Six New Coumarin Glycosides from the Aerial Parts of Gendarussa vulgaris
Abstract
:1. Introduction
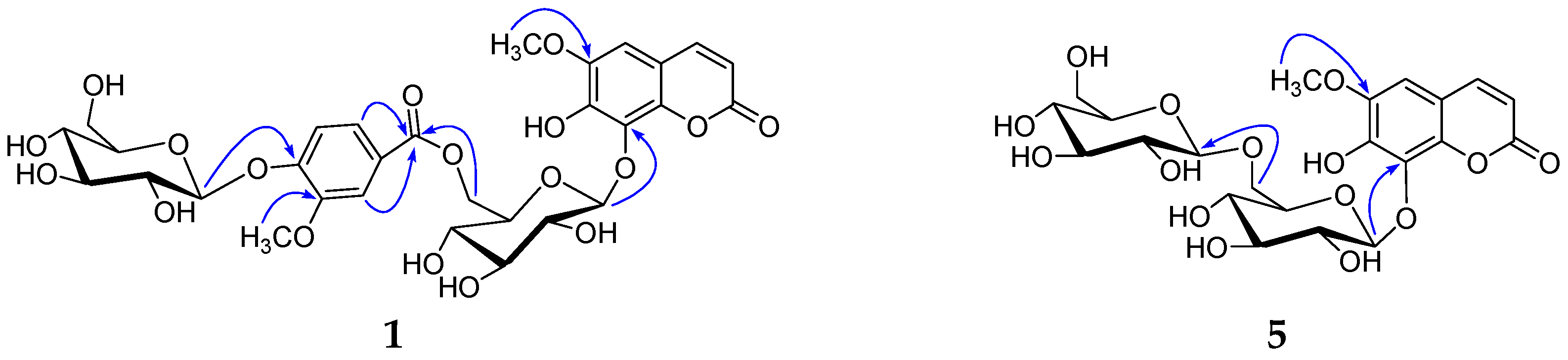
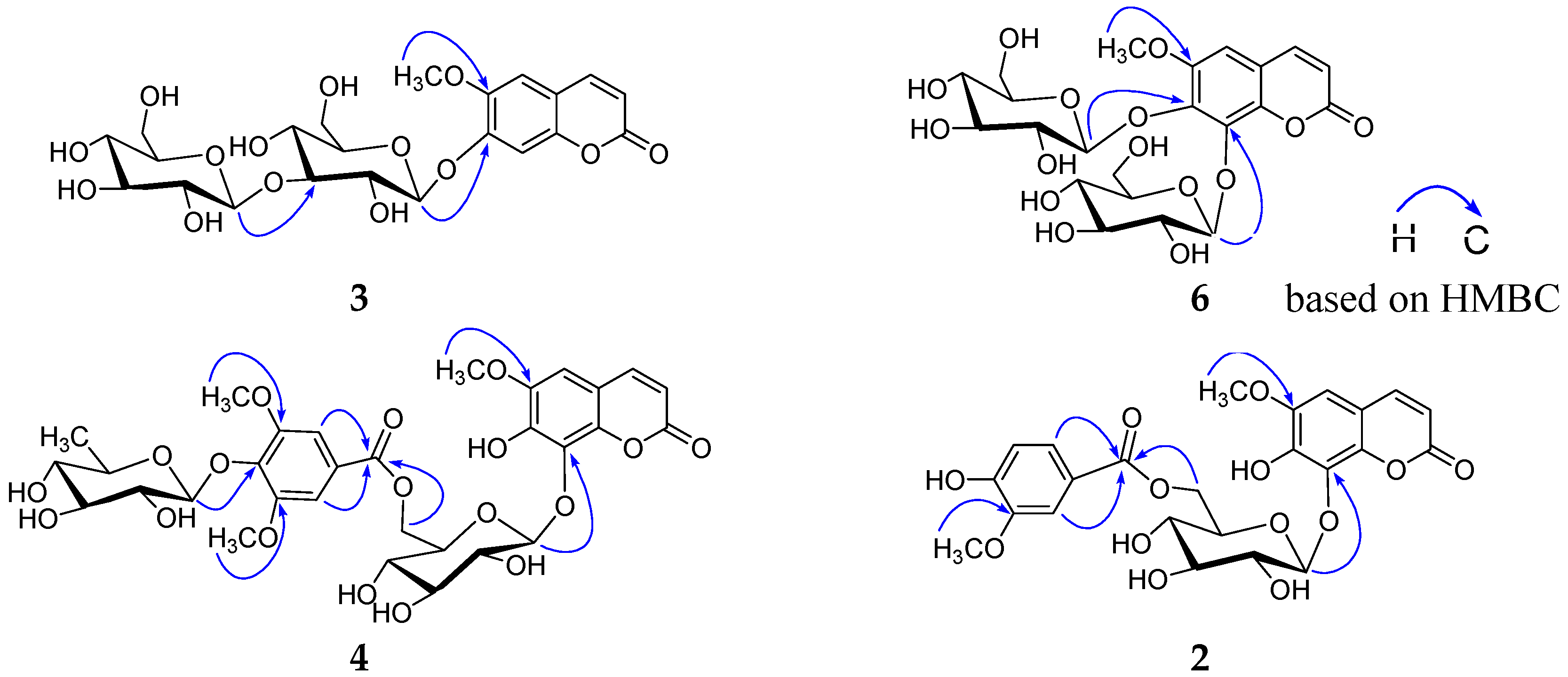
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic and Physical Data
3.5. Acid Hydrolysis and Sugar Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, S.M.; Zhang, G.L. Alkaloids from Gendarussa vulgaris Nees. Nat. Prod. Res. 2008, 22, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Zeng, J.; Wang, Y.C.; Huang, C. Advances on chemical constituents and pharmacological action of Gendarussa vulgar. Herald Med. 2014, 33, 477–480. [Google Scholar]
- Durić, K.; Besovic, E.E.K.; Niksic, H.; Muratovic, S.; Sofic, E. Anticoagulant activity of some Artemisia dracunculus leaf extracts. Bosn. J. Basic Med. Sci. 2015, 15, 9–14. [Google Scholar] [CrossRef]
- Mckee, T.C.; Fuller, R.W.; Covington, C.D.; Cardellina, J.H.; Gulakowski, R.J.; Krepps, B.L.; McMahon, J.B.; Boyd, M.R. New pyranocoumarins isolated from Calophyllum lanigerum and Calophyllum teysmannii. J. Nat. Prod. 1996, 59, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Cho, H.J.; Jeong, S.G.; Park, J.E.; Han, J.A.; Kang, H.R.; Lee, D.; Song, M.J. Antiviral activity of angelicin against gammaherpesviruses. Antivir. Res. 2013, 100, 75–83. [Google Scholar] [CrossRef]
- Mossa, A.T.; Heikal, T.M.; Belaiba, M.; Raoelison, E.G.; Ferhout, H.; Bouajila, J. Antioxidant activity and hepatoprotective potential of Cedrelopsis grevei on cypermethrin induced oxidative stress and liver damage in male mice. BMC Complem. Altern.Med. 2015, 15, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- Kumar, M.; Singla, R.; Dandriyal, J.; Jaitak, V. Coumarin derivatives as anticancer agents for lung cancer therapy: A review. Anti-Cancer Agents Med. Chem. 2018, 18, 1–21. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Marzouk, M.I.; EI-Kazak, A.M. Synthesis and characterization of some new coumarins with in vitro antitumor and antioxidant activity and high protective effects against DNA damage. Molecules 2016, 21, 249. [Google Scholar] [CrossRef]
