Evaluation of the Degree of Polymerization of the Proanthocyanidins in Cranberry by Molecular Sieving and Characterization of the Low Molecular Weight Fractions by UHPLC-Orbitrap Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Total Amount of PACs in Commercial Extracts
2.2. PACs Determination by UPLC-DAD-Orbitrap MS
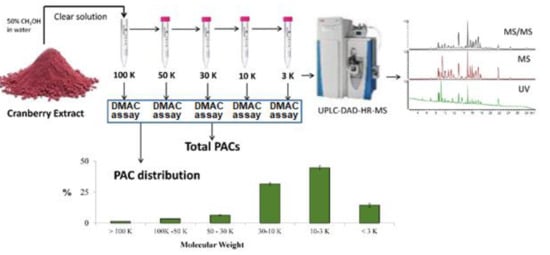
2.3. Proanthocyanidin Fractioning by Molecular Sieve
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Determination of Total Proanthocyanidins
3.3. Proanthocyanidin Determination by UHPLC-DAD-Orbitrap MS
3.4. Proanthocyanidin Fractioning by Ultra-Centrifugal Filter
3.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Howell, A.B.; Vorsa, N.; Der Marderosian, A.; Foo, L.Y. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N. Eng. J. Med. 1998, 339, 1085–1086. [Google Scholar] [CrossRef]
- Peron, G.; Pellizzaro, A.; Brun, P.; Schievano, E.; Mammi, S.; Sut, S.; Castagliuolo, I.; Dall’Acqua, S. Antiadhesive activity and metabolomics analysis of rat urine after cranberry (Vaccinium macrocarpon Aiton) administration. J. Agric. Food Chem. 2017, 65, 5657–5667. [Google Scholar] [CrossRef]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant activities and antitumor screening of extracts from cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, T.C.; Moura, E.G.; de Oliveira, E.; Soares, P.N.; Guarda, D.S.; Bernardino, D.N.; Ai, X.X.; Rodrigues, V.D.S.T.; de Souza, G.; da Silva, A.J.R.; et al. Cranberry (Vaccinium macrocarpon) extract treatment improves triglyceridemia, liver cholesterol, liver steatosis, oxidative damage and corticosteronemia in rats rendered obese by high fat diet. Eur. J. Nutr. 2018, 57, 1829–1844. [Google Scholar] [CrossRef] [PubMed]
- Weh, K.M.; Clarke, J.; Kresty, L.A. Cranberries and cancer: An update of preclinical studies evaluating the cancer inhibitory potential of cranberry and cranberry derived constituents. Antioxidants 2016, 5, 27. [Google Scholar] [CrossRef]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. North Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Wang, C.H.; Fang, C.C.; Chen, N.C.; Liu, S.S.; Yu, P.H.; Wu, T.Y.; Chen, W.; Lee, C.; Chen, S.C. cranberry-containing products for prevention of urinary tract infections in susceptible populations: A systemic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2012, 172, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.G.; Vannozzi, S.A.; Turk, R.; O’Shea, E.; Brilliant, K. Cranberry phytochemicals and their health benefits. In Nutraceutical Beverages. Chemistry, Nutrition, and Health Effects; Shahidi, F., Weerasinghe, D.K., Eds.; American Chemical Society: Washington, DC, USA, 2004; Volume 871, pp. 35–50. [Google Scholar]
- Pappas, E.; Schaich, K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef]
- Vvedenskaya, I.O.; Rosen, R.T.; Guido, J.E.; Russell, D.J.; Mills, K.A.; Vorsa, N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J. Agric. Food Chem. 2004, 52, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Johnson-Cicalese, J.; Singh, A.P.; Vorsa, N. Characterization and quantification of flavonoids and organic acids over fruit development in American cranberry (Vaccinium macrocarpon) cultivars using HPLC and APCI-MS/MS. Plant Sci. 2017, 262, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Shipley, P.R. Determination of anthocyanins in cranberry fruit and cranberry fruit products by high-performance liquid chromatography with ultraviolet detection: Single-laboratory validation. J. AOAC Int. 2011, 94, 459–466. [Google Scholar] [CrossRef]
