Copper-Catalyzed Redox Coupling of Nitroarenes with Sodium Sulfinates
Abstract
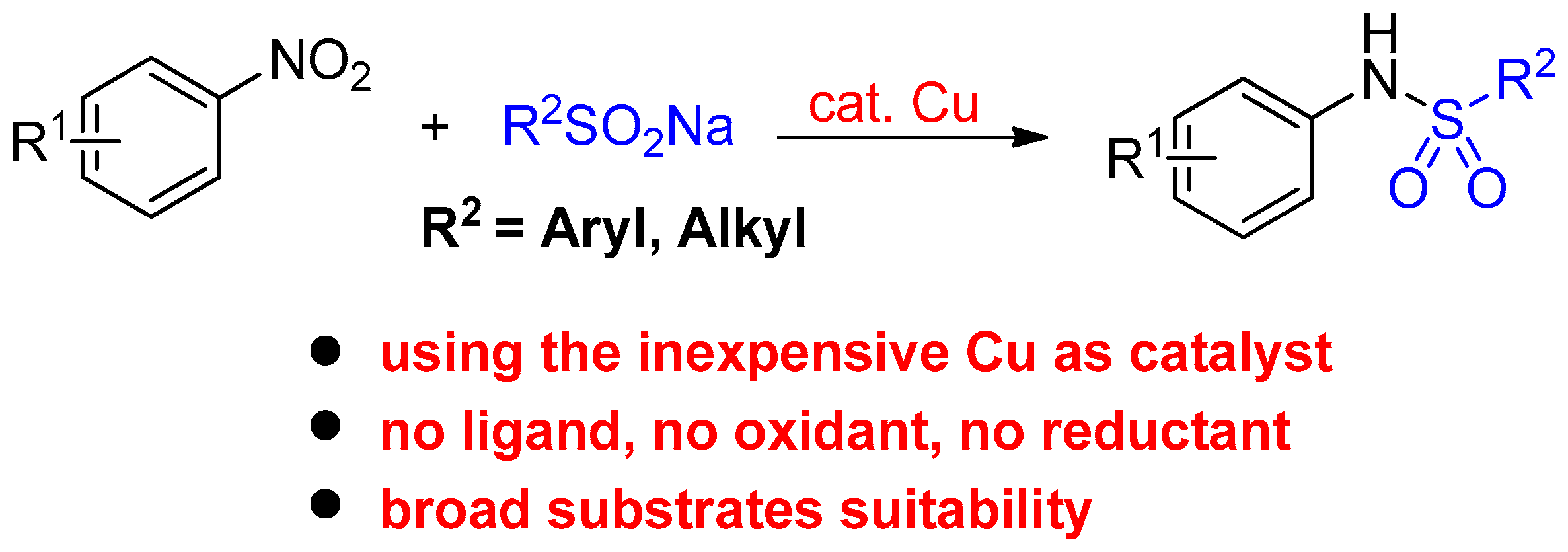
:1. Introduction
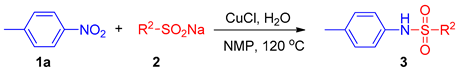
2. Results
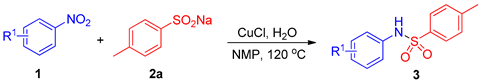
2.1. Optimization of Reaction Conditions for Synthesis of 4-Methyl-N-(p-tolyl)benzenesulfonamide 3a
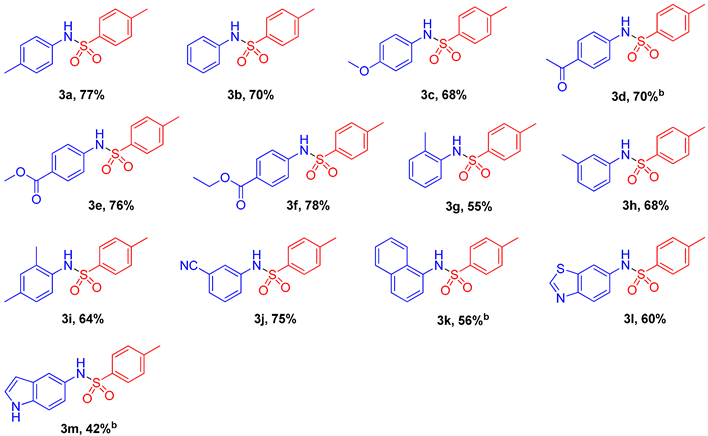
2.2. Substrate Scope for the Nitroarenes
2.3. Substrate Scope for the Sodium Sulfinates
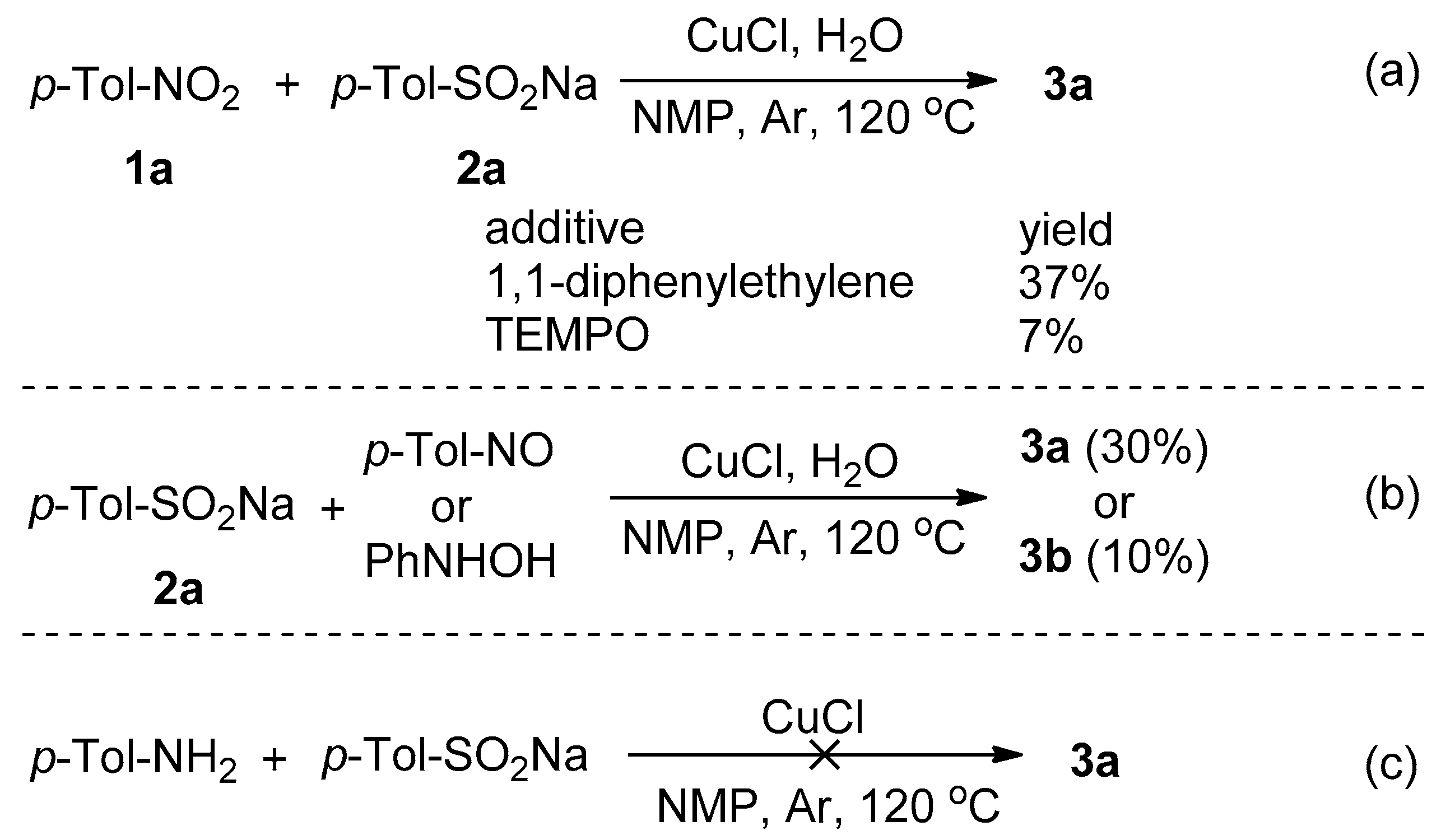
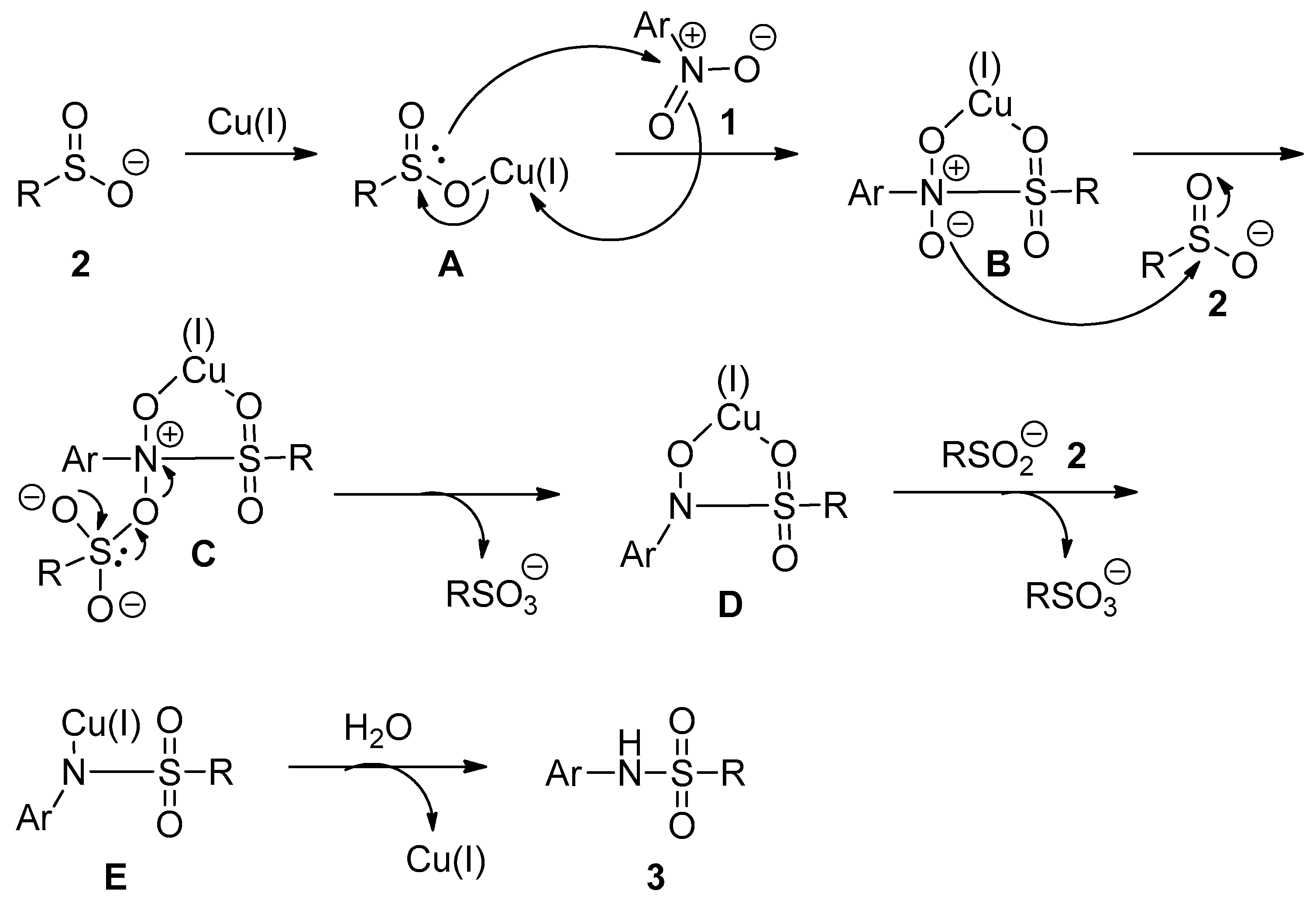
2.4. Mechanism
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Preparation of Sodium Sulfinates
3.3. General Procedure for the Synthesis of N-Arylsulfonamides
3.4. Product Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kerr, J.M.; Suckling, C.J.; Bamfield, P. Selective Hydrogenation by A Novel Palladium(II) Complex. Tetrahedron Lett. 1988, 29, 5545–5548. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Zhang, R.; Chatterjee, M.; Ikushima, Y.; Arai, M. Hydrogenation of Nitrobenzene with Supported Transition Metal Catalysts in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2004, 346, 661–668. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P.; Concepcion, P.; Calvino, J.J. Transforming Nonselective into Chemoselective Metal Catalysts for the Hydrogenation of Substituted Nitroaromatics. J. Am. Chem. Soc. 2008, 130, 8748–8753. [Google Scholar] [CrossRef]
- Cantillo, D.; Baghbanzadeh, M.; Kappe, C.O. In Situ Generated Iron Oxide Nanocrystals as Efficient and Selective Catalysts for the Reduction of Nitroarenes using a Continuous Flow Method. Angew. Chem. Int. Ed. 2012, 51, 10190–10193. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.E.; Junge, H.; Pohl, M.M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-Based Catalysts for Selective Hydrogenation of Nitroarenes to Anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.K.; Yang, H.L.; Li, Y.; Guo, X.W. Cobalt-Modified Molybdenum Carbide as an Efficient Catalyst for Chemoselective Reduction of Aromatic Nitro Compounds. Green Chem. 2014, 16, 1274–1281. [Google Scholar] [CrossRef]
- Ono, N. The Nitro Group in Organic Synthesis; Wiley-VCH: New York, NY, USA, 2001. [Google Scholar]