- He, R.J.; Huang, X.S.; Zhang, Y.J.; Wu, L.D.; Nie, H.; Zhou, D.X.; Liu, B.M.; Deng, S.P.; Yang, R.Y.; Huang, S.; et al. Structural characterization and assessment of the cytotoxicity of 2,3-dihydro-1H-indene derivatives and coumarin glucosides from the bark of Streblus indicus. J. Nat. Prod. 2016, 79, 2472–2478. [Google Scholar] [CrossRef]
- Li, Z.L.; Li, X.; Li, D.Y.; Gao, L.S.; Xu, J.; Wang, Y. A new coumarin glycoside from the husks of Xanthoceras sorbifolia. Fitoterapia 2007, 78, 605–606. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Xing, Y.C.; Tang, Y.Z.; Li, N.; Jiang, S. Isolation and identification of chemical constituents from the testa of Xanthoceras sorbifolia Bunge. Chin. J. Med. Chem. 2013, 23, 397–399. [Google Scholar]
- Liu, K.; Hu, H.G.; Wang, J.L.; Jia, Y.J.; Li, H.X.; Li, J. Chemical constituents from Phellinus robustus. Chin. Pharm. J. 2014, 49, 180–183. [Google Scholar]
- He, Y.; Zhao, M.; Zong, Y.Y.; Cai, S.X.; Che, Z.T. Chemical constituents from Eurycorymbus cavaleriei. China. Tradit. Herb Drugs 2010, 41, 36–39. [Google Scholar]
- Duan, Y.H.; Dai, Y.; Gao, H.; Ye, W.C.; Yao, X.S. Chemical constituents from Sarcandra glabra. China. Tradit. Herb Drugs 2010, 41, 29–32. [Google Scholar]
- Wu, Y.B.; Zheng, C.J.; Qin, L.P.; Sun, L.N.; Han, T.; Jiao, L.; Zhang, Q.Y.; Wu, J.Z. Antiosteoporotic activity of anthraquinones from Morinda officinalis on osteoblasts and osteoclasts. Molecules 2009, 14, 573–583. [Google Scholar] [CrossRef]
- Liu, W.W.; Zhang, Y.; Hao, X.J.; Wang, Q.; Li, S.L. Chemical constituents from the leaves and twigs of Jatropha podagrica. Nat. Prod. Res. Dev. 2014, 26, 1953–1956. [Google Scholar]