- Česonienė, L.; Jasutienė, I.; Šarkinas, A. Phenolics and anthocyanins in berries of European cranberry and their antimicrobial activity. Medicina (Kaunas) 2009, 45, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Lazarus, S.A.; Cao, G.; Muccitelli, H.; Hammerstone, J.F. Identification of procyanidins and anthocyanins in Blueberries and Cranberries (Vaccinium Spp.) using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 2001, 49, 1270–1276. [Google Scholar] [CrossRef]
- Howell, A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007, 51, 732–737. [Google Scholar] [CrossRef]
- Reed, J.D.; Krueger, C.G.; Vestling, M.M. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry 2005, 66, 2248–2263. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.R.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Colour reactions of flowers attributed to (a) flavanols and (b) carotenoid Oxides. J. Exp. Bot. 1953, 4, 1–9. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo da Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Cunningham, D.G.; Vannozzi, S.; O’Shea, E.; Turk, R. Analysis and standardization of cranberry products. In Quality Management of Nutraceuticals; Ho, C., Zheng, Q.Y., Eds.; ACS: Washington, DC, USA, 2002; Volume 803, pp. 152–167. [Google Scholar]
- Wang, Y.; Singh, A.P.; Hurst, W.J.; Glinski, J.; Koo, H.; Vorsa, N. Influence of degree-of-polymerization and linkage on the quantification of proanthocyanidins using 4-Dimethylaminocinnamaldehyde (DMAC) Assay. J. Agric. Food Chem. 2016, 64, 2190–2199. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Shea, M.P.; Shanmuganayagam, D.; Krueger, C.G.; Howell, A.B.; Reed, J.D. Comparison of isolated cranberry (Vaccinium macrocarpon Ait.) proanthocyanidins to catechin and procyanidins A2 and B2 for use as standards in the 4-(dimethylamino) cinnamaldehyde assay. J. Agric. Food Chem. 2012, 60, 4578–4585. [Google Scholar] [CrossRef]
- Ye, L.; Neilson, A.; Sarnoski, P.; Ray, W.K.; Duncan, S.; Boyer, R.; O’Keefe, S.F. Comparison of A-type Proanthocyanidins in cranberry and Peanut skin extracts using matrix assisted laser desorption ionization-time of flight mass spectrometry. J. Mol. Genet. Med. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Li, H.J.; Deinzer, M.L. Tandem mass spectrometry for sequencing proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Parets, L.; Alechaga, E.; Núñez, O.; Saurina, J.; Hernández-Cassou, S.; Lluis Puignou, L. Ultrahigh pressure liquid chromatography-atmospheric pressure photoionization-tandem mass spectrometry for the determination of polyphenolic profiles in the characterization and classification of cranberry-based pharmaceutical preparations and natural extracts. Anal. Methods 2016, 8, 4363–4378. [Google Scholar]