- Lawrence, S. Amines: Synthesis, Properties and Applications; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Arnold, H.; Döbert, F.; Gaube, J. In Handbook of Heterogeneous Catalysis; Wiley-Interscience: New York, NY, USA, 2008; pp. 3266–3284. [Google Scholar]
- Srivastava, R.S.; Nicholas, K.M. Iron-Catalyzed Allylic Amination by Nitroorganics. Chem. Comm. 1998, 24, 2705–2706. [Google Scholar] [CrossRef]
- Chen, J.Z.; Ling, G.; Lu, S.W. Synthesis of N-Phenyl-N’-pyrimidylurea Derivatives by Selenium- or Selenium Dioxide-Catalyzed Reductive Carbonylation of Nitroaromatics. Eur. J. Org. Chem. 2003, 17, 3446–3452. [Google Scholar] [CrossRef]
- Li, M.; Hu, L.Q.; Cao, X.H.; Hong, Y.; Lu, J.M.; Gu, H.W. Direct Hydrogenation of Nitroaromatics and One-Pot Amidation with Carboxylic Acids over Platinum Nanowires. Chem. Eur. J. 2011, 17, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Pan, C.; Jing, Y.; Qin, T.; Lo, J.; Lee, B.; Spergel, S.; Mertzman, M.; Pitts, W.; Cruz, T.; et al. Practical Olefin Hydroamination with Nitroarenes. Science 2015, 348, 886–891. [Google Scholar] [CrossRef]
- Oakdale, J.; Solano, D.; Fettinger, J.; Haddadin, M.; Kurth, M.J. An Oxazolo[3,2-b]indazole Route to 1H-Indazolones. Org. Lett. 2009, 11, 2760–2763. [Google Scholar] [CrossRef]
- Cui, X.J.; Zhang, Y.; Shi, F.; Deng, Y.Q. Ruthenium-Catalyzed Nitro and Nitrile Compounds Coupling with Alcohols: Alternative Route for N-Substituted Amine Synthesis. Chem. Eur. J. 2011, 17, 2587–2591. [Google Scholar] [CrossRef]
- Zanardi, A.; Mata, J.A.; Peris, E. One-Pot Preparation of Imines from Nitroarenes by a Tandem Process with an Ir-Pd Heterodimetallic Catalyst. Chem. Eur. J. 2010, 16, 10502–10506. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.H.; He, L.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Direct One-Pot Reductive N-Alkylation of Nitroarenes by using Alcohols with Supported Gold Catalysts. Chem. Eur. J. 2011, 17, 7172–7177. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Al-Mourabit, A. Iron Sulfide Catalyzed Redox/Condensation Cascade Reaction between 2-Amino/Hydroxy Nitrobenzenes and Activated Methyl Groups: A Straightforward Atom Economical Approach to 2-Hetaryl-benzimidazoles and -benzoxazoles. J. Am. Chem. Soc. 2013, 135, 118–121. [Google Scholar] [CrossRef]
