samples from Romania (other than SCDP) | RO-DB red kernel selection (n = 1) | −25.1 | −25.4 | −23.6 |
| RO-VL (n = 3) | −23.3 ± 1.0 | −24.8 ± 0.8 | −22.7 ± 0.7 | |
| Samples classified by groups | C1 (n = 23) | −27.0 ± 1.7 | −27.6 ± 1.3 | −25.4 ± 1.6 |
| C2 (n = 25) | −26.6 ± 1.2 | −27.4 ± 1.2 | −24.7 ± 1.3 | |
| C3 (n = 6) | −26.8 ± 1.4 | −27.4 ± 1.1 | −24.8 ± 1.1 | |
| C4 (n = 8) | −26.3 ± 1.5 | −26.8 ± 0.7 | −24.1 ± 1.0 | |
| C5 (n = 2) | −28.2 | −26.5 | −24.0 | |
 | ||||
|---|---|---|---|---|
| Group Labels | Group Components | |||
| SCDP–Cultivars | SCDP–Romanian Selections | SCDP–Romanian Hybrids | Other Geographical Origin | |
| C1 (n = 23) | USA: Chase D9-2016, Hartley 2015, Pedro 2015, Tehama 2015, Vina 2015 FR: Fernette 2015, Fernor 2015, Lara 2015, RO: Argesan 2015, Germisara 2015, Muscelean 2015, Orastie 2015, Roxana 2015, Sibisel 44-2015, Sibisel Precoce 2015, Valcris-2015 | Munteanu 2015, VL206S 2015, Vladesti 2015 | HC 02-2015, UC 4/12-2015, T 2/3-2015 | Fumureni 2-2016 (RO-VL2) |
| C1 compositional characteristics: high protein, linoleic, linolenic, IV | ||||
| C2 (n = 25) | USA: Adams 10-2015, Chase D9-2015, Howe 2016, Idaho 2016, Payne 2016, Pedro 2016, Serr 2016, Wilson Franquette 2015/2016 RO: Germisara 2016, Mihaela 2015, Muscelean 2016, Timval 2015, Valcor 2016, Velnita 2015 | Ignat Tudor 2015, VL 301B-2015 | C2-2015, HC3390-2015, UC 3/1-2015 | Brezoi-2015 (RO-VL2), Fumureni 1-2016 (RO-VL2), GR 1-2015, GR 2-2016, PRC 2-2016 (Zhong Lin no.1 cultivar) |
| C2 compositional characteristics: high linoleic, linolenic, IV | ||||
| C3 (n = 6) | USA: Payne 2015 FR: Ferjean 2016, Fernette 2016, Fernor 2016 RO: Jupanesti 2015 | - | - | Targoviste 1-red kernel 2016 (RO-DB) |
| C3 compositional characteristics: high SFA, linoleic, linolenic, IV | ||||
| C4 (n = 8) | USA: Adams 10-2016, FR: Lara 2016 RO: Roxana 2016, Sibisel 44-2016, Timval 2016, Unival 2016, Valmit 2016 | - | - | PRC 1-2016 (Jin Bo Feng no.1 cultivar) |
| C4 compositional characteristics: high fat, oleic, UFA/ SFA; low linoleic, linolenic, IV | ||||
| C5 (n = 2) | USA: Hartley 2016, Vina 2016 | - | - | - |
| C5 compositional characteristics: low fat, proteins; high SFA, linolenic | ||||
| Geographical Origin of the Cultivar | Description | Name | Genetic Origin | Harvest Year |
|---|---|---|---|---|
| Fruit Growing Location-Research Station (RO-VL1), Romania | ||||
| USA (Oregon) | Cultivar | Adams 10 | Open pollinated seedling | 2015 and 2016 |
| Cultivar | Chase D9 | Open pollinated seedling | 2015 and 2016 | |
| Cultivar | Howe | Chance seedling | 2016 | |
| USA (California) | Cultivar | Tehama | ‘Waterloo’ × ‘Payne’ | 2015 |
| Cultivar | Hartley | Open pollinated seedling | 2015 and 2016 | |
| Cultivar | Payne | Chance seedling | 2015 and 2016 | |
| Cultivar | Pedro | ‘Conway Mayette’ × ‘Payne’ | 2015 and 2016 | |
| Cultivar | Vina | ‘Franquette’ × ‘Payne’ | 2015 and 2016 | |
| Cultivar | Wilson Franquette | Selection of ‘Franquette’ | 2015 and 2016 | |
| Cultivar | Serr | ‘Payne’ × PI 159568 | 2016 | |
| USA (Idaho) | Cultivar | Idaho | Selection from local populations | 2016 |
| France | Cultivar | Fernette | ‘Franquette’ × ‘Lara’ | 2015 and 2016 |
| Cultivar | Fernor | ‘Franquette’ × ‘Lara’ | 2015 and 2016 | |
| Cultivar | Lara | Chance seedling of ‘Payne’ | 2015 and 2016 | |
| Cultivar | Ferjean | Grosvert’ × ‘Lara’ | 2016 | |
| Romania (Argeş) | Cultivar | Argesan | Selection from local populations | 2015 |
| Cultivar | Jupâneşti | Selection from local populations | 2015 | |
| Cultivar | Mihaela | Selection from local populations | 2015 | |
| Selection | Ignat Tudor | Selection from local populations | 2015 | |
| Selection | Munteanu | Selection from local populations | 2015 | |
| Selection | Vladesti | Selection from local populations | 2015 | |
| Cultivar | Muscelean | Selection from local populations | 2015 and 2016 | |
| Cultivar | Roxana | Selection from local populations | 2015 and 2016 | |