- van Dooren, I.; Foubert, K.; Theunis, M.; Naessens, T.; Pieters, L.; Apers, S. Advantages of a validated UPLC-MS/MS standard addition method for the quantification of A-type dimeric and trimeric proanthocyanidins in cranberry extracts in comparison with well-known quantification methods. J. Pharm. Biomed. Anal. 2018, 148, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Bartolomé, B.; Martín-Alvarez, P.J.; Anderson, M.; Howell, A.; Monagas, M. Comprehensive assessment of the quality of commercial cranberry products. Phenolic characterization and in vitro bioactivity. J. Agric. Food Chem. 2012, 60, 3396–3408. [Google Scholar]
- Lin, L.Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMSn profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [PubMed]
- del Mar Contreras, M.; Arráez-Román, D.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Nano-liquid chromatography coupled to time-of-flight mass spectrometry for phenolic profiling: A case study in cranberry syrups. Talanta 2015, 132, 929–938. [Google Scholar] [CrossRef]
- Barbosa, S.; Pardo-Mates, N.; Hidalgo-Serrano, M.; Saurina, J.; Puignou, L.; Núñez, O. Detection and quantitation of frauds in the authentication of cranberry-based extracts by UHPLC-HRMS (Orbitrap) polyphenolic profiling and multivariate calibration methods. J. Agric. Food Chem. 2018, 66, 9353–9936. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Wilson, T.; Kalk, A.J.; Cheong, J.; Vorsa, N. Isolation of specific cranberry flavonoids for biological activity assessment. Food Chem. 2009, 116, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Hellenbrand, N.; Sendker, J.; Lechtenberg, M.; Petereit, F.A. Isolation and quantification of oligomeric and polymeric procyanidins in leaves and flowers of Hawthorn (Crataegus spp.). Fitoterapia 2015, 104, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Contreras Mdel, M.; Uberos, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Assessment of the stability of proanthocyanidins and other phenolic compounds in cranberry syrup after gamma-irradiation treatment and during storage. Food Chem. 2015, 174, 392–399. [Google Scholar]
- Engström, M.T.; Pälijärvi, M.; Fryganas, C.; Grabber, J.H.; Mueller-Harvey, I.; Salminen, J.P. Rapid qualitative and quantitative analyses of proanthocyanidin oligomers and polymers by UPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 3390–3399. [Google Scholar]
- Cardellina, J.H.; Gafner, S. Cranberry product laboratory guidance document; UNPA: Austin, TX, USA, 2018. [Google Scholar]
- Koo, H.; Duarte, S.; Murata, R.M.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of Cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Caries Res. 2010, 44, 116–126. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples A1–A3 are available from the authors. |



| Analyte | A1 (%) | A2 (%) | A3 (%) |
|---|---|---|---|
| Total PACs | 27.1 ± 1.1a | 33.1 ± 2.1a | 14.7 ± 1.0a |
| Catechin | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.0 ± 0.0 |
| Epicatechin | 1.3 ± 0.1 | 0.1 ± 0.0 | 0.4 ± 0.0 |
| Dimers, A-type | 1.8 ± 0.1 | 3.3 ± 0.1 | 0.5 ± 0.0 |
| (PA2) | 1.8 ± 0.1 | 0.2 ± 0.0 | 0.5 ± 0.0 |
| Dimers, B-type | 0.5 ± 0.0 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| Trimers, AA-type | 0.1 ± 0.0 | 0.2 ± 0.1 | n.f. |
| Trimers, AB-type | 1.4 ± 0.1 | 2.5 ± 0.1 | 0.5 ± 0.0 |
| Trimers, BB-type | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| (PC1) | 0.2 ± 0.0 | n.f. | 0.1 ± 0.0 |
| Peak | RT | λmax | [M − H]− | Formula | Fragment Ions | Compound |
