Biological Activities of Euphorbia peplus Leaves Ethanolic Extract and the Extract Fabricated Gold Nanoparticles (AuNPs)
Abstract
1. Introduction
2. Results
2.1. Characterization of AuNPs
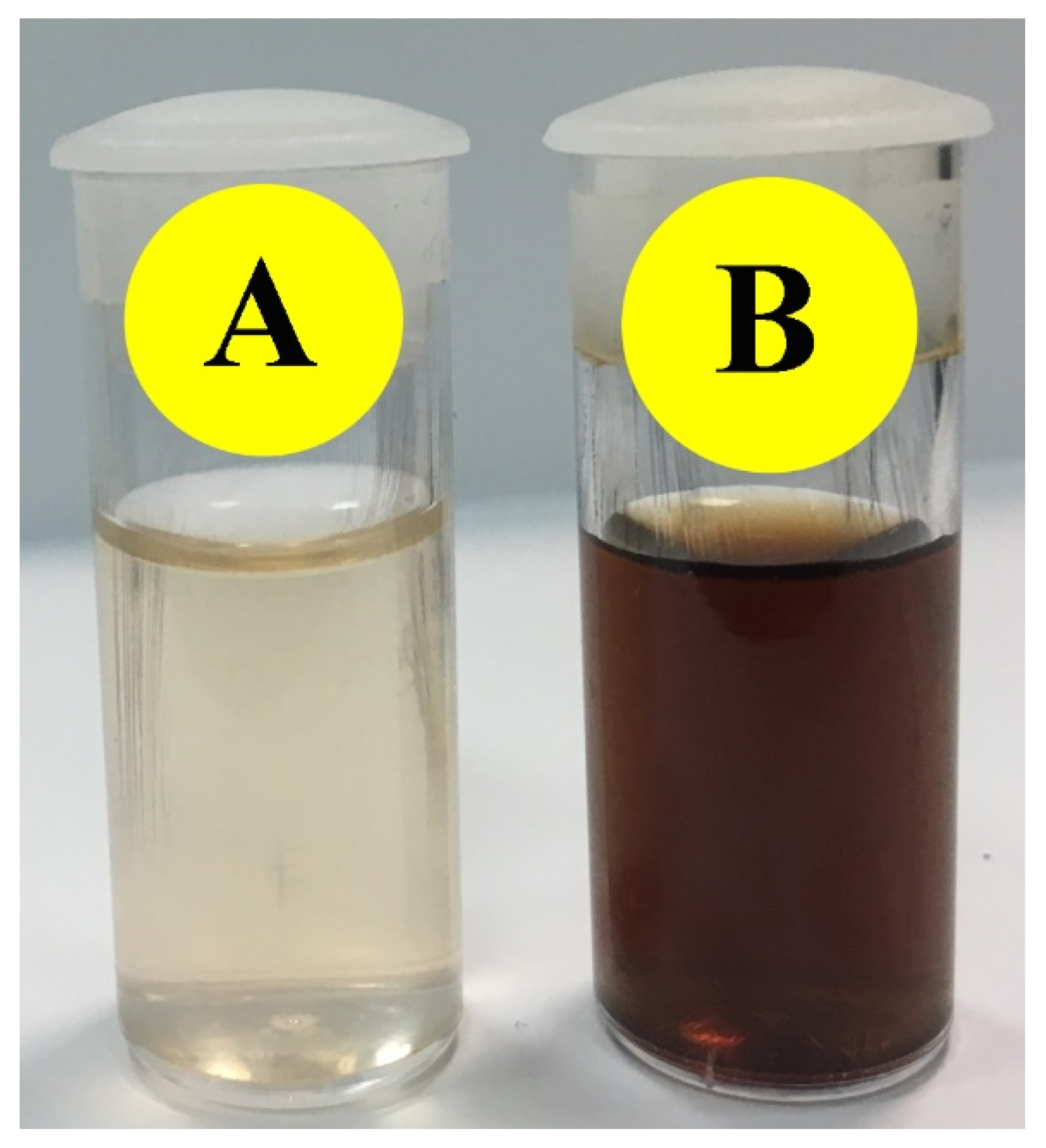
2.1.1. Change in Color
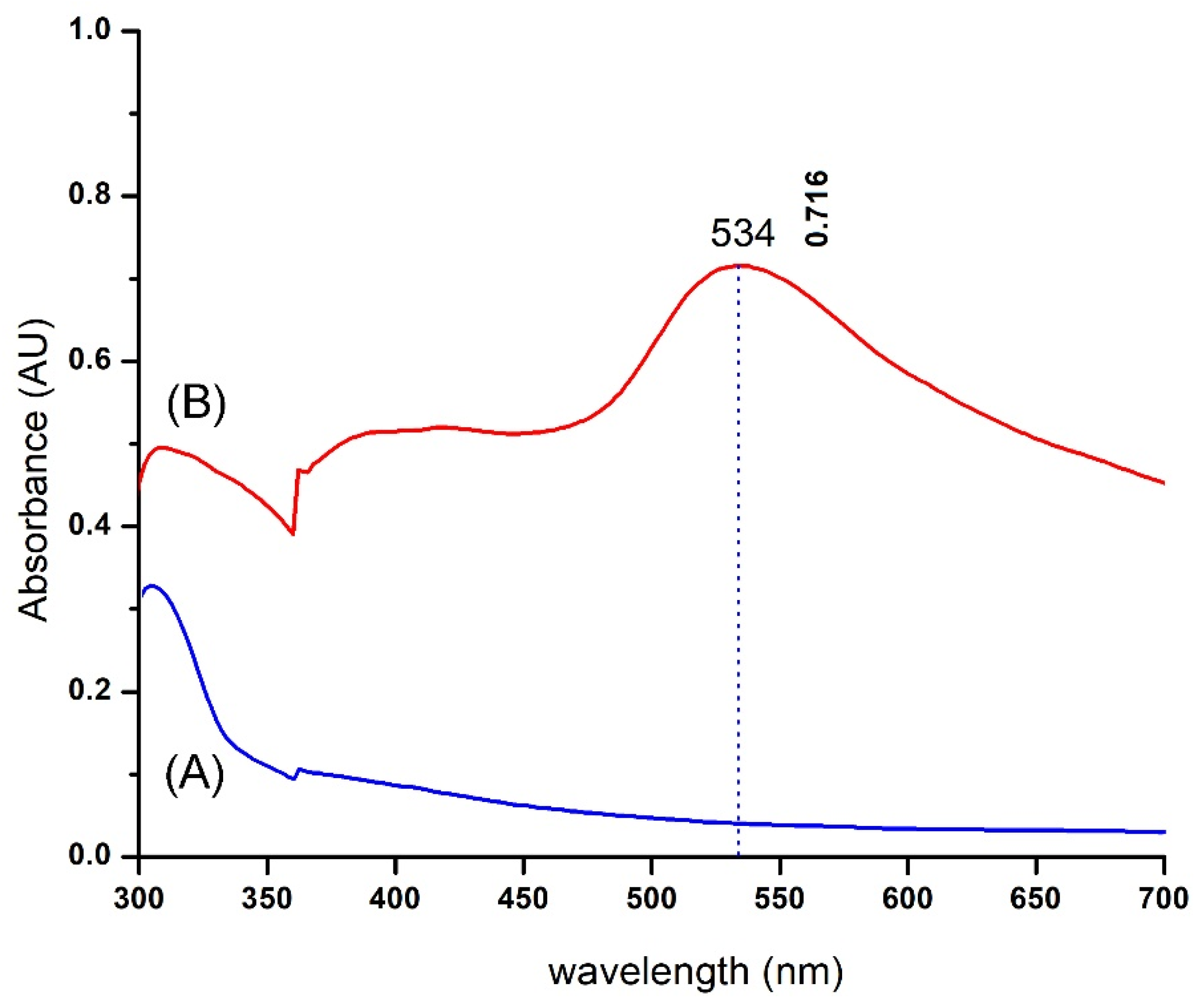
2.1.2. UV–Vis Spectrometry
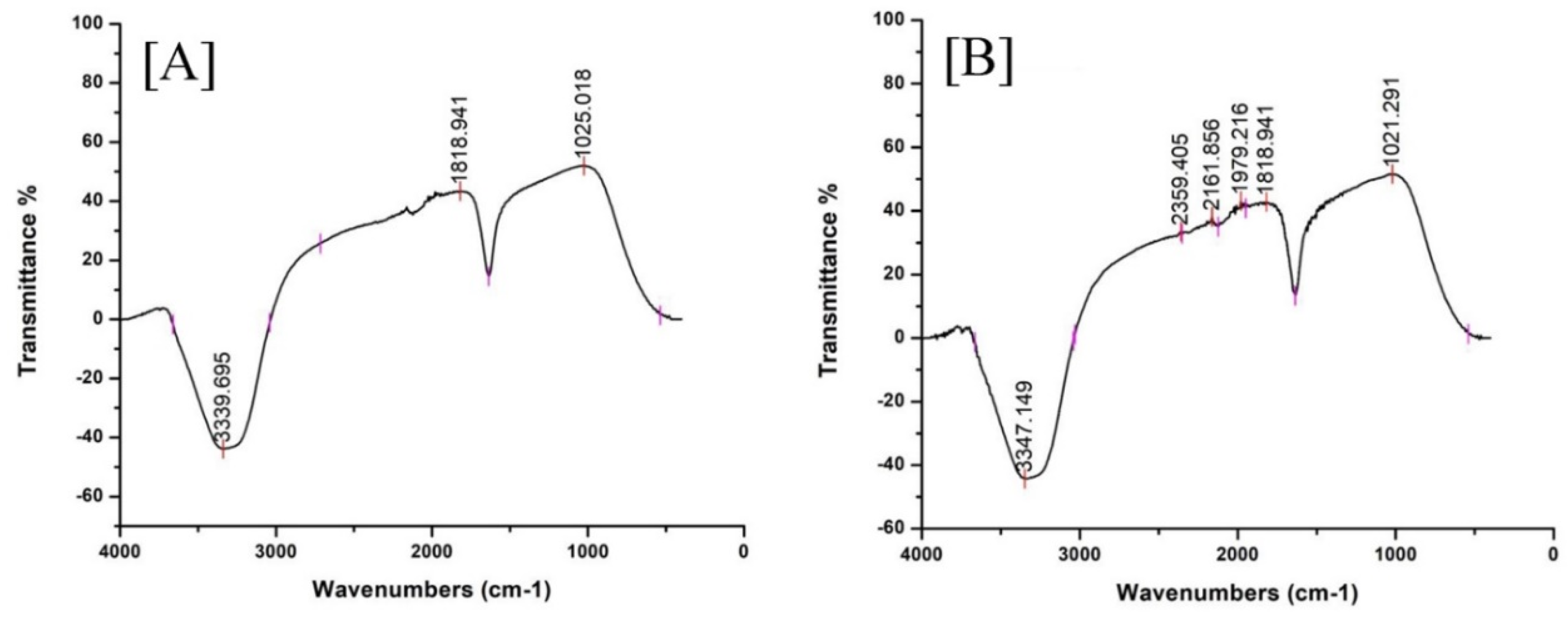
2.1.3. FT-IR of EpExt and EpExt-AuNPs
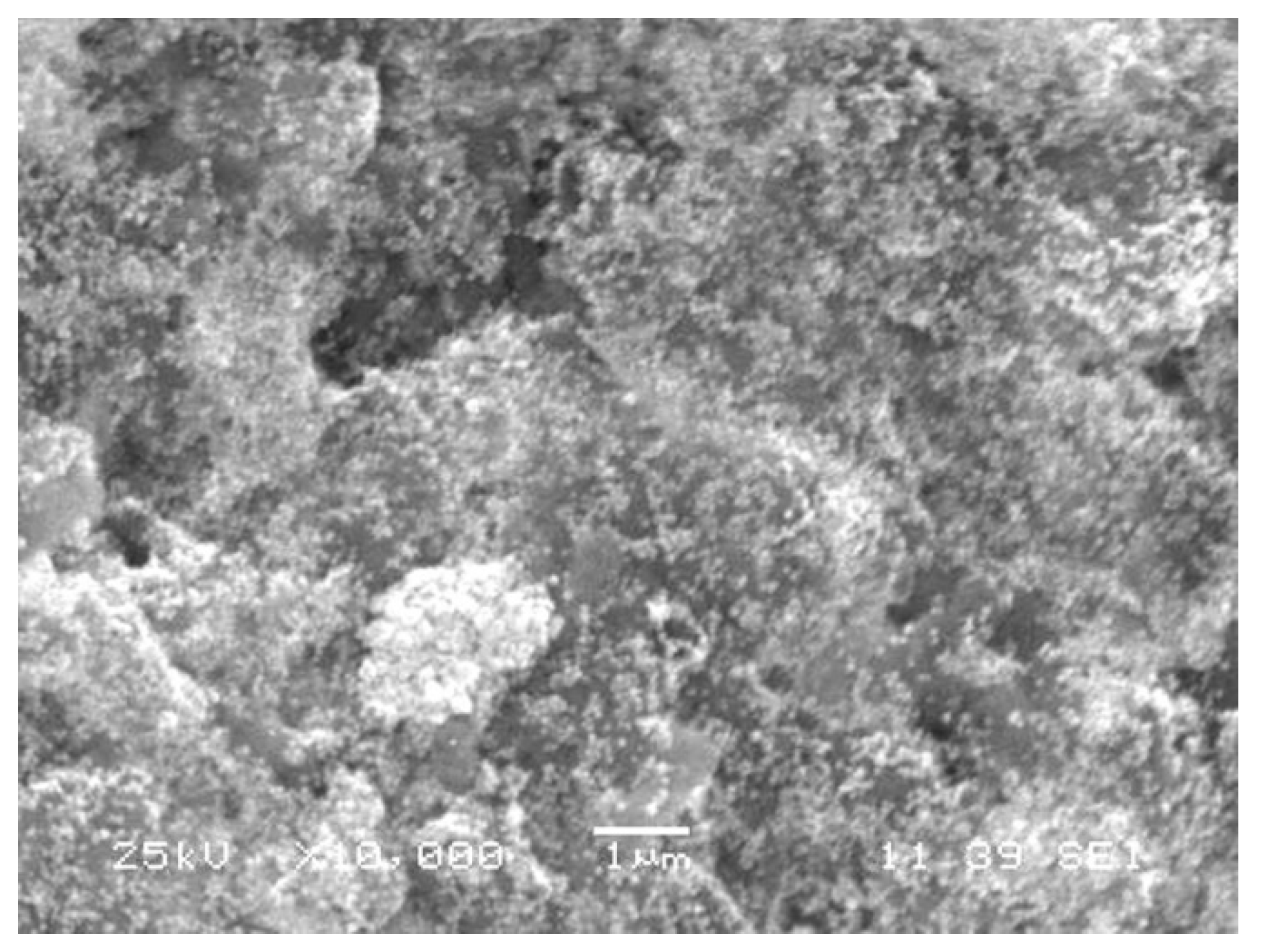
2.1.4. Morphological Characterization Using Scanning Electron Microscopy (SEM)
2.1.5. XRD Analysis of AuNPs Phytofabricated by EpExt
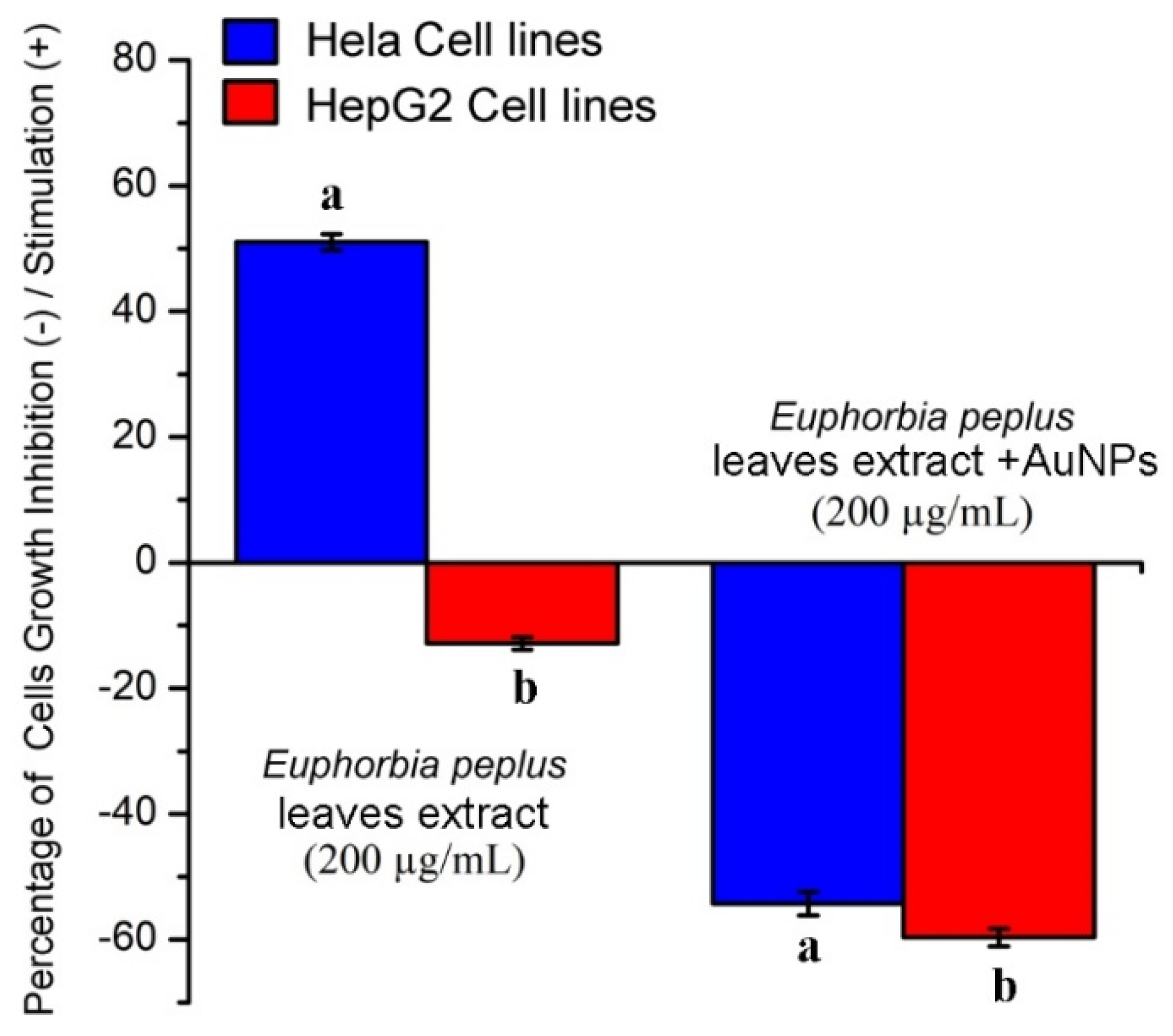
2.2. Effects of EpExt and EpExt-AuNPs on Hela and HepG2 Cancer Cell Lines
2.3. Antimicrobial Activity
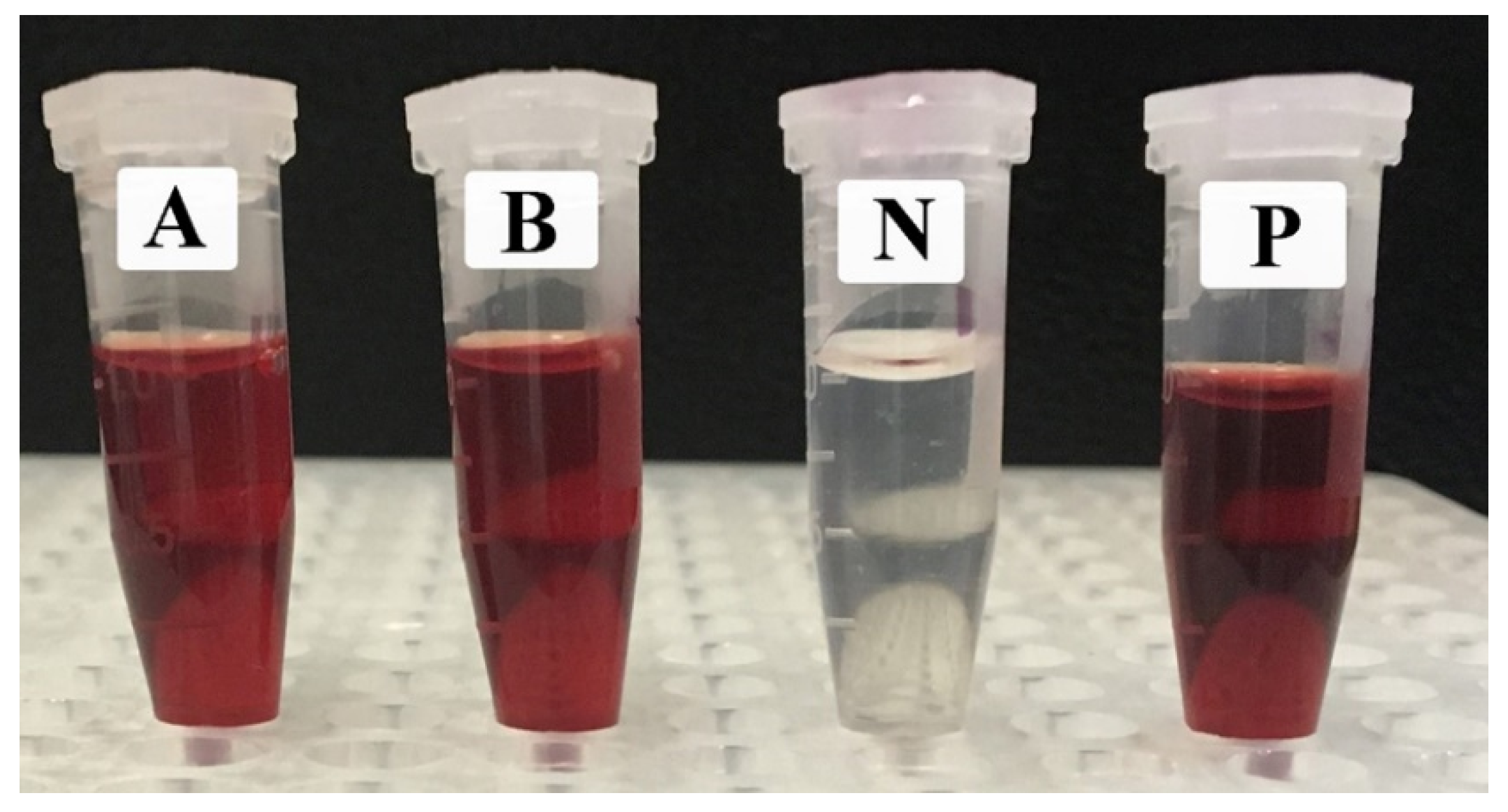
2.4. The Lytic Effects of EpExt and EpExt-AuNPs on Red Blood Cells
2.5. Effects of EpExt and EpExt-AuNPs on Rat Splenic Cell Proliferation
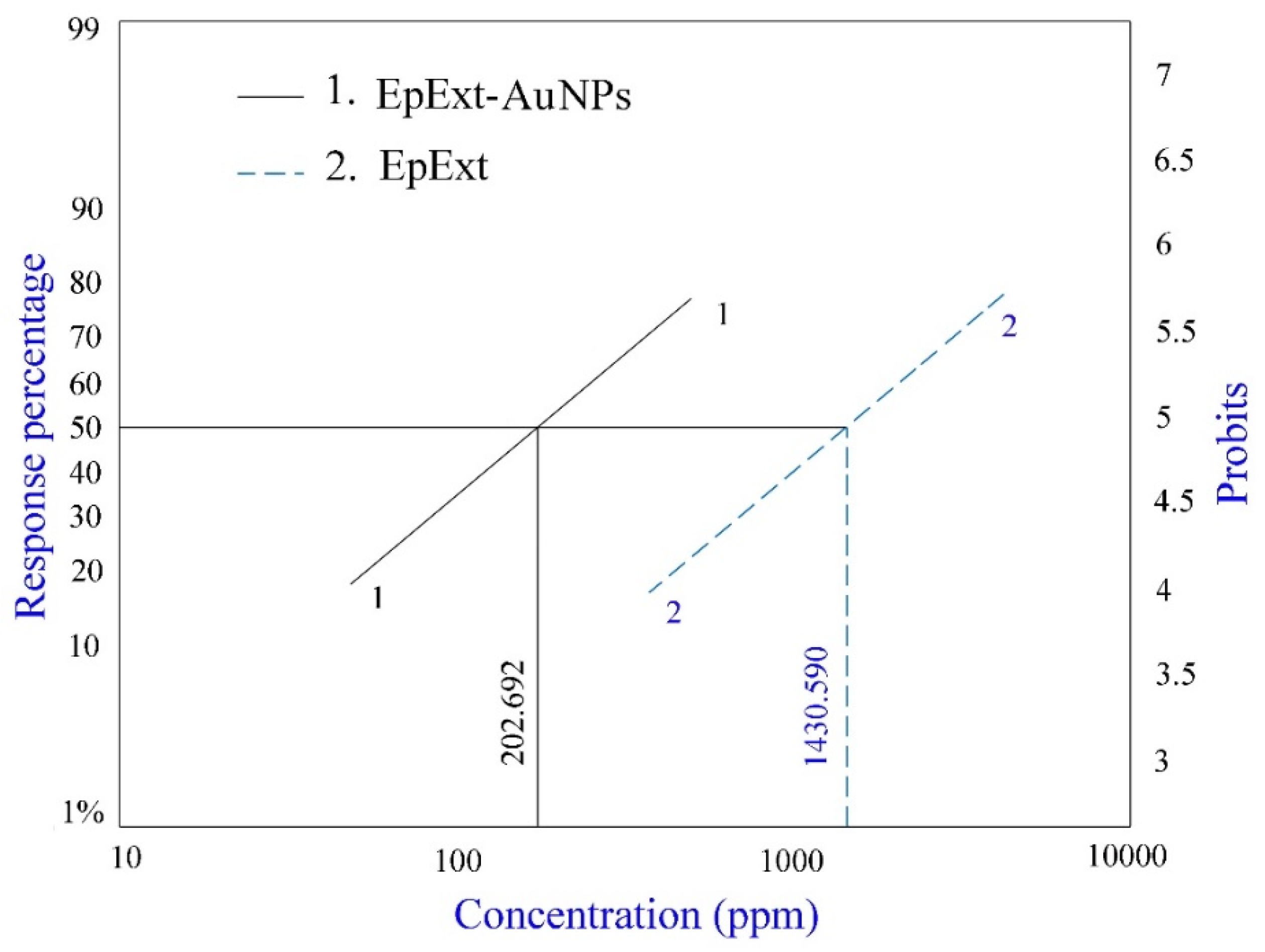
2.6. Effects of EpExt and EpExt-AuNPs on Culex pipiens Larvae
3. Discussion
4. Materials and Methods
4.1. Plant Extract Preparation
4.2. Biosynthesis of Gold Nanoparticles (AuNPs)
4.3. Characterization of Gold Nanoparticles
4.4. Effects of EpExt and EpExt-AuNPs Against Hela and HepG2 Cancer Cell Lines
4.5. Antimicrobial Activity of EpExt and EpExt-AuNPs
4.5.1. Microbial Strains and Media
4.5.2. Well Diffusion Assay for Antimicrobial Activity
4.6. Lytic Effects of EpExt and EpExt-AuNPs on Red Blood Cells (RBCs)
4.7. In Vitro Effects of EpExt and EpExt-AuNPs on Splenic Cells Proliferation
Splenic Cells Culture Preparation
4.8. Insecticidal Effect of EpExt and EpExt-AuNPs on Mosquito (Culex pipiens) Larvae
4.8.1. Collection of Mosquito (Cx. pipiens) Larvae
4.8.2. Larval Bioassay
4.9. Statistical Analysis
4.9.1. Statistical Analysis of Antimicrobial Activity
4.9.2. Statistical Analysis of Larvicidal Bioassay
