Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-Vis Spectra
2.2. Fluorescence Spectra
2.3. Infrared Spectra
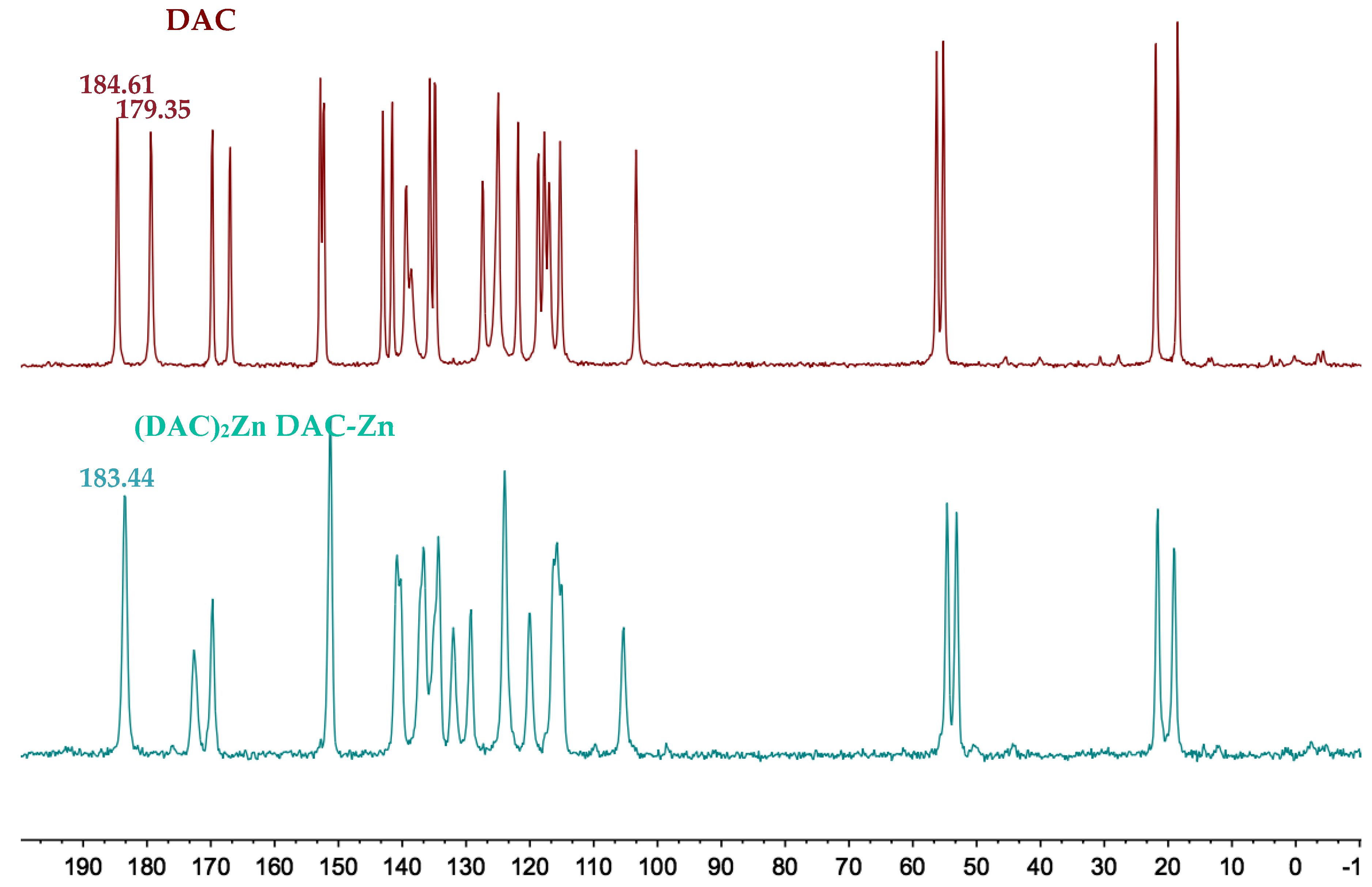
2.4. NMR Spectra
2.5. NMR Solid State Spectra
2.6. EPR Spectra
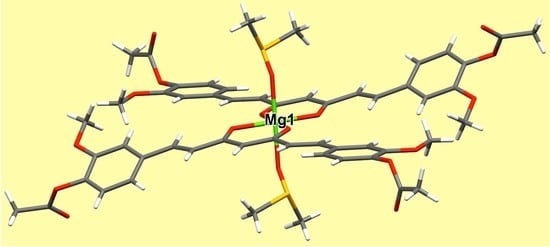
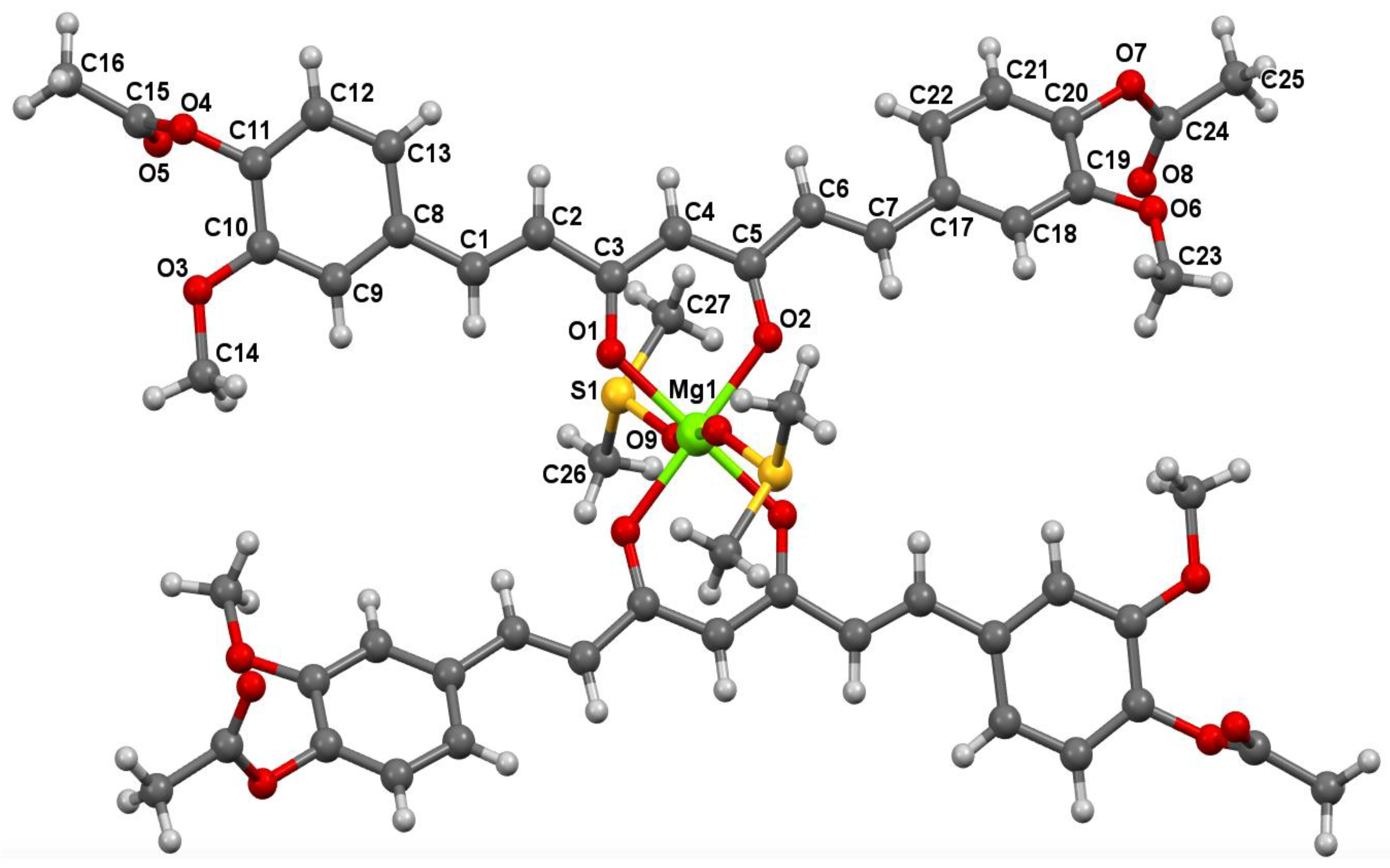
2.7. X-ray Diffraction
2.8. Inhibition of Lipid Peroxidation (LP) in Rat Brain Homogenate
2.9. Cytotoxic Activity
2.10. Acute Toxicity Study in Mice
3. Materials and Methods
3.1. Physical Measurements
3.2. Spectroscopic Determinations
3.3. Inhibition of Lipid Peroxidation in Rat Brain
3.3.1. Animals
3.3.2. Rat Brain Homogenate Preparation
3.3.3. Induction of Lipid Peroxidation and Thiobarbituric Acid Reactive Substances (TBARS) Quantification
3.4. Citotoxic Activity in Human Tumor Cells
3.5. Acute Toxicity Study In Mice
3.6. Synthesis of Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin-synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef]
- Ghosh, S.; Beberjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Sarkar, T.; Butcher, R.; Banerjee, S.; Murkherjee, S.; Hussain, A. Visible light-induced cytotoxicity of a dinuclear iron(III) complex of curcumin with low-micromolar IC50 value in cancer cells. Inorg. Chim. Acta. 2016, 439, 8–17. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Mohammadi, K.; Deilami, I.; Zandi, K.; Foiladvand, M.; Ramedani, E.; Asayesh, G. Antibacterial activity of indium curcumin and indium diacetylcurcumin. Afr. J. Biotechnol. 2008, 7, 3832–3835. [Google Scholar]
- Vreese, R.D.; Grootaert, C.; D´hoore, S.; Theppawong, A.; Van Damme, S.; Van Bogaert, M.; Van Camp, J.; D´hooghe, M. Synthesis and anticancer assessment of novel curcuminoides accommodating a central β-enaminone motif and their impact on cell growth and oxidative stress. Eur. J. Med. Chem. 2016, 123, 727–736. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, X.; Jiang, B.; Tian, Y. Metal complexes of a novel bis-β-diketone-type ligand and its copper(II) complexes of two-photon biological imaging. Sci. China Chem. 2012, 55, 334–340. [Google Scholar] [CrossRef]