- Liu, D.; Shi, N.N.; Wu, Y.H.; Li, W.H.; Zhang, M.L.; Shi, Q.W. Chemical constituents from plant of Artemisia frigida. China. Tradit. Herb Drugs 2017, 48, 5090–5098. [Google Scholar]
- Wang, X.Y.; Zhang, W.; Gao, K.; Lu, Y.Y.; Tang, H.F.; Sun, X.L. Oleanane-type saponins from Anemone taipaiensis and their cytotoxic activities. Fitoterapia 2013, 89, 224–230. [Google Scholar] [CrossRef]
- Sun, Y.J.; Gao, M.L.; Zhang, Y.L.; Wang, J.M.; Wu, Y.; Wang, Y.; Liu, T. Labdane diterpenes from the fruits of Sinopodophyllum emodi. Molecules 2016, 21, 434. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Robert, L.; Arvind, S.M. Flavonoids nutraceuticals in prevention and treatment of cancer: A review. Asian. J. Pharm. Clin. Res. 2018, 11, 1–6. [Google Scholar]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [Green Version]
- Li, S.H. Chemical constituents of Gendarussa vulgaris. China. Tradit. Herb Drugs 2018, 49, 3998–4002. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| No. | 1 a | 2 a | 3 a | 4 b | 5 a | 6 a |
|---|---|---|---|---|---|---|
| 3 | 6.13 d (9.5) | 6.08 d (9.5) | 6.33 d (9.6) | 5.97 d (9.3) | 6.22 d (9.5) | 6.39 d (9.5) |
| 4 | 7.80 d (9.5) | 7.79 d (9.5) | 7.96 d (9.6) | 7.56 d (9.3) | 7.88 d (9.5) | 7.94 d (9.5) |
| 5 | 6.93 s | 6.92 s | 7.30 s | 6.65 s | 7.02 s | 7.14 s |
| 8 | 7.17 s | |||||
| 6-OCH3 | 3.77 s | 3.76 s | 3.81 s | 3.80 s | 3.81 s | 3.81 s |
| 1′ | 5.06 d (5.3) | 4.94 d (7.7) | 5.21d (7.4) | 5.11 d (7.8) | 4.97 d (7.8) | 5.26 d (7.8) |
| 2′ | 3.47 m | 3.44 m | 3.50 m | 3.59 m | 3.37 m | 3.38 m |
| 3′ | 3.31 m | 3.31 m | 3.50 m | 3.60 m | 2.97 m | 3.20 m |
| 4′ | 3.23 m | 3.23 m | 3.23 m | 4.30 m | 3.24 m | 3.10 m |
| 5′ | 3.49 m | 3.47 m | 3.47 m | 3.51 m | 2.89 m | 3.09 m |
| 6′ | 4.43 dd (11.8, 2.0) 4.19 dd (11.8, 7.5) | 4.46 dd (11.8, 1.9) 4.14 dd (11.8, 7.5) | 3.61 m 3.48 m | 4.60 m 4.41 m | 3.87 m 3.60 m | 3.58 m 3.41 m |
| 1′′ | 4.35 d (7.8) | 4.06 d (7.8) | 5.18 d (7.8) | |||
| 2′′ | 7.28 d (1.9) | 7.29 d (1.9) | 3.10 m | 7.03 s | 2.84 m | 3.38 m |