4.9.3. Statistical Analysis of Biological Activities
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patiño Herrera, R.; Robles-Martínez, M.; González, J.F.C.; Pérez-Vázquez, F.J.; Montejano-Carrizales, J.M.; Pérez, E. Antimycotic activity potentiation of Allium sativum extract and silver nanoparticles against Trichophyton rubrum. Chem. Biodivers. 2019. [Google Scholar] [CrossRef]
- Barla, A.; Bİrman, H.; Kültür, Ş.; Öksüz, S. Secondary metabolites from Euphorbia helioscopia and their vasodepressor activity. Turk. J. Chem. 2006, 30, 325–332. [Google Scholar]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M.; Salaryan, P.; Enayati, A.; Sajjadi, S.A.; Naderi, K. Optimal extraction method of phsenolics from the root of Euphorbia condylocarpa. Chem. Nat. Compd. 2011, 47, 434–435. [Google Scholar] [CrossRef]
- Noori, M.; Chehreghani, A.; Kaveh, M. Flavonoids of 17 species of Euphorbia (Euphorbiaceae) in Iran. Toxicol. Environ. Chem. 2009, 91, 631–641. [Google Scholar] [CrossRef]
- Ali, A.A.; Sayed, H.M.; Ibrahim, S.R.; Zaher, A.M. Chemical constituents, antimicrobial, analgesic, antipyretic, and anti-inflammatory activities of Euphorbia peplus L. Phytopharmacology 2013, 4, 69–80. [Google Scholar]
- Khafagy, S.; Gharbo, S.; Salam, N.A. Phytochemical study of Euphorbia peplus. Planta Med. 1975, 27, 387–394. [Google Scholar] [CrossRef]
- Annamalai, A.; Christina, V.; Sudha, D.; Kalpana, M.; Lakshmi, P. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf. B Biointerfaces 2013, 108, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ikram, S. Synthesis of gold nanoparticles using plant extract: An overview. Nano Res. Appl. 2015, 1, 1–6. [Google Scholar]