- Rajesh, J.; Gubendran, A.; Rajagopal, G.; Athappan, P. Synthesis, spectra and DNA interactions of certain mononuclear transition metal(II) complexes of macrocyclic tetraaza diacetyl curcumin ligand. J. Mol. Struct. 2012, 1010, 169–178. [Google Scholar] [CrossRef]
- Wang, J.; Wei, D.; Jiang, B.; Liu, T.; Ni, J.; Zhou, S. Two copper(II) complexes of curcumin derivatives: Synthesis, crystal structure and in vitro antitumor activity. Transit. Met. Chem. 2014, 39, 553–558. [Google Scholar] [CrossRef]
- Pi, Z.; Wang, J.; Jiang, B.; Cheng, G.; Zhou, S. A curcumin-based TPA four-branched copper(II) complex probe for in vivo early tumor detection. Mater. Sci. Eng. C Mater. Biol Appl. 2015, 46, 565–571. [Google Scholar] [CrossRef]
- Thomachan, S.; Sindhu, S.; John, V.D. Synthesis, Characterization, Antibacterial, Antifungal and Cytotoxic Activity of Curcuminoid Analogues with Trisubstituted Phenyl and Anthracenyl ring and their Zinc(II), Copper(II) and Vanadyl(IV) Chelates. Int. J. Pharmac. Chem. 2016, 6, 78–86. [Google Scholar]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin–magnesium complex. Acta Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef]
- Pucci, D.; Bellini, T.; Crispini, A.; D’Agnano, I.; Liguori, P.; Garcia-Orduña, P.; Pirillo, S.; Valentini, A.; Zanchetta, G. DNA binding and cytotoxicity of fluorescent curcumin-based Zn(II) complexes. Med. Chem. Commun. 2012, 3, 462–468. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.; Shi, H.; Yang, M.; Liu, Y.; Ji, P.; Chen, H.; Tan, R.X.; Li, E. Curcumin is a biologically active copper chelator with antitumor activity. Phytomedicine 2016, 23, 1–8. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Watanabe, H.; Tohda, M.; Kummasud, N.; Sumanont, Y. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef]
- Kaur, A.; Banipal, P.K.; Banipal, T.S. Study on the interactional behaviour of transition metal ions with myoglobin: A detailed calorimetric, spectroscopic and light scattering analysis. Spectrochim. Acta Mol. Biomol. Spectrosc. 2017, 174, 236–244. [Google Scholar] [CrossRef]
- Aliaga-Alcalde, N.; Marqués-Gallego, P.; Kraaijkamp, M.; Herranz-Lancho, C.; Dulk, H.D.; Görner, H.; Roubeau, O.; Teat, S.J.; Weyhermüller, T.; Reedijk, J. Copper Curcuminoids Containing Anthracene Groups: Fluorescent Molecules with Cytotoxic Activity. Inorg. Chem. 2010, 49, 9655–9663. [Google Scholar] [CrossRef] [Green Version]
