PEGylated Polyurea Bearing Hindered Urea Bond for Drug Delivery
Abstract
:1. Introduction
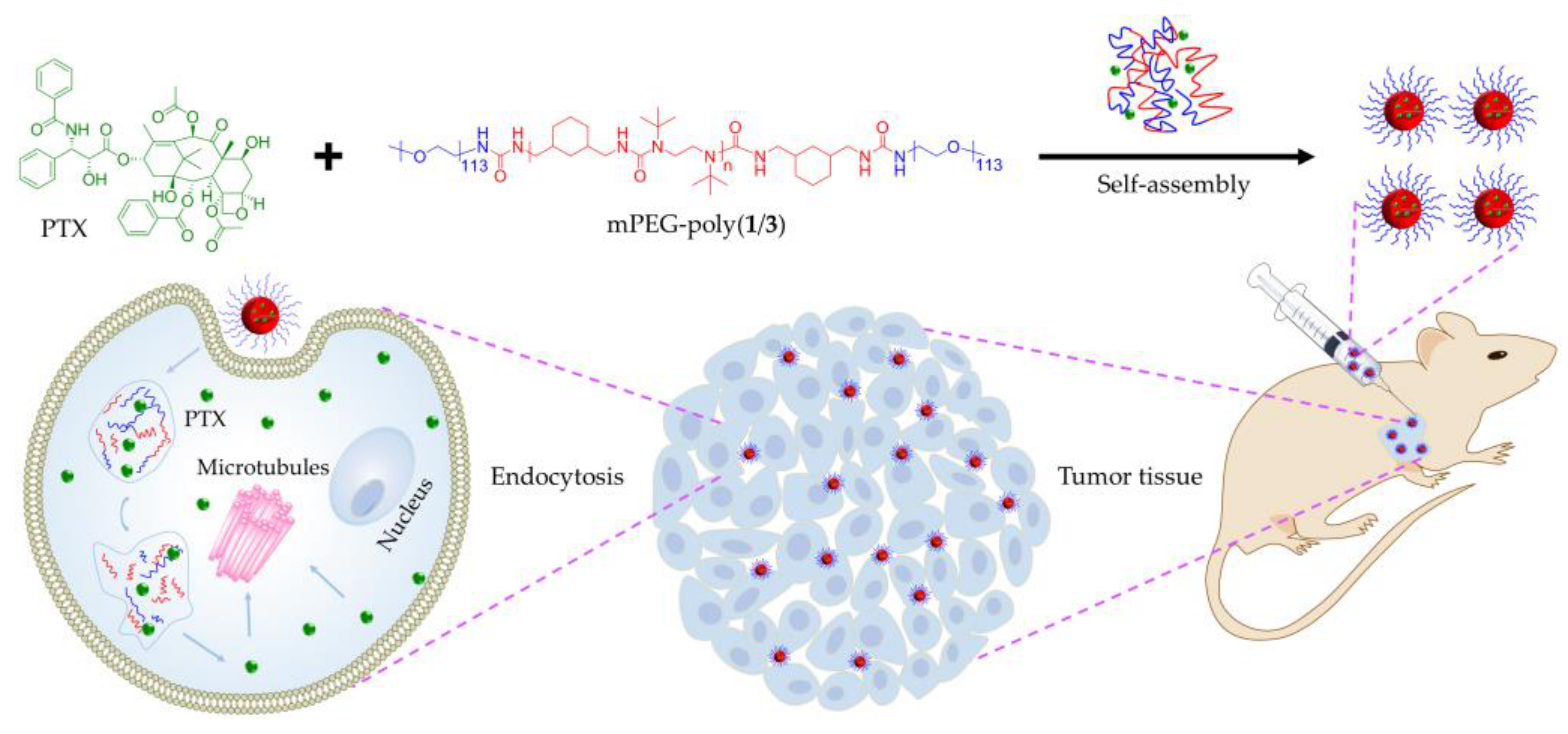
2. Results and Discussion
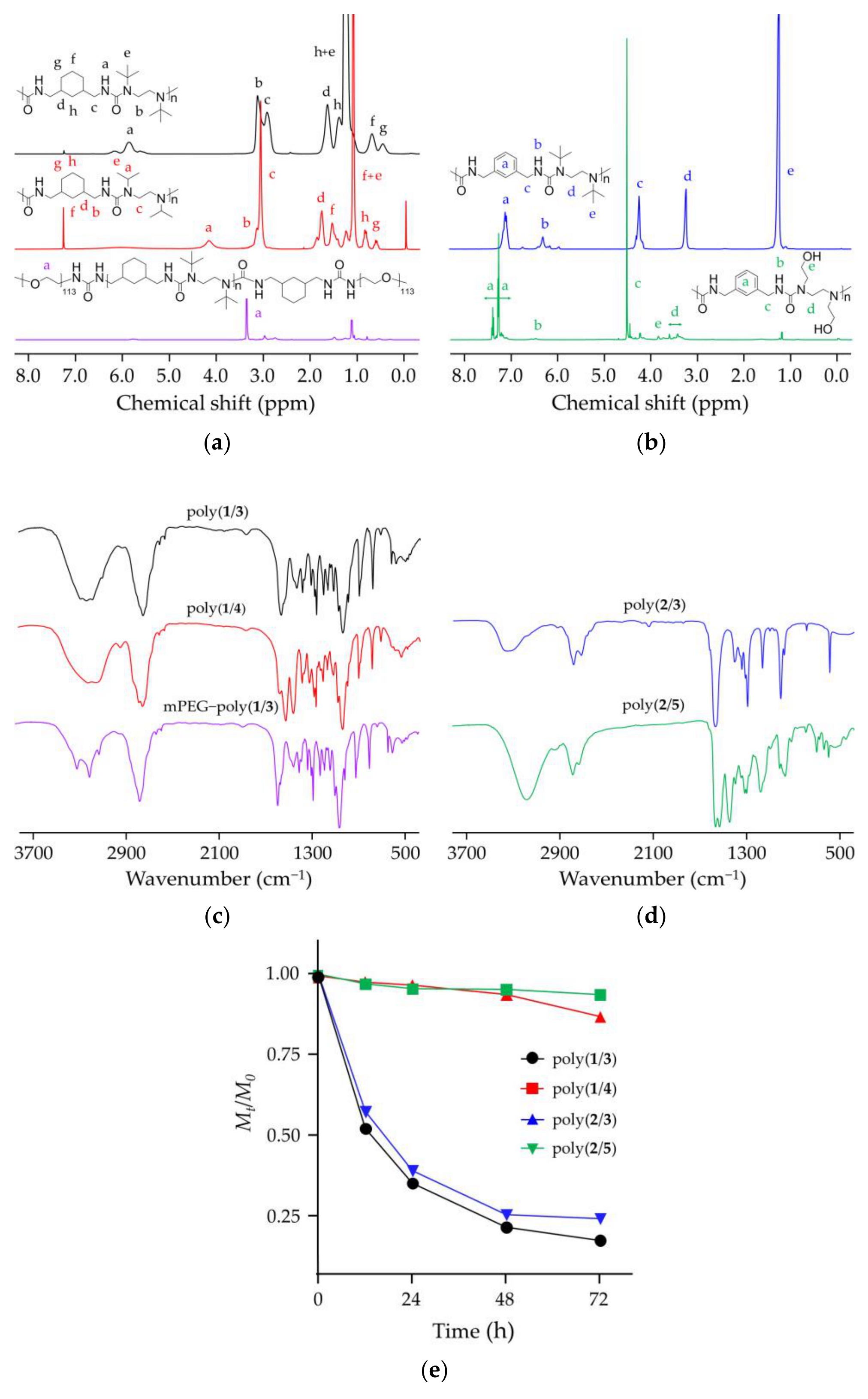
2.1. Syntheses and Characterizations
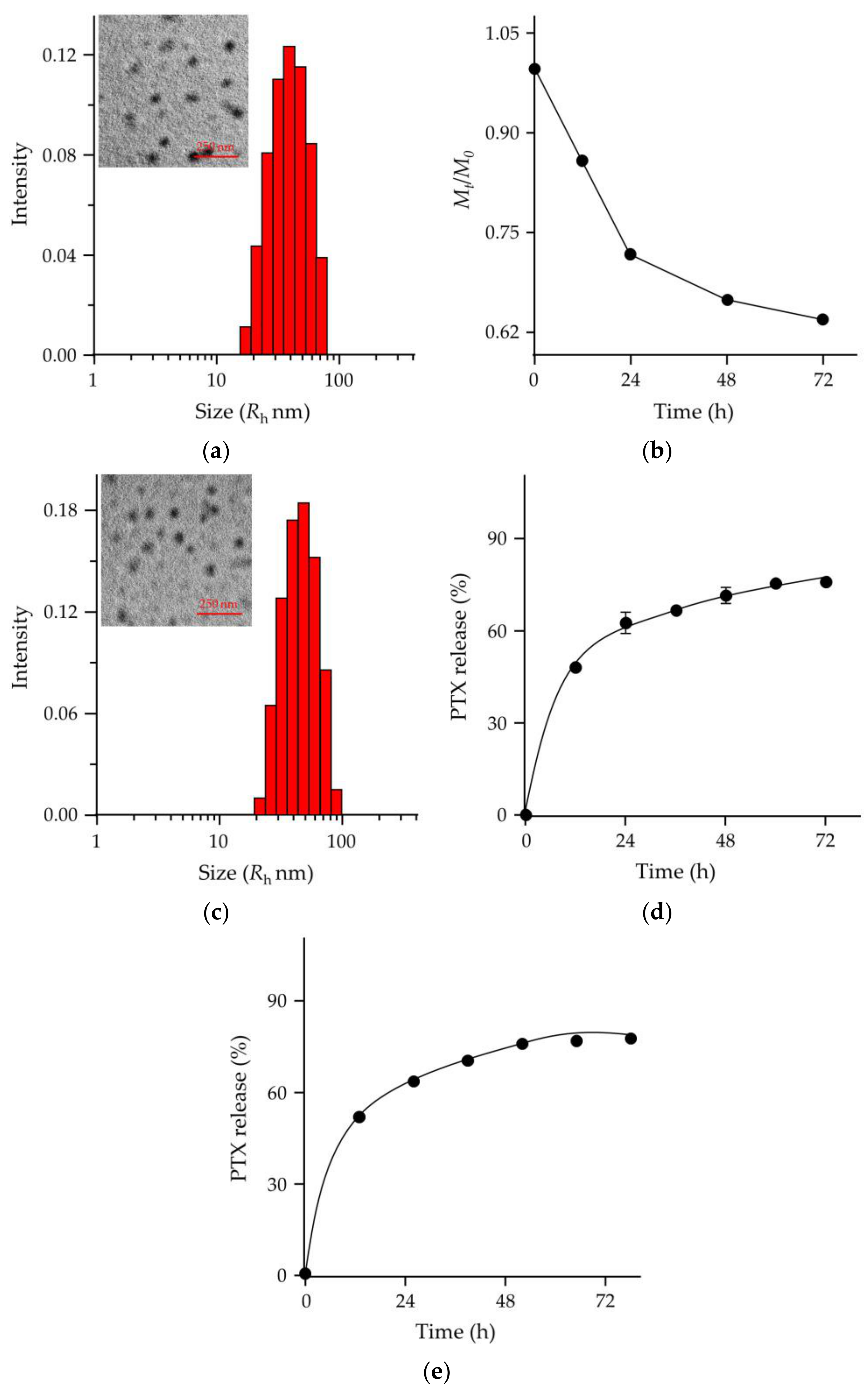
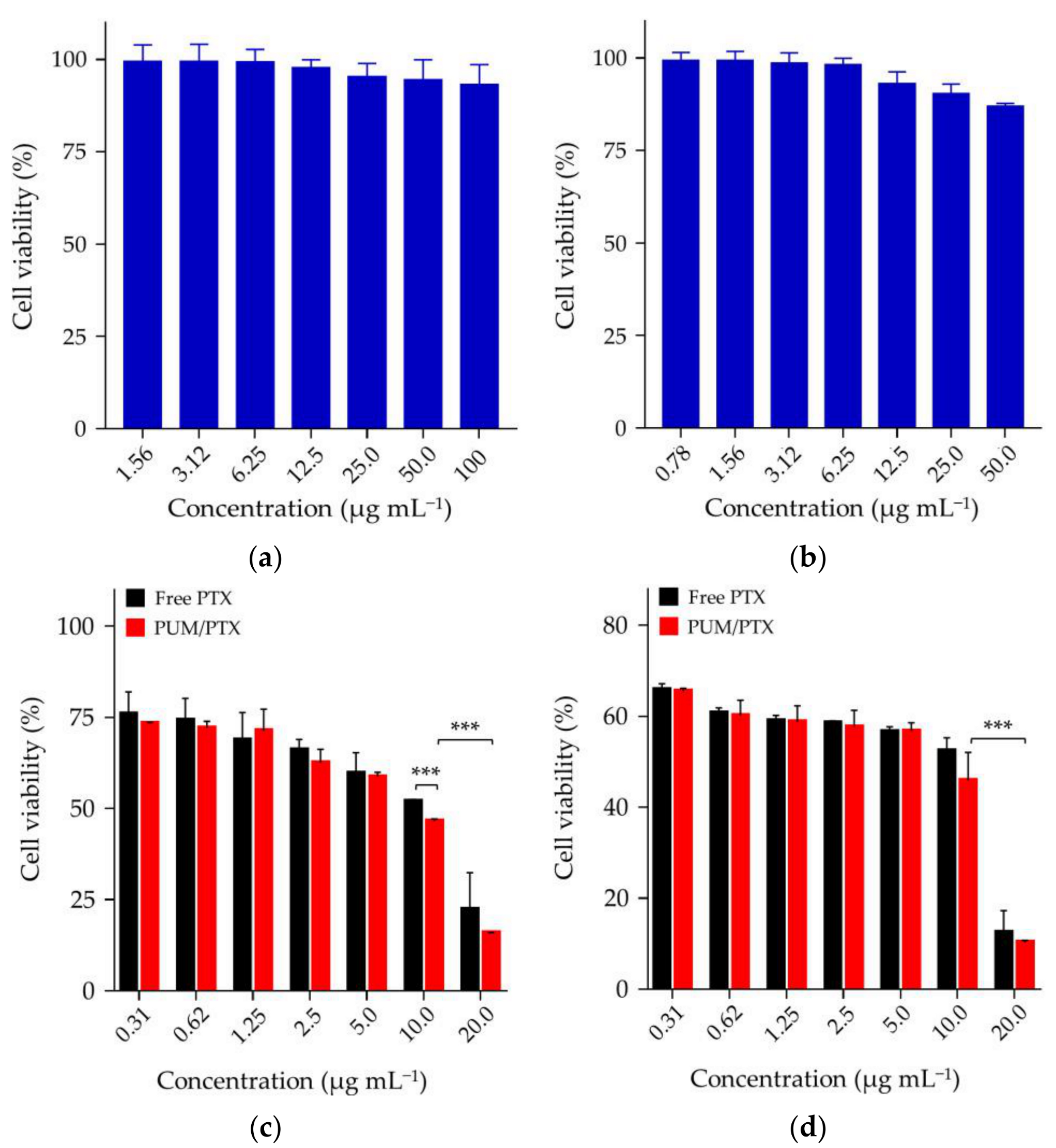
2.2. PTX Release, In Vitro Cell Uptake, and Cell Proliferation Inhibition
2.3. In Vivo Antitumor Efficacy
3. Materials and Methods
3.1. Materials
3.2. Syntheses of Four Different Polyureas
3.3. Synthesis of mPEG−poly(1/3)
3.4. Preparation of PUM
3.5. Preparation of PUM/PTX
3.6. Characterizations
3.7. In Vitro PTX Release
3.8. Cell Cultures
3.9. Cell Uptakes
3.9.1. FCM
3.9.2. CLSM
3.10. Cytotoxicity Assays
3.11. Animal Procedures
3.12. In Vivo Antitumor Efficacy
3.13. Histopathological and Immunofluorescence Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cai, K.M.; Ying, H.Z.; Cheng, J.J. Dynamic ureas with fast and pH-independent hydrolytic kinetics. Chem. Eur. J. 2018, 24, 7345–7348. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.Z.; Zhang, Y.F.; Cheng, J.J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218–3226. [Google Scholar] [CrossRef] [PubMed]