- Ganeshkumar, M.; Sastry, T.P.; Kumar, M.S.; Dinesh, M.G.; Kannappan, S.; Suguna, L. Sun light mediated synthesis of gold nanoparticles as carrier for 6-mercaptopurine: Preparation, characterization and toxicity studies in zebrafish embryo model. Mater. Res. Bull. 2012, 47, 2113–2119. [Google Scholar] [CrossRef]
- Thakor, A.; Jokerst, J.; Zavaleta, C.; Massoud, T.; Gambhir, S. Gold nanoparticles: A revival in precious metal administration to patients. Nano Lett. 2011, 11, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 2014, 510246. [Google Scholar] [CrossRef]
- Mittal, J.; Batra, A.; Singh, A.; Sharma, M.M. Phytofabrication of nanoparticles through plant as nanofactories. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043002. [Google Scholar] [CrossRef]
- Ghule, K.; Ghule, A.V.; Liu, J.-Y.; Ling, Y.-C. Microscale Size Triangular Gold Prisms Synthesized Using Bengal Gram Beans (Cicer arietinum L.) Extract and HAuCl4·3H2O: A Green Biogenic Approach. J. Nanosci. Nanotechnol. 2006, 6, 3746–3751. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- Song, J.Y.; Kwon, E.-Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst. Eng. 2010, 33, 159. [Google Scholar] [CrossRef]
- Monica, R.C.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ankamwar, B.; Singh, A.; Ahmad, A.; Sastry, M. Biological synthesis of triangular gold nanoprisms. Nat. Mater. 2004, 3, 482. [Google Scholar] [CrossRef]
- Ranganathan, R.; Madanmohan, S.; Kesavan, A.; Baskar, G.; Krishnamoorthy, Y.R.; Santosham, R.; Ponraju, D.; Rayala, S.K.; Venkatraman, G. Nanomedicine: Towards development of patient-friendly drug-delivery systems for oncological applications. Int. J. Nanomed. 2012, 7, 1043. [Google Scholar]
- Manivasagan, P.; Venkatesan, J.; Senthilkumar, K.; Sivakumar, K.; Kim, S.-K. Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. Biomed. Res. Int. 2013, 2013, 287638. [Google Scholar] [CrossRef]