| 3′′ | 3.21 m | 3.24 m | 3.20 m | |||
| 4′′ | 3.02 m | 2.99 m | 3.10 m | |||
| 5′′ | 7.05 d (8.5) | 6.79 d (8.2) | 3.23 m | 3.32 m | 3.12 m | |
| 6′′ | 7.18 dd (8.5, 1.9) | 7.22 dd (8.2, 1.9) | 3.61 m 3.43 m | 7.03 s | 3.38 m 3.57 m | 3.58m 3.40 m |
| 3′′-OCH3 | 3.74 s | 3.75 s | 3.79 s | |||
| 5′′-OCH3 | 3.79 s | |||||
| 1′′′ | 5.12 d (5.3) | 5.38 br.s | ||||
| 2′′′ | 3.25 m | 3.89 m | ||||
| 3′′′ | 3.32 m | 3.48 m | ||||
| 4′′′ | 3.22 m | 3.40 m | ||||
| 5′′′ | 3.47 m | 4.15 m | ||||
| 6′′′ | 3.71 m 3.48 m | 1.25 d (6.2) |
| No. | 1a | 2 a | 3 a | 4 b | 5 a | 6 a | No. | 1 a | 2 a | 3 a | 4 b | 5 a | 6 a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 160.1 s | 160.2 s | 160.4 s | 163.4 s | 160.2 s | 159.9 s | 1′′ | 122.8 s | 123.4 s | 103.9 d | 126.9 s | 103.0 s | 102.5 d |
| 3 | 111.9 d | 115.0 d | 113.4 d | 112.4 d | 111.2 d | 114.9 d | 2′′ | 112.4 d | 112.4 d | 73.8 d | 107.6 d | 73.5 d | 74.0 d |
| 4 | 144.6 d | 144.5 d | 144.1 d | 146.0 d | 144.8 d | 144.2 d | 3′′ | 148.4 s | 147.2 s | 75.9 d | 154.3 s | 76.2 d | 76.4 d |
| 5 | 104.8 d | 103.8 d | 110.0 d | 105.3 d | 105.0 d | 105.9 d | 4′′ | 150.5 s | 151.4 s | 70.1 d | 139.7 s | 69.8 d | 69.9 s |
| 6 | 145.3 s | 145.8 s | 146.0 s | 147.2 s | 145.3 s | 149.6 s | 5′′ | 114.0 d | 115.0 d | 76.9 d | 154.3 s | 76.6 d | 77.5 d |
| 7 | 148.4 s | 147.2 s | 148.9 s | 146.0 s | 143.7 s | 141.3 s | 6′′ | 122.6 d | 120.5 d | 60.4 t | 107.6 d | 60.8 d | 60.7 t |
| 8 | 131.3 s | 131.5 s | 103.1d | 132.2 s | 131.3 s | 136.1 s | 7′′ | 165.0 s | 165.3 s | 167.0 s | |||
| 9 | 142.8 s | 143.2 s | 148.9 s | 144.6 s | 142.7 s | 142.4 s | 3′′-OCH3 | 55.5 q | 55.5 q | 56.5 q | |||
| 10 | 110.0 s | 112.4 s | 112.4 s | 110.2 s | 110.1 s | 114.4 s | 5′′-OCH3 | 56.5 q | |||||
| 6-OCH3 | 56.1 q | 56.0 q | 56.1 q | 56.8 q | 56.1 q | 56.6 q | 1′′′ | 99.6 d | 99.6 d | 103.4 d | |||