- Cui, X.J.; Deng, Y.Q.; Shi, F. Reductive N-Alkylation of Nitro Compounds to N-Alkyl and N,N-Dialkyl Amines with Glycerol as the Hydrogen Source. ACS Catal. 2013, 3, 808–811. [Google Scholar] [CrossRef]
- Feng, C.; Liu, Y.; Peng, S.M.; Shuai, Q.; Deng, G.J.; Li, C.J. Ruthenium-Catalyzed Tertiary Amine Formation from Nitroarenes and Alcohols. Org. Lett. 2010, 12, 4888–4891. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Feng, C.; Deng, G.J. Ruthenium-Catalyzed One-Pot Aromatic Secondary Amine Formation from Nitroarenes and Alcohols. Chem. Asian J. 2011, 6, 1142–1146. [Google Scholar] [CrossRef]
- Cano, R.; Ramón, D.J.; Yus, M. Impregnated Ruthenium on Magnetite as a Recyclable Catalyst for the N-Alkylation of Amines, Sulfonamides, Sulfinamides, and Nitroarenes Using Alcohols as Electrophiles by a Hydrogen Autotransfer Process. J. Org. Chem. 2011, 76, 5547–5557. [Google Scholar] [CrossRef]
- Xiao, F.H.; Liu, Y.; Tang, C.L.; Deng, G.J. Peroxide-Mediated Transition-Metal-Free Direct Amidation of Alcohols with Nitroarenes. Org. Lett. 2012, 14, 984–987. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hu, X.; Liu, J.; Liao, Y.F.; Deng, G.J. Iron-Catalyzed 2-Arylbenzoxazole Formation from o-Nitrophenols and Benzylic Alcohols. Org. Lett. 2012, 14, 2722–2725. [Google Scholar] [CrossRef]
- Wang, H.M.; Cao, X.X.; Xiao, F.H.; Liu, S.W.; Deng, G.J. Iron-Catalyzed One-Pot 2,3-Diarylquinazolinone Formation from 2‑Nitrobenzamides and Alcohols. Org. Lett. 2013, 15, 4900–4903. [Google Scholar] [CrossRef]
- Wang, H.M.; Chen, H.; Chen, Y.; Deng, G.J. Palladium-Catalyzed One Pot 2-Arylquinazoline Formation via Hydrogen-Transfer Strategy. Org. Biomol. Chem. 2014, 12, 7792–7799. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Al-Mourabit, A. Nitro-Methyl Redox Coupling: Efficient Approach to 2-Hetarylbenzothiazoles from 2-Halonitroarene, Methylhetarene, and Elemental Sulfur. Org. Lett. 2013, 15, 4218–4221. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Retailleau, P.; Al-Mourabit, A. A Simple and Straightforward Approach to Quinoxalines by Iron/Sulfur-Catalyzed Redox Condensation of o-Nitroanilines and Phenethylamines. Org. Lett. 2013, 15, 5238–5241. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Bescont, J.L.; Ermolenko, L.; Al-Mourabit, A. Cobalt- and Iron-Catalyzed Redox Condensation of o-Substituted Nitrobenzenes with Alkylamines: A Step- and Redox-Economical Synthesis of Diazaheterocycles. Org. Lett. 2013, 15, 6218–6221. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Pasturaud, K.; Ermolenko, L.; Al-Mourabit, A. Concise Access to 2‑Aroylbenzothiazoles by Redox Condensation Reaction between o‑Halonitrobenzenes, Acetophenones, and Elemental Sulfur. Org. Lett. 2015, 17, 2562–2565. [Google Scholar] [CrossRef]
- Xing, Q.Y.; Ma, Y.F.; Xie, H.; Xiao, F.H.; Zhang, F.; Deng, G.J. Iron-Promoted Three-Component 2-Substituted Benzothiazole Formation via Nitroarene ortho C-H Sulfuration with Elemental Sulfur. J. Org. Chem. 2019, 84, 1238–1246. [Google Scholar] [CrossRef]
- Ganguly, A.K.; Alluri, S.S.; Caroccia, D.; Biswas, D.; Wang, C.H.; Kang, E.; Zhang, Y.; McPhail, A.T.; Carroll, S.S.; Burlein, C.; et al. Design, Synthesis, and X-ray Crystallographic Analysis of a Novel Class of HIV-1 Protease Inhibitors. J. Med. Chem. 2011, 54, 7176–7183. [Google Scholar] [CrossRef]
- Gilbert, A.M.; Caltabiano, S.; Koehn, F.E.; Chen, Z.-J.; Francisco, G.D.; Ellingboe, J.W.; Kharode, Y.; Mangine, A.; Francis, R.; TrailSmith, M.; et al. Pyrazolopyrimidine-2,4-dione Sulfonamides: Novel and Selective Calcitonin Inducers. J. Med. Chem. 2002, 45, 2342–2345. [Google Scholar] [CrossRef]
- Masereel, B.; Rolin, S.; Abbate, F.; Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors: Anticonvulsant Sulfonamides Incorporating Valproyl and Other Lipophilic Moieties. J. Med. Chem. 2002, 45, 312–320. [Google Scholar] [CrossRef]
- Drew, J. Drug Discovery: A Historical Perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef]
- Wilden, J.; Geldeard, L.; Lee, C.; Judd, D.; Caddick, S. Trichlorophenol (TCP) Sulfonate Esters: A Selective Alternative to Pentafluorophenol (PFP) Esters and Sulfonyl Chlorides for The Preparation of Sulfonamides. Chem. Commun. 2007, 10, 1074–1076. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.; Soheilizad, M. Direct Conversion of Thiols to Sulfonyl Chlorides and Sulfonamides. J. Org. Chem. 2009, 74, 9287–9291. [Google Scholar] [CrossRef]