- Wiley, B.J.; Im, S.H.; Li, Z.-Y.; McLellan, J.; Siekkinen, A.; Xia, Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B 2006, 110, 15666–15675. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Jagtap, U.B.; Bapat, V.A. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind. Crops Prod. 2013, 46, 132–137. [Google Scholar] [CrossRef]
- Giner, J.-L.; Berkowitz, J.D.; Andersson, T. Nonpolar Components of the Latex of Euphorbia p eplus. J. Nat. Prod. 2000, 63, 267–269. [Google Scholar] [CrossRef]
- Doan, H.Q.; Gulati, N.; Levis, W.R. Ingenol mebutate: Potential for further development of cancer immunotherapy. J. Drugs Dermatol. JDD 2012, 11, 1156. [Google Scholar]
- Fallen, R.S.; Gooderham, M. Ingenol mebutate: An introduction. Skin Ther. Lett. 2012, 17, 1–3. [Google Scholar]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B Biol. 2018, 184, 71–79. [Google Scholar] [CrossRef]
- Vimalraj, S.; Ashokkumar, T.; Saravanan, S. Biogenic gold nanoparticles synthesis mediated by Mangifera indica seed aqueous extracts exhibits antibacterial, anticancer and anti-angiogenic properties. Biomed. Pharmacother. 2018, 105, 440–448. [Google Scholar] [CrossRef]
- Kumar, P.; Vijay, P.N.; Ummey, S.; Ruddaraju, L.K.; Pammi, S.; Yoon, S.-G. Ultra Small, mono dispersed green synthesized silver nanoparticles using aqueous extract of Sida cordifolia plant and investigation of antibacterial activity. Microb. Pathog. 2018, 124, 63–69. [Google Scholar]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Chau, C.-F.; Wu, S.-H.; Yen, G.-C. The development of regulations for food nanotechnology. Trends Food Sci. Technol. 2007, 18, 269–280. [Google Scholar] [CrossRef]
- Mocan, T. Hemolysis as expression of nanoparticles-induced cytotoxicity in red blood cells. Biotechnol. Mol. Biol. Nanomed. 2013, 1, 7–12. [Google Scholar]