|---|---|---|---|---|---|---|
| 1 | 3.2 | 270 | 169.0146 | C7 H5 O5 | 125.0246 | GA |
| 2 | 4.7 | 315 | 315.0735 | C13 H15 O9 | 152.0118 | DHBA-Hex |
| 3 | 5.3 | 277 | 329.0883 | C14 H17 O9 | 167.0353, 152.0118, 123.0454 | VA-Hex |
| 4 | 5.4 | 263 | 299.0784 | C13 H15 O8 | 137.0247 | HBA-Hex |
| 5 | 5.6 | 302 | 315.1091 | C14 H19 O8 | 163.0404, 153.056, 145.0298, 123.0454 | VH-Hex |
| 6 | 6.8 | 295 | 503.1428 | C21 H27 O14 | 341.0883, 179.0354, 161.0249, 135.0455 | CA-di-Hex |
| 7 | 7.0 | 328 | 341.0884 | C15 H17 O9 | 179.0354, 161.0249, 133.0298 | CA-Hex |
| 8 | 7.7 | 277 | 577.1363 | C30 H25 O12 | 407.0792, 289.0731, 125.0247 | DP2, B |
| 9 | 8.1 | 310 | 341.0884 | C15 H17 O9 | 179.0354, 161.0249, 133.0298 | CA-Hex |
| 10 | 8.4 | 227, 277 | 289.0726 | C15 H13 O6 | 159.0456, 137.0247, 123.0455 | CAT |
| 11 | 8.5 | 320 | 353.0884 | C16 H17 O9 | 191.0567, 163.0567 | CHL |
| 12 | 8.7 | 313 | 325.0938 | C15 H17 O8 | 163.0404, 145.0298 | pC-Hex |
| 13 | 9.1 | 234, 277 | 591.1147 | C30 H23 O13 | 447.0947, 347.0576, 284.0339 | DP2 A1 |
| 14 | 9.4 | 234 | 461.1307 | C19 H25 O13 | 121.0297 | HBA-di-Hex |
| 15 | 9.7 | 241 | 445.1360 | C19 H25 O12 | 323.0989, 121.0297 | BA-di-Hex |
| 16 | 10.0 | 234, 277 | 865.2005 | C45 H37 O18 | 407.0791, 287.0574, 577.1368 160.0170, 125.0247 | DP3, BB |
| 17 | 10.1 | 234 | 461.1307 | C19 H25 O13 | 121.0297 | HBA-di-Hex |
| 18 | 10.9 | 227, 277 | 289.0726 | C15 H13 O6 | 159.0456, 137.0247, 123.0455 | EC |
| 19 | 11.4 | 234 | 371.0991 | C16 H19 O10 | 359.1515, 344.1277, 249.0625, 121.0298 | BA-X |
| 20 | 11.9 | 234, 278 | 863.1857 | C45 H35 O18 | 573.1061, 451.1053, 411.0742, 289.0732 | DP3, AB |
| 20a | 12.1 | 234, 280 | 865.2007 | C45 H37 O18 | 407.0791, 287.0574, 577.1368, 125.0247 | PC1 |
| 21 | 13.1 | 255, 356 | 479.0841 959.1754 | C22 H19 O13 [2M-H]- | 316.0237, 271.026 | M-Glc |
| 22 | 13.6 | 230, 280 | 863.1853 | C45 H35 O18 | 575.1894, 449.1099 | DP3, AB |
| 23 | 13.8 | 230, 275 | 577.2055 | C32 H33 O10 | 397.1423, 373.1157, 203.0833 | N.I. |
| 23a | 14.8 | 254, 311 | 535.1469 | C25 H27 O13 | 316.0238, 271.0262, 191.0356, 163.0405, 147.0455 | N.I. |
| 24 | 15.0 | 255, 356 | 463.0893 927.1860 | C21 H19 O12 [2M-H]- | 300.0290, 271.0261, 151.0040 | Q-Glc |
| 25 | 15.3 | 254, 312 | 535.1469 | C25 H27 O13 | 407.0789, 289.0730, 191,0356, 163.0405, 147.0455 | N.I. |
| 26 | 15.5 | 493.1008 | C22 H21 O13 | 330.0395, 315.0161, 163.0404 | methyl-M | |
| 27 | 15.6 | 230, 280 | 575.1200 1151.2485 | C30 H23 O12 [2M-H]- | 285.0417 | PA2 |
| 28 | 16.0 | 313 | 537.1624 1075.3317 | C25 H19 O13 [2M-H]- | 163.0405, 149.0611 | dh-MT-pC |
| 29 | 16.1 | 433.0783 | C20 H17 O11 | 300.0289, 271.0261 | Q-Ara | |
| 30 | 16.4 | 255, 352 | 433.0787 | C20 H17 O11 | 300.0288, 271.0260, 255.0310, 151.0040 | Q-Ara |
| 31 | 16.9 | 255, 352 | 433.0787 | C20 H17 O11 | 300.0288, 271.0260, 255.0310 | Q-Ara |
| 32 | 17.4 | 255, 348 | 447.0943 | C21 H19 O11 | 300.0288, 271.0259, 255.0310, 151.0040 | Q-Rha |
| 33 | 17.8 | 252, 359 | 507.1155 | C23 H23 O13 | 344.0551, 329.0319, 316.0601, 301.0367, 273.0419 | dm-M-Hex |
| 34 | 17.9 | 252, 377 | 317.031 | C15 H19 O8 | 271.0259, 151.004 | M |
| 35 | 19.2 | 254, 352 | 447.0951 | C21 H19 O11 | 314.0447, 285.0419, 271.0262, 243.0309 | I-Ara |