- Zhu, M.; Wei, W.; Yang, D.; Cui, H.; Wang, L.; Meng, G.; Wang, H. Metal-Free I2O5-Mediated Direct Construction of Sulfonamides from Thiols and Amines. Org. Biomol. Chem. 2017, 15, 4789–4793. [Google Scholar] [CrossRef]
- Sheykhan, M.; Khani, S.; Abbasnia, M.; Shaabanzadeh, S.; Joafshan, M. An Approach to C–N Activation: Coupling of Arenesulfonyl Hydrazides and Arenesulfonyl Chlorides with Tert-Amines via A Metal-, Oxidant- and Halogen-free Anodic Oxidation. Green Chem. 2017, 19, 5940–5948. [Google Scholar] [CrossRef]
- DeBergh, J.R.; Niljianskul, N.; Buchwald, S.L. Synthesis of Aryl Sulfonamides via Palladium-Catalyzed Chlorosulfonylation of Arylboronic Acids. J. Am. Chem. Soc. 2013, 135, 10638–10641. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Huang, L.; Qi, C.; Wu, X.; Wu, W.; Jiang, H. Copper-Catalyzed Sulfonamides Formation from Sodium Sulfinates and Amines. Chem. Commun. 2013, 49, 6102–6104. [Google Scholar] [CrossRef]
- Buathongjan, C.; Beukeaw, D.; Yotphan, S. Iodine-Catalyzed Oxidative Amination of Sodium Sulfinates: A Convenient Approach to the Synthesis of Sulfonamides under Mild Conditions. Eur. J. Org. Chem. 2015, 7, 1575–1582. [Google Scholar] [CrossRef]
- Pan, X.; Gao, J.; Liu, J.; Lai, J.; Jiang, H.; Yuan, G. Synthesis of Sulfonamides via I2-Mediated Reaction of Sodium Sulfinates with Amines in An Aqueous Medium at Room Temperature. Green. Chem. 2015, 17, 1400–1403. [Google Scholar] [CrossRef]
- Yang, K.; Ke, M.; Lin, Y.; Song, Q. Sulfonamide Formation from Sodium Sulfinates and Amines or Ammonia under Metal-free Conditions at Ambient Temperature. Green Chem. 2015, 17, 1395–1399. [Google Scholar] [CrossRef]
- Sarkar, D.; Ghosh, M.K.; Rout, N. PTAB Mediated Open Air Synthesis of Sulfonamides, Thiosulfonates and Symmetrical Disulfanes. Tetrahedron Lett. 2018, 59, 2360–2364. [Google Scholar] [CrossRef]
- Baffoe, J.; Hoe, M.; Touré, B. Copper-Mediated N-Heteroarylation of Primary Sulfonamides: Synthesis of Mono-N-heteroaryl Sulfonamides. Org. Lett. 2010, 12, 1532–1535. [Google Scholar] [CrossRef]