- Hutchby, M.; Houlden, C.E.; Ford, J.G.; Tyler, S.N.G.; Gagne, M.R.; Lloyd-Jones, G.C.; Booker-Milburn, K.I. Hindered ureas as masked isocyanates: Facile carbamoylation of nucleophiles under neutral conditions. Angew. Chem. Int. Ed. 2009, 48, 8721–8724. [Google Scholar] [CrossRef]
- Rocas, P.; Cusco, C.; Rocas, J.; Albericio, F. On the importance of polyurethane and polyurea nanosystems for future drug delivery. Curr. Drug Delivery 2018, 15, 37–43. [Google Scholar] [CrossRef]
- Ying, H.Z.; Cheng, J.J. Hydrolyzable polyureas bearing hindered urea bonds. J. Am. Chem. Soc. 2014, 136, 16974–16977. [Google Scholar] [CrossRef]
- Florez-Grau, G.; Rocas, P.; Cabezon, R.; Espana, C.; Panes, J.; Rocas, J.; Albericio, F.; Benitez-Ribas, D. Nanoencapsulated budesonide in self-stratified polyurethane-polyurea nanoparticles is highly effective in inducing human tolerogenic dendritic cells. Int. J. Pharm. 2016, 511, 785–793. [Google Scholar] [CrossRef] [PubMed]
- John, J.V.; Seo, E.J.; Augustine, R.; Jang, I.H.; Kim, D.K.; Kwon, Y.W.; Kim, J.H.; Kim, I. Phospholipid end-capped bioreducible polyurea micelles as a potential platform for intracellular drug delivery of doxorubicin in tumor cells. ACS Biomater. Sci. Eng. 2016, 2, 1883–1893. [Google Scholar] [CrossRef]