| 36 | 19.8 | 252, 348 | 447.0951 | C21 H19 O11 | 314.0447, 285.0419, 271.0262, 243.0310 | I-Ara |
| 37 | 20.3 | 262, 359 | 477.1049 | C22 H21 O12 | 344.0551, 329.0317, 316.0601, 301.0367, 273.0418 | dm-M-Pentose |
| 38 | 20.8 | 280, 316 | 445.1158 | C22 H21 O10 | 179.0355, 135.0455, 121.0297 | CA-Hex-BA |
| 39 | 21.6 | 285, 310 | 609.1270 | C30 H25 O14 | 463.0902, 300.0288, 163.0041, 151.0040 | Q-Hex-pC |
| 40 | 21.8 | 255, 370 | 301.0363 | C15 H9 O7 | 151.0040 | Q |
| 41 | 22.3 | 254, 373 | 331.0468 | C16 H11 O8 | 316.0239, 271.0262, 164.0120, 151.0041 | I |
| 42 | 24.5 | 255, 352 | 567.1158 | C28 H23 O13 | 300.0289 | Q-Hex-BA |
| 43 | 28.1 | 255, 366 | 345.0622 | C217 H13 O8 | 315.0519, 300.0288, 287.0211, 271.0260, 151.0041 | dm-M |
| 44 | 28.8 | 266, 252 | 593.1318 | C30 H25 O13 | 300.0288, 271.0261 | Q-Rha-pC |
| 45 | 3.2 | 235 | 391.1254 783.2287 | C16 H23 O11 [2M-H]- | 229.0724 (A), 211.0618, 167.0717, 149.0611, 123.0454 | A-Hex |
| 46 | 10.1 | 230, 277 | 415.1257 461.1311 | C18 H23 O11 [M+COOH]- | 284.0340, 121.0298 | N.I. |
| 47 | 10.5 | 248, 352 | 385.1151 | C17 H21 O10 | 205.0513, 190.0287, 175.0042, 149.0248 | SA-Hex |
| 48 | 10.9 | 385.1877 431.1931 | C19 H29 O8 [M+COOH]- | 298.0495, 283.0260, 205.1240, 153.0925, 125.0247 | N.I. | |
| 49 | 14.4 | 260, 359 | 449.0737 | C20 H17 O12 | 316.0238, 287.0211, 271.0262 | M-Pentose |
| 50 | 14.6 | 266, 348 | 449.0737 | C20 H17 O12 | 316.0238, 287.0212, 271.0262 | M-Pentose |
| 51 | 14.8 | 248, 309 | 535.1471 1071.3013 | C25 H27 O13 [2M-H]- | 316.0236, 271.0264, 191.0356, 163.0405, 147.0455 | M-Pentose-X |
| M1 | 1.8 | 341.1093 665.2165 | C12 H21 O11, C24 H41 O21 | 161.0456 | Maltodextrin | |
| M2 | 2.1 | 989.3204 1151.3730 1313.4260 | C36 H61 O31 C42 H71 O36 C48 H81 O41 | 341.1093, 827.2680, 665.2165, 161.0458 | Maltodextrin | |
| 52 | 5.0 | 230, 277 | 865.2011 | C45 H37 O18 | 407.0791, 287.0574, 577.1368 | DP3, BB |
| 53 | 6.8 | 230, 277 | 1439.3153 719.1533 | C75 H59 O30 [2M-H]- | 573.1053, 411.0738, 289.0731, 125.0247 | DP5, ABBB |
| 54 | 7.7 | 230, 277 | 577.1367 | C30 H25 O12 | 407.0791, 289.0730, 245.0830, 125.0247 | DP2, B |
| 55 | 8.2 | 230, 277 | 577.1367 | C30 H25 O12 | 407.0791, 289.0730, 245.0830, 125.0247 | DP2, B |
| 56 | 9.6 | 230, 277 | 577.1367 | C30 H25 O12 | 447.0944, 284.0341, 125.0248 | DP2, B |
| 57 | 10.0 | 230, 277 | 1151.2487 575.1208 | C60 H47 O24 [M-2H]2- | 447.0943, 285.0419, 125.0248 | DP4, ABB |
| 58 | 10.2 | 230, 277 | 1151.2489 575.1208 | C60 H47 O24 [M-2H]2- | 863.1873, 285.0418, 125.0248 | DP4, ABB |
| 59 | 10.5 | 230, 277 | 863.1857 | C45 H35 O18 | 693.1282, 575.1221, 449.0899, 423.0743, 407.0791, 285.0419, 125.0247 | DP3, AB |
| 60 | 11.2 | 234, 277 | 863.1857 | C45 H35 O18 | 411.0742, 289.0732 | DP3, AB |
| 61 | 12.1 | 237, 278 | 863.1857 | C45 H35 O18 | 693.1282, 575.1221, 449.0899, 289.0732, 125.0247 | DP3, AB |
| 62 | 13.3 | 232, 278 | 863.1850 | C45 H35 O18 | 693.1282, 575.1221, 449.0899, 289.0732, 125.0247 | DP3, AB |
| 63 | 13.6 | 231, 277 | 575.1221 | C30 H23 O12 | 449.0899, 289.0732, 125.0247 | DP2, A |
| 64 | 13.9 | 234, 278 | 863.1850 | C45 H35 O18 | 575.1221, 449.0899, 289.0732, 125.0247 | DP3, AB |
| 65 | 14.2 | 236, 280 | 1726.2633 862.1778 | C90 H69 O36 | 1153.2630, 863.1860, 411.0741, 289.0731 | DP6, AABBB |