- Burton, G.; Cao, P.; Li, G.; Rivero, R. Palladium-Catalyzed Intermolecular Coupling of Aryl Chlorides and Sulfonamides under Microwave Irradiation. Org. Lett. 2003, 5, 4373–4376. [Google Scholar] [CrossRef]
- Rosen, B.R.; Ruble, J.C.; Beauchamp, T.J.; Navarro, A. Mild Pd-Catalyzed N-Arylation of Methanesulfonamide and Related Nucleophiles: Avoiding Potentially Genotoxic Reagents and Byproducts. Org. Lett. 2011, 13, 2564–2567. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Ehsani, A.; Maham, M. Copper-Catalyzed N-Arylation of Sulfonamides with Boronic Acids in Water under Ligand-Free and Aerobic Conditions. Synlett 2014, 25, 505–508. [Google Scholar] [CrossRef]
- Nasrollahzadeha, M.; Rostami-Vartooni, A.; Ehsani, A.; Moghadam, M. Fabrication, Characterization and Application of Nanopolymer Supported Copper (II) Complex as an Effective and Reusable Catalyst for the C-N Bond Cross-Coupling Reaction of Sulfonamides with Arylboronic Acids in Water under Aerobic Conditions. J. Mol. Catal. A: Chem. 2014, 387, 123–129. [Google Scholar] [CrossRef]
- Cao, X.; Bai, Y.; Xie, Y.; Deng, G.J. Palladium-Catalyzed Arylation of Aryl Sulfonamides with Cyclohexanones. J. Mol. Catal. A: Chem. 2014, 383, 94–100. [Google Scholar] [CrossRef]
- Qu, P.; Sun, C.; Ma, J.; Li, F. The N-Alkylation of Sulfonamides with Alcohols in Water Catalyzed by the Water-Soluble Iridium Complex {Cp*Ir[6,6 (OH)2bpy](H2O)}[OTf]2. Adv. Synth. Catal. 2014, 356, 447–459. [Google Scholar] [CrossRef]
- Ramachandran, R.; Prakash, G.; Selvamurugan, S.; Viswanathamurthi, P.; Malecki, J.G.; Ramkumar, V. Efficient and Aersatile Catalysis of N-alkylation of Heterocyclic Amines with Alcohols and One-Pot Synthesis of 2-Aryl Substituted Benzazoles with Newly Designed Ruthenium(II) Complexes of PNS Thiosemicarbazones. Dalton Trans. 2014, 43, 7889–7902. [Google Scholar] [CrossRef]
- Li, Q.Q.; Xiao, Z.F.; Yao, C.Z.; Zheng, H.X.; Kang, Y.B. Direct Alkylation of Amines with Alcohols Catalyzed by Base. Org. Lett. 2015, 17, 5328–5331. [Google Scholar] [CrossRef]
- Shekhar, S.; Dunn, T.B.; Kotechi, B.; Montavon, D.; Cullen, S.C. A General Method for Palladium-Catalyzed Reactions of Primary Sulfonamides with Aryl Nonaflates. J. Org. Chem. 2011, 76, 4552–4563. [Google Scholar] [CrossRef]