- Valerio, A.; Feuser, P.E.; Bubniak, L.D.; Dos Santos-Silva, M.C.; de Araujo, P.H.H.; Sayer, C. In vitro biocompatibility and macrophage uptake assays of poly(urea-urethane) nanoparticles obtained by miniemulsion polymerization. J. Nanosci. Nanotechnol. 2017, 17, 4955–4960. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Rahman, M.S.U.; Iqbal, S.; Arshad, M.I.; Tahir, M.A.; Mahmood, T. Sustained drug delivery of doxorubicin as a function of pH, releasing media, and NCO contents in polyurethane urea elastomers. J. Drug Deliv. Sci. Tec. 2017, 39, 277–282. [Google Scholar] [CrossRef]
- Morral-Ruiz, G.; Melgar-Lesmes, P.; Lopez-Vicente, A.; Solans, C.; Garcia-Celma, M.J. Biotinylated polyurethane-urea nanoparticles for targeted theranostics in human hepatocellular carcinoma. Nano Res. 2015, 8, 1729–1745. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Ying, H.Z.; Hart, K.R.; Wu, Y.X.; Hsu, A.J.; Coppola, A.M.; Kim, T.A.; Yang, K.; Sottos, N.R.; White, S.R.; et al. Malleable and recyclable poly(urea-urethane) thermosets bearing hindered urea bonds. Adv. Mater. 2016, 28, 7646–7651. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Chen, J.J.; Cui, L.G.; Zhuang, X.L.; Ding, J.X.; Chen, X.S. Advances in stimuli-responsive polypeptide nanogels. Small Methods 2018, 2, 307–321. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Y.; Feng, X.; Ding, J. Functional polypeptide nanogels. J. Funct. Polym. 2019, 32, 13–27. [Google Scholar]
- Tang, B.Q.; Zaro, J.L.; Shen, Y.; Chen, Q.; Yu, Y.L.; Sun, P.P.; Wang, Y.Q.; Shen, W.C.; Tu, J.S.; Sun, C.M. Acid-sensitive hybrid polymeric micelles containing a reversibly activatable cell-penetrating peptide for tumor-specific cytoplasm targeting. J. Controlled Release 2018, 279, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Li, S.; Qiu, H.; Li, Z.; Wang, C.; Wang, X.; Ding, J. Polylactide-cholesterol stereocomplex micelle encapsulating chemotherapeutic agent for improved antitumor efficacy and safety. J. Biomed. Nanotechnol. 2018, 14, 2102–2113. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.H.; Ding, J.X.; Xiao, C.S.; Zhuang, X.L.; He, C.L.; Chen, L.; Chen, X.S. Intracellular microenvironment responsive PEGylated polypeptide nanogels with ionizable cores for efficient doxorubicin loading and triggered release. J. Mater. Chem. 2012, 22, 14168–14179. [Google Scholar] [CrossRef]
- Chu, B.Y.; Qu, Y.; Huang, Y.X.; Zhang, L.; Chen, X.X.; Long, C.F.; He, Y.Q.; Ou, C.W.; Qian, Z.Y. PEG-derivatized octacosanol as micellar carrier for paclitaxel delivery. Int. J. Pharm. 2016, 500, 345–359. [Google Scholar] [CrossRef]
- Cheng, Y.L.; He, C.L.; Ding, J.X.; Xiao, C.S.; Zhuang, X.L.; Chen, X.S. Thermosensitive hydrogels based on polypeptides for localized and sustained delivery of anticancer drugs. Biomaterials 2013, 34, 10338–10347. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, L.L.; Li, D.; Lao, Y.H.; Liu, D.Z.; Li, M.Q.; Ding, J.X.; Chen, X.S. Tumor microenvironment-responsive hyaluronate-calcium carbonate hybrid nanoparticle enables effective chemotherapy for primary and advanced osteosarcomas. Nano Res. 2018, 11, 4806–4822. [Google Scholar] [CrossRef]
- Wang, Y.P.; Gao, W.Q.; Shi, X.Y.; Ding, J.J.; Liu, W.; He, H.B.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Chen, J.J.; Ding, J.X.; Wang, Y.C.; Cheng, J.J.; Ji, S.X.; Zhuang, X.L.; Chen, X.S. Sequentially responsive shell-stacked nanoparticles for deep penetration into solid tumors. Adv. Mater. 2017, 29, 170–177. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.; Xu, W.; Yang, Y.; Zhuang, X.; Ding, J.; Chen, X. Chiral Polypeptide Thermogels Induce Controlled Inflammatory Response as Potential Immunoadjuvants. ACS Appl. Mater. Interfaces 2019, 11, 8725–8730. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.G.; Ding, J.X.; Xiao, C.S.; Li, L.Y.; Zhuang, X.L.; Chen, X.S. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Xu, C.; Zhao, X.; Lin, C.; Yang, X.; Xin, X.; Zhang, L.; Qn, C.; Han, X.; Yang, L.; et al. Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Feng, X.; Xu, W.; Wang, Y.; Yang, Y.; Jiang, Z.; Ding, J. PEGylated Polyurea Bearing Hindered Urea Bond for Drug Delivery. Molecules 2019, 24, 1538. https://doi.org/10.3390/molecules24081538
Chen M, Feng X, Xu W, Wang Y, Yang Y, Jiang Z, Ding J. PEGylated Polyurea Bearing Hindered Urea Bond for Drug Delivery. Molecules. 2019; 24(8):1538. https://doi.org/10.3390/molecules24081538
Chicago/Turabian StyleChen, Meishan, Xiangru Feng, Weiguo Xu, Yanqiao Wang, Yanan Yang, Zhongyu Jiang, and Jianxun Ding. 2019. "PEGylated Polyurea Bearing Hindered Urea Bond for Drug Delivery" Molecules 24, no. 8: 1538. https://doi.org/10.3390/molecules24081538
APA StyleChen, M., Feng, X., Xu, W., Wang, Y., Yang, Y., Jiang, Z., & Ding, J. (2019). PEGylated Polyurea Bearing Hindered Urea Bond for Drug Delivery. Molecules, 24(8), 1538. https://doi.org/10.3390/molecules24081538