- Aliaga-Alcalde, N.; Rodríquez, L.; Febinteanu, M.; Höfer, P.; Weyhermüller, T. Crystal Structure, Fluorescence, and Nanostructuration Studies of the First ZnII Anthracene-Based Curcuminoid. Inorg. Chem. 2012, 51, 864–873. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, D. Curcumin, a Biological Wonder Molecule: A Crystal Engineering Point of View. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Machado-Rodriguez, J.C.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Nieto-Camacho, A.; Toscano, R.A.; Soriano-García, M.; Cassani, J.; Enríquez, R.G. A New Family of Homoleptic Copper Complexes of Curcuminoids: Synthesis, Characterization and Biological Properties. Molecules 2019, 24, 910. [Google Scholar] [CrossRef]
- Aiyub, Z.; Abdullah, Z.; Yaakob, B.H.; Abu Bakar, M.A. Fluorescence characteristic of 2-chloropyrazine: Effect of solvents and concentration of Cu(II). MJAC 2007, 11, 105–109. [Google Scholar]
- Badaruddin, E.; Aiyub, Z.; Abdullah, A. Fluorescence properties of metal complexes of 2-N-anilinopyrimidine. MJAC 2008, 12, 285–290. [Google Scholar]
- Zhao, X.Z.; Jiang, T.; Wang, L.; Yang, H.; Zhang, S.; Zhou, P.J. Interaction of curcumin with Zn(II) and Cu(II) ions based on experiment and theoretical calculation. J. Mol. Struct. 2010, 984, 316–325. [Google Scholar] [CrossRef]
- John, V.D.; Krishnankutty, K. Antitumour activity of synthetic curcuminoid analogues (1,7-diaryl-1,6-heptadiene-3,5-diones) and their copper complexes. Appl. Organometal Chem. 2006, 20, 477–482. [Google Scholar] [CrossRef]
- Asti, M.; Ferrari, E.; Croci, S.; Atti, G.; Rubagotti, S.; Iori, M.; Cappon, P.C.; Zerbini, A.; Saladini, M.; Versari, A. Synthesis and Characterization of 68Ga-Labeled Curcumin and Curcuminoid Complexes as Potential Radiotracers for Imaging of Cancer and Alzheimer’s Disease. Inorg. Chem. 2014, 53, 4922–4933. [Google Scholar] [CrossRef]
- Rapheal, P.F.; Manoj, E.; Prathapachandra Kurup, M.R. Syntheses and EPR spectral studies of manganese(II) complexes derived from pyridine-2-carbaldehyde based N(4)-substituted thiosemicarbazones: Crystal structure of one complex. Polyhedron 2007, 26, 5088–5094. [Google Scholar] [CrossRef]