- Xiao, B.; Gong, T.J.; Xu, J.; Liu, Z.J.; Liu, L. Palladium-Catalyzed Intermolecular Directed C−H Amidation of Aromatic Ketones. J. Am. Chem. Soc. 2011, 133, 1466–1474. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Shang, Y.P.; Su, W.P. Rhodium(III)-Catalyzed Intermolecular N‑Chelator-Directed Aromatic C-H Amidation with Amides. Org. Lett. 2013, 15, 5106–5109. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, J.; Rao, B.; Luo, M. Iron-Catalyzed N‑Arylsulfonamide Formation through Directly Using Nitroarenes as Nitrogen Sources. J. Org. Chem. 2015, 80, 3504–3511. [Google Scholar] [CrossRef]
- Li, X.X.; Chen, F.; Lu, G.P. Fe-Based Metal-Organic Frameworks for The Synthesis of N-Arylsulfonamides via The Reactions of Sodium Arylsulfinates or Arylsulfonyl Chlorides with Nitroarenes in Water. Tetrahedron Lett. 2018, 59, 4226–4230. [Google Scholar] [CrossRef]
- Nadim, E.; Karamé, I.; Andrioletti, B. Straightforward and Sustainable Synthesis of Sulfonamides in Water under Mild Conditions. Eur. J. Org. Chem. 2018, 5016–5022. [Google Scholar]
- Jiang, J.; Zeng, S.; Chen, D.; Cheng, C.; Deng, W.; Xiang, J. Synthesis of N-Arylsulfonamides via Fe-Promoted Reaction of Sulfonyl Halides with Nitroarenes in an Aqueous Medium. Org. Biomol. Chem. 2018, 16, 5016–5020. [Google Scholar] [CrossRef]
- Zhao, F.; Li, B.; Huang, H.W.; Deng, G.J. Palladium-Catalyzed N-Arylsulfonamide Formation from Arylsulfonyl Hydrazides and Nitroarenes. RSC Adv. 2016, 6, 13010–13013. [Google Scholar] [CrossRef]
- Yang, B.; Lian, C.; Yue, G.L.; Liu, D.Y.; Wei, L.Y.; Ding, Y.; Zheng, X.C.; Lu, K.; Qiu, D.; Zhao, X. Synthesis of N-Arylsulfonamides Through A Pd-Catalyzed Reduction Coupling Reaction of Nitroarenes with Sodium Arylsulfinates. Org. Biomol. Chem. 2018, 16, 8150–8154. [Google Scholar] [CrossRef]
- Deruer, E.; Coulibali, S.; Boukercha, S.; Canesi, S. Carbon−Phosphorus Bond Formation on Anilines Mediated by a Hypervalent Iodine Reagent. J. Org. Chem. 2017, 82, 11884–11890. [Google Scholar] [CrossRef]
- Downer, N.K.; Jackson, Y.A. Synthesis of Benzothiazoles via Ipso Substitution of Orthomethoxythiobenzamides. Org. Biomol. Chem. 2004, 2, 3039–3043. [Google Scholar] [CrossRef]