| 66 | 14.3 | 234, 278 | 1437.2966 718.1456 | C75 H57 O30 [M-2H]2- | 1149.2328, 862.1768, 575.1221, 411.0740, 285.0419, 125.0247 | DP5, AABB |
| 67 | 14.9 | 234, 278 | 1149.2328 | C60 H45 O24 | 575.1221, 411.0740, 285.0419, 125.0247 | DP4, AAB |
| 68 | 15.1 | 237, 278 | 1437.2878 | C75 H57 O30 | 863.1839, 575.1221, 411.074, 285.0419, 125.0247 | DP5, AABB |
| 69 | 15.7 | 234, 279 | 575.1212 | C30 H23 O12 | 447.0743. 411.0741, 285.0419, 125.0247 | DP2, A |
| 70 | 15.7 | 234, 279 | 575.1212 | C30 H23 O12 | 4479.0902. 411.0745, 285.0419, 125.0247 | DP2, A |
| 71 | 16.0 | 237, 278 | 1149.2328 | C60 H45 O24 | 575.1221, 449.0899, 411.0740, 285.0419, 125.0247 | DP4, AAB |
| 72 | 16.4 | 237, 279 | 1149.2323 | C60 H45 O24 | 575.1221, 449.0899, 411.0740, 285.0419, 125.0247 | DP4, AAB |
| 73 | 17.0 | 234, 277 | 575.1212 | C30 H23 O12 | 285.0419, 125.0247 | DP2, A |
| 74 | 17.4 | 234, 278 | 1149.2323 | C60 H45 O24 | 575.1221, 449.0899, 411.0740, 285.0419, 125.0247 | DP4, AAB |
| 75 | 17.9 | 1149.2323 | C60 H45 O24 | 575.1221, 411.0740, 285.0419, 125.0247 | DP4, AAB | |
| 76 | 18.1 | 1149.2323 | C60 H45 O24 | 861.1715, 573.1060, 411.0740, 125.0247 | DP4, AAB | |
| 77 | 18.4 | 1149.2323 | C60 H45 O24 | 575.1221, 497.2778, 411.0740, 285.0419 | DP4, AAB | |
| 78 | 18.9 | 1149.2323 | C60 H45 O24 | 575.1221, 411.0740, 285.0419, 125.0247 | DP4, AAB | |
| 79 | 19.6 | 1149.2323 | C60 H45 O24 | 575.1219, 411.0740, 285.0419 | DP4, AAB | |
| 80 | 28.5 | 965.4270 | C44 H69 O23 | 803.3750, 641.3204 | N.I. | |
| 81 | 28.5 | 327.2186 | C18 H31 O5 | N.I. | ||
| 82 | 29.6 | 329.2344 | C18 H33 O5 | 283.0671, 211.1347, 183.1394, 171.1031, 139.1131, 127.1131 | N.I. |
| NMWL | A1 | A2 | A3 |
|---|---|---|---|
| >100 K | 0.1 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.1 |
| 100–50 K | 2.5 ± 0.1 | 0.6 ± 0.0 | 3.8 ± 0.2 |
| 50–30 K | 4.9 ± 0.2 | 8.3 ± 0.4 | 9.2 ± 0.5 |
| 30–10 K | 21.6 ± 1.1 | 11.6 ± 0.6 | 22.4 ± 1.1 |
| 10–3 K | 36.3 ± 1.4 | 5.7 ± 0.3 | 37.8 ± 1.6 |
| <3 K | 34.6 ± 1.4 | 72.9 ± 3.6 | 25.7 ± 1.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardana, C.; Simonetti, P. Evaluation of the Degree of Polymerization of the Proanthocyanidins in Cranberry by Molecular Sieving and Characterization of the Low Molecular Weight Fractions by UHPLC-Orbitrap Mass Spectrometry. Molecules 2019, 24, 1504. https://doi.org/10.3390/molecules24081504
Gardana C, Simonetti P. Evaluation of the Degree of Polymerization of the Proanthocyanidins in Cranberry by Molecular Sieving and Characterization of the Low Molecular Weight Fractions by UHPLC-Orbitrap Mass Spectrometry. Molecules. 2019; 24(8):1504. https://doi.org/10.3390/molecules24081504
Chicago/Turabian StyleGardana, Claudio, and Paolo Simonetti. 2019. "Evaluation of the Degree of Polymerization of the Proanthocyanidins in Cranberry by Molecular Sieving and Characterization of the Low Molecular Weight Fractions by UHPLC-Orbitrap Mass Spectrometry" Molecules 24, no. 8: 1504. https://doi.org/10.3390/molecules24081504
APA StyleGardana, C., & Simonetti, P. (2019). Evaluation of the Degree of Polymerization of the Proanthocyanidins in Cranberry by Molecular Sieving and Characterization of the Low Molecular Weight Fractions by UHPLC-Orbitrap Mass Spectrometry. Molecules, 24(8), 1504. https://doi.org/10.3390/molecules24081504