- Attanasio, D.; Collamati, I.; Ercolani, C. Ligand arrangement in tetragonally CuO4N and CuO4N2 chromophores formed from copper(II) α-nitroketonates and sterically hindered N-bases. Dalton Trans. 1974, 0, 2442–2448. [Google Scholar] [CrossRef]
- Garribba, E.; Micera, G. The Determination of the Geometry of Cu(II) Complexes an EPR Spectroscopy Experiment. J. Chem. Educ. 2006, 83, 1229–1232. [Google Scholar] [CrossRef]
- Reed, G.H.; Cohn, M. Electron Paramagnetic Resonance Studies of Manganese(II)-Pyruvate Kinase-Substrate Complexes. J. Biol. Chem. 1973, 18, 6436–6442. [Google Scholar]
- Rajagopal, G.; Prasanna, N.; Athappan, P. Copper(II) and Ruthenium(II)/(III) Schiff base complexes. Trans. Metal Chem. 1999, 24, 251–257. [Google Scholar] [CrossRef]
- Nishio, M.; Umezawa, Y.; Hirota, M.; Takeuchi, Y. The CH/π interaction: Significance in molecular recognition. Tetrahedron 1995, 51, 8665–8701. [Google Scholar] [CrossRef]
- Clegg, J.K.; Lindoy, L.F.; McMurtrie, J.C.; Schilter, D. Dinuclear bis-β-diketonato ligand derivatives of iron(III) and copper(II) and use of the latter as components for the assembly of extended metallo-supramolecular structures. Dalton Trans. 2005, 5, 857–864. [Google Scholar] [CrossRef]
- Shiga, T.; Ohba, M.; Okawa, H. A Series of Trinuclear CuIILnIIICuII Complexes Derived from 2,6-Di(acetoacetyl)pyridine: Synthesis, Structure, and Magnetism. Inorg. Chem. 2004, 43, 4435–4446. [Google Scholar] [CrossRef]
- Morosin, B. The crystal structure of diaquobis(acetylacetonato)magnesium(II). Acta Cryst. 1967, 22, 316–320. [Google Scholar] [CrossRef]
- Bennett, M.J.; Cotton, F.A.; Eiss, R. The crystal and molecular structure of trimeric bis(acetylacetonato) zinc(II). Acta Cryst. 1968, B24, 904–913. [Google Scholar] [CrossRef]
- Lebrun, P.C.; Lyon, W.D.; Kuska, H.A. Crystal structure of bis(2,4-pentanedionato) copper(II). J. Chem. Crystallogr. 1986, 16, 889–893. [Google Scholar] [CrossRef]
- Onuma, S.; Shibata, S. The crystal and molecular structure of bis (acetylacetonato)manganese(II) dehydrate. Bull. Chem. Soc. Jpn. 1970, 43, 2395–2397. [Google Scholar] [CrossRef]