| Romania (Hunedoara) | Cultivar | Sibisel Precoce | Selection from local populations | 2015 |
| Cultivar | Orastie | Selection from local populations | 2015 | |
| Cultivar | Germisara | Selection from local populations | 2015 and 2016 | |
| Cultivar | Sibişel 44 | Selection from local populations | 2015 and 2016 | |
| Romania (Vâlcea) | Cultivar | Valcris (syn. VL202 PO) | Selection from local populations | 2015 |
| Selection | VL 206 S | Selection from local populations | 2015 | |
| Cultivar | Timval (syn. VL 54 B) | Selection from local populations | 2015 and 2016 | |
| Cultivar | Unival | Selection from local populations | 2016 | |
| Cultivar | Valcor | Selection from local populations | 2016 | |
| Cultivar | Valmit | Selection from local populations | 2016 | |
| Romania (Craiova) | Hybrid | C2 | Open pollinated seedling of ‘Ideal’ | 2015 |
| Hybrid | HC 02 | Open pollinated seedling of ‘Ideal’ | 2015 | |
| Hybrid | HC 3390 | Open pollinated seedling of ‘Ideal’ | 2015 | |
| Hybrid | T2/3 | Open pollinated seedling of ‘Ideal’ | 2015 | |
| Hybrid | UC 3/1 | Open pollinated seedling of ‘Ideal’ | 2015 | |
| Hybrid | UC 4/12 | Open pollinated seedling of ‘Ideal’ | 2015 | |
| Romania (Bucureşti) | Selection | VL 301 B | Selection from local populations | 2015 |
| Romania (Iaşi) | Cultivar | Velniţa | Selection from local populations | 2015 |
| Fruit Growing Location-Vâlcea county (RO-VL2), Romania | ||||
| Romania (Vâlcea) | Selection | Brezoi 1 | Selection from local populations | 2016 |
| Selection | Fumureni 1 | Selection from local populations | 2016 | |
| Selection | Fumureni 2 | Selection from local populations | 2016 | |
| Fruit Growing Location-Dâmboviţa county (RO-DB), Romania | ||||
| Romania (Dâmboviţa) | Selection | Târgovişte 1-red kernel | Selection from local populations | 2016 |
| Fruit Growing Location-Tripoli (GR), Greece | ||||
| Greece (Tripoli) | Cultivar | Nut sample 1 | Franquette’ × ‘Hartley’ x ‘Chandler’ × ‘Meylannaise’ | 2015 |
| Greece (Tripoli) | Cultivar | Nut sample 2 | 2016 | |
| Fruit Growing Location-PRC | ||||
| China (Fruit Growing Institute in Taigu-Shanxi) | Cultivar | Jin Bo Feng no.1 | Unknown | 2016 |
| China (Taiyuan market, Shanxi) | Cultivar | Zhong Lin no.1 | Unknown | 2016 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, R.; Ionete, R.E.; Botoran, O.R.; Costinel, D.; Bucura, F.; Geana, E.I.; ’Alabedallat, Y.F.J.; Botu, M. 1H-NMR Profiling and Carbon Isotope Discrimination as Tools for the Comparative Assessment of Walnut (Juglans regia L.) Cultivars with Various Geographical and Genetic Origins—A Preliminary Study. Molecules 2019, 24, 1378. https://doi.org/10.3390/molecules24071378
Popescu R, Ionete RE, Botoran OR, Costinel D, Bucura F, Geana EI, ’Alabedallat YFJ, Botu M. 1H-NMR Profiling and Carbon Isotope Discrimination as Tools for the Comparative Assessment of Walnut (Juglans regia L.) Cultivars with Various Geographical and Genetic Origins—A Preliminary Study. Molecules. 2019; 24(7):1378. https://doi.org/10.3390/molecules24071378
Chicago/Turabian StylePopescu, Raluca, Roxana Elena Ionete, Oana Romina Botoran, Diana Costinel, Felicia Bucura, Elisabeta Irina Geana, Yazan Falah Jadee ’Alabedallat, and Mihai Botu. 2019. "1H-NMR Profiling and Carbon Isotope Discrimination as Tools for the Comparative Assessment of Walnut (Juglans regia L.) Cultivars with Various Geographical and Genetic Origins—A Preliminary Study" Molecules 24, no. 7: 1378. https://doi.org/10.3390/molecules24071378
APA StylePopescu, R., Ionete, R. E., Botoran, O. R., Costinel, D., Bucura, F., Geana, E. I., ’Alabedallat, Y. F. J., & Botu, M. (2019). 1H-NMR Profiling and Carbon Isotope Discrimination as Tools for the Comparative Assessment of Walnut (Juglans regia L.) Cultivars with Various Geographical and Genetic Origins—A Preliminary Study. Molecules, 24(7), 1378. https://doi.org/10.3390/molecules24071378