- Natarajan, A.; Guo, Y.H.; Harbinski, F.; Fan, Y.H.; Chen, H.; Luus, L.; Diercks, J.; Aktas, H.; Chorev, M.; Halperin, J.A. Novel Arylsulfoanilide−Oxindole Hybrid as an Anticancer Agent That Inhibits Translation Initiation. J. Med. Chem. 2004, 47, 4979–4982. [Google Scholar] [CrossRef]
- Aissaoui, H.; Koberstein, R.; Zumbrunn, C.; Gatfield, J.; Brisbare-Roch, C.; Jenck, F.; Treiber, A.; Boss, C. N-Glycine-sulfonamides as Potent Dual Orexin 1/Orexin 2 Receptor Antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 5729–5733. [Google Scholar] [CrossRef]
- Sadaf, A.; Priyanka, C.; Popuri, S.; Shahulhameed, S.; Jeyakumar, K. tert-Butyl Nitrite Mediated Nitrogen Transfer Reactions: Synthesis of Benzotriazoles and Azides at Room Temperature. Org. Biomol. Chem. 2018, 16, 8280–8285. [Google Scholar]
Sample Availability: Not available. |
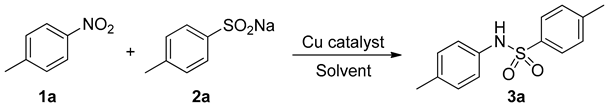
| Entry | Catalyst | Solvent | Yield b (%) |
|---|---|---|---|
| 1 | - | NMP | 10 |
| 2 | FeCl2·4H2O | NMP | 20 |
| 3 | FeSO4·7H2O | NMP | 11 |
| 4 | Cu2(OH)2CO3 | NMP | 31 |
| 5 | Cu(OTf)2 | NMP | 45 |
| 6 | CuCl2 | NMP | 30 |
| 7 | CuI | NMP | 46 |
| 8 | CuBr | NMP | 82 |
| 9 | CuCl | NMP | 92 |
| 10 | Cu | NMP | 86 |
| 11 | CuCl | DMF | 78 |
| 12 | CuCl | DMSO | 75 |
| 13 | CuCl | Anisole | 0 |
| 14 | CuCl | Dioxane | 8 |
| 15 | CuCl | Toluene | 0 |
| 16 | CuCl | Diglyme | 15 |
| 17 | CuCl | NMP | 52 c |
 |
 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Chen, R.; Zhang, J. Copper-Catalyzed Redox Coupling of Nitroarenes with Sodium Sulfinates. Molecules 2019, 24, 1407. https://doi.org/10.3390/molecules24071407
Liu S, Chen R, Zhang J. Copper-Catalyzed Redox Coupling of Nitroarenes with Sodium Sulfinates. Molecules. 2019; 24(7):1407. https://doi.org/10.3390/molecules24071407
Chicago/Turabian StyleLiu, Saiwen, Ru Chen, and Jin Zhang. 2019. "Copper-Catalyzed Redox Coupling of Nitroarenes with Sodium Sulfinates" Molecules 24, no. 7: 1407. https://doi.org/10.3390/molecules24071407
APA StyleLiu, S., Chen, R., & Zhang, J. (2019). Copper-Catalyzed Redox Coupling of Nitroarenes with Sodium Sulfinates. Molecules, 24(7), 1407. https://doi.org/10.3390/molecules24071407