- Renfrew, A.K.; Bryce, N.S.; Hambley, T.W. Delivery and release of curcumin by hypoxia-activated cobalt chaperone: A XANES and FLIM study. Chem. Sci. 2013, 3, 3731. [Google Scholar] [CrossRef]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutla, S.; Zhang, H.; Priyadarsini, K.L. Evaluation of a new copper(II) curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radical Biol. Med. 2005, 39, 811–822. [Google Scholar] [CrossRef]
- Banarjee, S.; Chakravarty, A.R. Metal complexes of curcumin for cellular imaging, targeting, and photoinduced anticancer activity. Acc. Chem. Res. 2012, 48, 2075–2083. [Google Scholar] [CrossRef]
- Wilson, J.J.; Lippard, S.J. Synthetic methods for the preparation of platinum anticancer complexes. Chem. Rev. 2014, 114, 4470–4495. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 6th ed.; Butterworth Heinemann: Oxford, UK, 2009; pp. 138–159. [Google Scholar]
- Bruker, APEX2 and SAINT-Plus; Bruker AXS Inc.: Madison, WI, USA, 2004.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sec. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, M.; Nieto, A.; Marín, J.C.; Keck, A.S.; Jeffery, E.; Céspedes, C.L. Antioxidant activities of extractsfrom Barkleyanthus salicifolius (Asteraceae) and Penstemon gentianoides (Scrophulariaceae). J. Agric. Food Chem. 2005, 53, 5889–5895. [Google Scholar] [CrossRef]
- Rossato, J.I.; Ketzer, L.A.; Centuriao, F.B.; Silva, S.J.; Lüdtke, D.S.; Zeni, G.; Braga, A.L.; Rubin, M.A.; Rocha, B.T. Antioxidant properties of new chalcogenides against lipid peroxidation in rat brain. Neurochem. Res. 2002, 27, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehyde lipid peroxidation products. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paul, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 38, 757–766. [Google Scholar] [CrossRef]
- Sumantran, V.N. Cellular chemosensitivity assays: An overview. In Cancer Cell Culture: Methods and Protocols, 2nd ed.; Cree, I.A., Ed.; Humana Press: New York, NY, USA, 2011; pp. 219–236. [Google Scholar]