- Bigoniya, P.; Rana, A. Hemolytic and In-vitro Antioxidant Activity of Saponin Isolated from Euphorbia neriifolia Leaf. Recent Prog. Med. Plants Nat. Prod. II 2006, 2, 359–376. [Google Scholar]
- Kviecinski, M.R.; dos Santos, J.R.; Magagnin, B.G.; Correa, M.R.; Clarinda, M.M.; Trabold, L.A.; David, I.M.; Fernandes, F.S.; da Silva, J.; Kanis, L.A. Ehrlich ascites tumor-bearing mice treated with aqueous ethanol plant extract from Euphorbia tirucalli showed signs of systemic toxicity. Trop. J. Pharm. Res. 2016, 15, 2595–2602. [Google Scholar] [CrossRef][Green Version]
- Oliveira, V.M.A.D.; Carneiro, A.L.B.; Cauper, G.S.D.B.; Pohlit, A.M. In vitro screening of Amazonian plants for hemolytic activity and inhibition of platelet aggregation in human blood. Acta Amazon. 2009, 39, 973–980. [Google Scholar] [CrossRef][Green Version]
- Gandhi, V.; Cherian, K. Red cell haemolysis test as an in vitro approach for the assessment of toxicity of karanja oil. Toxicol. In Vitro 2000, 14, 513–516. [Google Scholar] [CrossRef]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of silver nanoparticles to red blood cells: Size dependent adsorption, uptake, and hemolytic activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef]
- Sap-Iam, N.; Homklinchan, C.; Larpudomlert, R.; Warisnoicharoen, W.; Sereemaspun, A.; Dubas, S. UV irradiation-induced silver nanoparticles as mosquito larvicides. J. Appl. Sci. 2010, 10, 3132–3136. [Google Scholar] [CrossRef]
- Raffi, M.; Hussain, F.; Bhatti, T.; Akhter, J.; Hameed, A.; Hasan, M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Shockman, G.D.; Barren, J. Structure, function, and assembly of cell walls of gram-positive bacteria. Annu. Rev. Microbiol. 1983, 37, 501–527. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.-J.; Ross, L., Jr.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- Kvitek, L.; Panácek, A.; Soukupova, J.; Kolář, M.; Veceřová, R.; Prucek, R.; Holecova, M.; Zbořil, R. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Sareen, S.; Pillai, R.; Chandramohanakumar, N.; Balagopalan, M. Larvicidal potential of biologically synthesised silver nanoparticles against Aedes Albopictus. Res. J. Rec. Sci. 2012, 1, 52–56. [Google Scholar]
- Salunkhe, R.B.; Patil, S.V.; Patil, C.D.; Salunke, B.K. Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera: Culicidae). Parasitol. Res. 2011, 109, 823–831. [Google Scholar] [CrossRef]
- Kumar, P.M.; Murugan, K.; Kovendan, K.; Subramaniam, J.; Amaresan, D. Mosquito larvicidal and pupicidal efficacy of Solanum xanthocarpum (Family: Solanaceae) leaf extract and bacterial insecticide, Bacillus thuringiensis, against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2012, 110, 2541–2550. [Google Scholar] [CrossRef]
- Veerakumar, K.; Govindarajan, M.; Rajeswary, M. Green synthesis of silver nanoparticles using Sida acuta (Malvaceae) leaf extract against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2013, 112, 4073–4085. [Google Scholar] [CrossRef]
- Ibrahim, E.H.; Kilany, M.; Ghramh, H.A.; Khan, K.A.; ul Islam, S. Cellular proliferation/cytotoxicity and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J. Biol. Sci. 2018. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef]
- Harley, J.P.; Prescott, L.M. Laboratory Exercises in Microbiology; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Barry, A.; Thorsberry, C. Susceptibility test: Diffusion Tests Procedures. In Manual of Clinical Microbiology, 4th ed.; Lennette, E.H., Balows, A., Haussler, W.J., Shadomy, H.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1985; pp. 978–987. [Google Scholar]
- Oves, M.; Khan, M.S.; Zaidi, A.; Ahmed, A.S.; Ahmed, F.; Ahmad, E.; Sherwani, A.; Owais, M.; Azam, A. Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonas maltophilia. PLoS ONE 2013, 8, e59140. [Google Scholar] [CrossRef]