| 1′ | 103.2 d | 104.8 d | 99.0 d | 104.4 d | 103.6 d | 102.6 d | 2′′′ | 73.1 d | 73.1 d | 72.3 d | |||
| 2′ | 73.7 d | 73.8 d | 71.8 d | 75.3 d | 73.8 d | 74.0 d | 3′′′ | 76.8 d | 76.8 d | 73.6 d | |||
| 3′ | 76.2 d | 76.3 d | 87.4 d | 77.9 d | 76.4 d | 76.4 d | 4′′′ | 69.7 d | 69.7 d | 72.2 d | |||
| 4′ | 70.4 d | 70.2 d | 67.9 d | 71.4 d | 69.5 d | 69.9 d | 5′′′ | 77.3 d | 77.3 d | 72.0 d | |||
| 5′ | 74.2 d | 74.3 d | 76.5 d | 75.7 d | 76.5 d | 77.6 d | 6′′′ | 60.8 t | 60.8 t | 18.0 q | |||
| 6′ | 64.0 t | 63.7 t | 61.1 t | 65.2 t | 67.7 t | 60.7 t |
| Compound | Eca-109 | MCF-7 | HepG2 | HUVEC |
|---|---|---|---|---|
| 11 | 21.04 ± 1.85 | 35.29 ± 2.61 | 43.72 ± 3.97 | >100 |
| 12 | 20.38 ± 1.94 | 28.61 ± 1.37 | 30.27 ± 1.18 | >100 |
| 13 | 45.72 ± 3.55 | 61.59 ± 5.70 | 53.74 ± 4.09 | >100 |
| 14 | 41.09 ± 3.78 | 59.59 ± 5.24 | >100 | >100 |
| etoposide | 20.48 ± 1.82 | 5.82 ± 0.49 | 1.15 ± 0.09 | 41. 65 ± 0.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Gao, M.; Chen, H.; Han, R.; Chen, H.; Du, K.; Zhang, Y.; Li, M.; Si, Y.; Feng, W. Six New Coumarin Glycosides from the Aerial Parts of Gendarussa vulgaris. Molecules 2019, 24, 1456. https://doi.org/10.3390/molecules24081456
Sun Y, Gao M, Chen H, Han R, Chen H, Du K, Zhang Y, Li M, Si Y, Feng W. Six New Coumarin Glycosides from the Aerial Parts of Gendarussa vulgaris. Molecules. 2019; 24(8):1456. https://doi.org/10.3390/molecules24081456
Chicago/Turabian StyleSun, Yanjun, Meiling Gao, Haojie Chen, Ruijie Han, Hui Chen, Kun Du, Yanli Zhang, Meng Li, Yingying Si, and Weisheng Feng. 2019. "Six New Coumarin Glycosides from the Aerial Parts of Gendarussa vulgaris" Molecules 24, no. 8: 1456. https://doi.org/10.3390/molecules24081456
APA StyleSun, Y., Gao, M., Chen, H., Han, R., Chen, H., Du, K., Zhang, Y., Li, M., Si, Y., & Feng, W. (2019). Six New Coumarin Glycosides from the Aerial Parts of Gendarussa vulgaris. Molecules, 24(8), 1456. https://doi.org/10.3390/molecules24081456