- Lozada, C.; Soria-Arteche, O.; Ramírez-Apan, M.T.; Nieto-Camacho, A.; Enríquez, R.G.; Izquierdo, T.; Jiménez-Corona, A. Synthesis, cytotoxic and antioxidant evaluations of amino derivatives from perezone. Bioorg. Med. Chem. 2012, 20, 5077–5084. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D. A New approach to practical acute toxicity testing. Arch. Toxicol. 1963, 54, 275–287. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animal. 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Lozada, C. Study of the reactivity of β-diketones against nucleophilic molecules: Synthesis of new heterocyclic derivatives of curcumin and 2,4-pentanodione. Ph.D. Thesis, National Autonomous University of Mexico, Mexico, 2005. [Google Scholar]
Sample Availability: Not available. |



| Ligand & Complexes | π–π* (nm) | ɛmax (L/cm mol) | n–π* (nm) | ɛ max (L/cm mol) | CT (nm) | ɛ max (L/cm mol) |
|---|---|---|---|---|---|---|
| DAC (1) | 408 | 349,187.06 | 328 | 34,955.35 | - | - |
| (DAC)2Mg (2) | 414 | 587,658.78 | 328 | 137,652.06 | - | - |
| (DAC)2Zn (3) | 415 | 250,821.69 | 338 | 211,677.96 | 439 | 161,111.68 |
| (DAC)2Cu (4) | 414 | 387,091.23 | 338 | 128,582.44 | 438 | 266,119.18 |
| (DAC)2Mn (5) | 418 | 300,551.07 | 328 | 111,206.39 | 440 | 213,207.90 |
| Ligand & Complexes | Excitation Wavelength (nm) | Fluorescence Wavelength (nm) | Intensity (I) |
|---|---|---|---|
| DAC (1) | 408 | 457.77 | 280,465.66 |
| (DAC)2Mg (2) | 414 | 458.64 | 412,828.49 |
| (DAC)2Zn (3) | 415 | 459.06 | 175,399.40 |
| (DAC)2Cu (4) | 414 | 458.50 | 230,331.13 |
| (DAC)2Mn (5) | 418 | 459.08 | 188,354.49 |
| Ligand & Complexes | δ (ppm) of Methine in DMSO-d6 |
|---|---|
| DAC (1) | 6.20 |
| (DAC)2Mg (2) | 5.71 |
| (DAC)2Zn (3) | 5.84 |
| (DAC)2Cu (4) | (Not observed) |
| (DAC)2Mn (5) | 5.32 |
| Complex | g║ | g┴ | A║ (10−4 cm−1) | A┴ (10−4 cm−1) | g║/A║ (cm−1) | µeffect |
|---|---|---|---|---|---|---|
| (DAC)2Cu (4) | 2.29 | 2.06 | 162.3 | 1.13 | 141.1 | 1.7 |
| Complex | g | A (10−4 cm−1) | g/A (cm−1) | µeffect |
|---|---|---|---|---|
| (DAC)2Mn (5) | 2.00 | 85 | 235.3 | 6.2 |
| Compound | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| CCDC deposit No. | 1,418,638 | 1,453,160 | 1,506,152 | 1,534,704 |
| Crystal size (mm) | 0.23 × 0.05 × 0.04 | 0.20 × 0.18 × 0.04 | 0.10 × 0.04 × 0.040 | 0.29 × 0.11 × 0.03 |