- Yashi, S.; Yoshiie, K.; Oda, H.; Sugano, M.; Imaizumi, K. Dietary Curcuma xanthorrhiza Roxb. increases mitogenic responses of splenic lymphocytes in rats, and alters populations of the lymphocytes in mice. J. Nutr. Sci. Vitaminol. 1993, 39, 345–354. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rahuman, A.; Bagavan, A.; Kamaraj, C.; Saravanan, E.; Zahir, A.; Elango, G. Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol. Res. 2009, 104, 1365. [Google Scholar] [CrossRef]
- WHO. Report of the World Health Organization (WHO) Informal Consultation on the “Evaluation and Testing of Insecticides", WHO/HQ, Geneva, 7 to 11 October 1996; World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Treatments | Zones of Inhibition (mm) | ||||
|---|---|---|---|---|---|
| Escherichia coli | Proteus mirabilis | Staphylococcus aureus | Shigella flexneri | Candida albicans | |
| Plant extract | 5.67 ± 1.15 c | 6.00 ± 1.73 c | 6.00 ± 1.00 c | 6.33 ± 0.58 c | 5.33 ± 0.58 c |
| Extract + AuNPs | 11.67 ± 1.57 b | 14.33 ± 1.53 b | 12.00 ± 1.00 b | 12.33 ± 1.15 b | 12.67 ± 0.58 b |
| HAuCl4 3 H2O (1mM) | 12.67 ± 1.15 b | 6.67 ± 1.15 c | 14.33 ± 1.53 b | 12.67 ± 0.58 b | 6.33 ± 0.58 c |
| Control | 22.67 ± 1.53 a | 19.00 ± 1.00 a | 23.67 ± 1.53 a | 18.67 ± 1.53 a | 24.33 ± 2.08 a |
| No. | Treatment | Absorbance at the Wavelength of 576 nm | Hemolysis (%) |
|---|---|---|---|
| 1 | EpExt | 3.00 ± 0.000 a | 100 ± 0.00 a |
| 2 | EpExt -AuNPs | 3.00 ± 0.000 a | 100 ± 0.00 a |
| 3 | Control (Negative) | 0.0123 ± 0.002 b | 0 ± 0.00 b |
| 4 | Control (Positive) | 3.00 ± 0.000 a | 100 ± 0.00 a |
| Concentration (µg/mL) | Percent (%) of Splenic Cells Growth Inhibition (−)/Stimulation (+) | |
|---|---|---|
| EpExt | EpExt-AuNPs | |