| Color/shape | Colorless/prism | Yellow/prism | Yellow/prism | Colorless/prism |
| Empirical formula | C54H58 Mg O18S2 | C52H52 Zn O17S | C53H53CuNO17 | C54H58MnO18S2 |
| Formula weight | 1083.43 | 1046.36 | 1039.50 | 1114.06 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | P-1 | P-1 | P-1 | P-1 |
| Unit cell dimensions | ||||
| a, Å | 7.4419(5) | 7.4706(2) | 12.7683(7) | 7.513(5) |
| b, Å | 12.1480(7) | 11.7637(4) | 13.2372(7) | 12.165(14) |
| c, Å | 15.8082(10) | 30.7699(10) | 15.3255(8) | 15.688(17) |
| α, deg | 80.701(2) | 81.6770(10) | 99.5684(18) | 80.87(10) |
| β, deg | 85.653(2) | 86.8260(10) | 103.0083(19) | 85.68(6) |
| γ, deg | 81.390(2) | 79.6230(10) | 94.303(2) | 80.40(7) |
| Volume, Å3 | 1392.50(15) | 2630.68(14) | 2471.6(2) | 1394(2) |
| Z | 1 | 2 | 2 | 1 |
| Density (calculated), Mg /m3 | 1.292 | 1.321 | 1.397 | 1.327 |
| Absorption coefficient, mm−1 | 0.177 | 0.576 | 0.517 | 0.382 |
| F (000) | 570 | 1092 | 1086 | 583 |
| Θ range for data collection (°) | 2.31 to 25.37 | 2.07 to 25.36 | 2.41 to 25.37 | 2.31 to 25.40 |
| Index ranges | −8 ≤ η ≤ 8, −14 ≤ κ ≤ 14, −19 ≤ λ ≤ 18 | −8 ≤ η ≤ 8, −14 ≤ κ ≤ 14, −36 ≤ λ ≤ 37 | −15 ≤ η ≤ 15, −15 ≤ κ ≤ 15, −18 ≤ λ ≤ 16 | −8 ≤ η ≤ 9, −14 ≤ κ ≤ 14, −18 ≤ λ ≤ 18 |
| Reflections collected | 20,701 | 43,039 | 40,595 | 22,968 |
| Independent reflections | 5086 (Rint = 0.1991) | 9550 (Rint = 0.0736) | 9019 (Rint = 0.16875) | 5086 (Rint = 0.1893) |
| Data/restraints/parameters | 5086/0/346 | 9550/90/676 | 9019/0/659 | 5086/20/357 |
| Goodness-of-fit on F2 | 0.859 | 1.054 | 1.096 | 1.034 |
| Final R índices [(Ι) > 2σ (Ι)] | R1 = 0.0676, wR2 = 0.1330 | R1 = 0.0685, wR2 = 0.1517 | R1 = 0.0889, wR2 = 0.1580 | R1 = 0.0768, wR2 = 0.1538 |
| R indices (all data) | R1 = 0.1798, wR2 = 0.1812 | R1 = 0.1073, wR2 = 0.1714 | R1 = 0.1644, wR2 = 0.1949 | R1 = 0.1571, wR2 = 0.1939 |
| Largest diff. peak and hole (ε/A−3) | 0.300 and −0.430 | 1.354 and −0.495 | 0.539 and −0.606 | 0.337 and −0.373 |
| Compounds | M-O (Å) | Melting Point | Geometry |
|---|---|---|---|
| (DAC)2Mg (2) | Mg(1)-O(1) 2.022 Mg(1)-O(2) 2.053 | 245.6 °C | octahedral |
| acac-Mg | Mg(1)-O(1) 2.040 Mg(1)-O(2) 2.027 | 265 °C | octahedral |