| 25 | −48.55 ± 0.45 b | −32.64 ± 0.19 a |
| 50 | −55.23 ± 1.06 b | −32.11 ± 0.87 a |
| 100 | −62.31 ± 0.58 b | −48.31 ± 1.22 a |
| 200 | −92.14 ± 1.55 b | −69.06 ± 1.21 a |
| Bio Insecticide | Conc. (ppm) | Mortality (%) | LC50 (ppm) | LC90 (ppm) | Chi-Square | Slope | RRa |
|---|---|---|---|---|---|---|---|
| EpExt | 500 | 24.96 ± 5.15 f | 1430.590 | 5805.463 | 18.97 | 3.073 ± 0.272 | 7.058 |
| 1000 | 30.55 ± 6.52 e,f | ||||||
| 1500 | 40.68 ± 3.94 d,e | ||||||
| 2000 | 60.49 ± 8.19 b,c | ||||||
| 2500 | 82.15 ± 5.58 a | ||||||
| 0 | 6.04 ± 2.21 g | ||||||
| EpExt -AuNPs | 100 | 20.33 ± 5.91 f | 202.692 | 529.463 | 3.684 | 2.107 ± 0.254 | |
| 200 | 47.62 ± 10.18 c,d | ||||||
| 300 | 64.18 ± 3.88 b | ||||||
| 400 | 83.45 ± 5.69 a | ||||||
| 500 | 92.23 ± 4.81 a | ||||||
| 0 | 0.00 ± 0.00 g |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghramh, H.A.; Khan, K.A.; Ibrahim, E.H. Biological Activities of Euphorbia peplus Leaves Ethanolic Extract and the Extract Fabricated Gold Nanoparticles (AuNPs). Molecules 2019, 24, 1431. https://doi.org/10.3390/molecules24071431
Ghramh HA, Khan KA, Ibrahim EH. Biological Activities of Euphorbia peplus Leaves Ethanolic Extract and the Extract Fabricated Gold Nanoparticles (AuNPs). Molecules. 2019; 24(7):1431. https://doi.org/10.3390/molecules24071431
Chicago/Turabian StyleGhramh, Hamed A., Khalid Ali Khan, and Essam H. Ibrahim. 2019. "Biological Activities of Euphorbia peplus Leaves Ethanolic Extract and the Extract Fabricated Gold Nanoparticles (AuNPs)" Molecules 24, no. 7: 1431. https://doi.org/10.3390/molecules24071431
APA StyleGhramh, H. A., Khan, K. A., & Ibrahim, E. H. (2019). Biological Activities of Euphorbia peplus Leaves Ethanolic Extract and the Extract Fabricated Gold Nanoparticles (AuNPs). Molecules, 24(7), 1431. https://doi.org/10.3390/molecules24071431