| (DAC)2Zn (3) | Zn(1)-O(1) 2.021 Zn(1)-O(2) 1.987 Zn(1)-O(9) 2.031 Zn(1)-O(10) 1.991 | 247.7 °C | trigonal bipyramid |
| acac-Zn | Zn(1)-O(11) 2.002 Zn(1)-O(12) 1.971 Zn(1)-O(21) 2.008 Zn(1)-O(22) 2.096 | 136.5 °C | trigonal bipyramid |
| (DAC)2Cu (4) | Cu(1)-O(1) 1.906 Cu(1)-O(2) 1.900 Cu(1)-O(9) 1.916 Cu(1)-O(10) 1.913 | 242.5 °C | square planar |
| acac-Cu | Cu(1)-O(1) 1.914 Cu(1)-O(2) 1.912 | 285.5 °C | square planar |
| (DAC)2Mn (5) | Mn(1)-O(1) 2.108 Mn(1)-O(2) 2.132 | 191 °C | octahedral |
| acac-Mn | Mn(1)-O(1) 2.150 Mn(1)-O(2) 2.129 | 249 °C | octahedral |
| Compounds | Concentration (μM) | TBARS (nmol/mg Protein) | % Inhibition | IC50 (μM) |
|---|---|---|---|---|
| Basal | - | 0.19 ± 0.03 | - | - |
| Control FeSO4 | - | 10.11 ± 0.56 | - | - |
| α−Tocoferol (n = 4) | Basal Control 0.1 0.32 1 3.16 10 31.62 100 | 0.200 ± 0.011 6.589 ± 0.213 6.283 ± 0.188 6.048 ± 0.242 5.211 ± 0.332 * 3.676 ± 0.569 ** 2.725 ± 0.335 ** 1.849 ± 0.319 ** 1.408 ± 0.364 ** | - - 4.62 ± 0.57 8.26 ± 1.31 21.13 ± 2.56 * 44.84 ± 6.74 ** 59.00 ± 3.71 ** 72.3 ± 3.87 ** 79.09 ± 4.79 ** | 6.78 ± 2.16 |
| BHT (n = 5) | Basal Control 0.32 0.42 0.56 0.75 1 1.33 1.78 2.37 3.16 | 0.268 ± 0.053 7.384 ± 0.630 6.690 ± 0.655 6.510 ± 0.551 6.098 ± 0.353 5.559 ± 0.294 * 4.457 ± 0.283 ** 3.228 ± 0.572 ** 1.315 ± 0.489 ** 0.487 ± 0.075 ** 0.53 ± 0.044 ** | - - 9.53 ± 2.91 11.62 ± 2.78 16.64 ± 2.86 23.92 ± 2.69 * 37.14 ± 7.44 ** 53.59 ± 8.93 ** 81.59 ± 6.89 ** 93.16 ± 1.16 ** 95.08 ± 0.68 ** | 1.22 ± 0.44 |
| DAC (1) | 0.1 0.32 1 3.16 10 | 9.68 ± 0.43 9.29 ± 0.67 7.78 ± 0.53 * 5.22 ± 0.35 ** 1.43 ± 0.08 ** | 4.16 ± 1.97 8.32 ± 1.49 23.19 ± 0.92 * 48.46 ± 1.53 ** 85.82 ± 0.60 ** | 3.21 ± 0.16 |
| (DAC)2Mg (2) | 0.1 0.33 1 3.28 10.38 | 9.50 ± 0.63 8.48 ± 0.78 6.55 ± 0.67 ** 3.56 ± 0.37 ** 0.34 ± 0.02 ** | 6.20 ± 1.00 16.41 ± 3.31 35.52 ± 3.78 ** 64.52 ± 4.42 ** 96.63 ± 0.08 ** | 2.03 ± 0.27 |
| (DAC)2Zn (3) | 0.1 0.32 1 3.22 10.20 | 9.16 ± 0.90 8.02 ± 0.72 6.22 ± 0.67 ** 2.87 ± 0.14 ** 0.35 ± 0.02 ** | 9.87 ± 3.73 20.99 ± 2.56 38.85 ± 3.04 ** 71.53 ± 1.67 ** 96.53 ± 0.41 ** | 1.58 ± 0.07 |
| (DAC)2Cu (4) | 0.1 0.33 1 3.22 10.20 | 9.08 ± 0.66 8.04 ± 0.70 6.24 ± 0.71 ** 3.08 ± 0.21 ** 1.32 ± 0.48 ** | 10.34 ± 1.48 20.78 ± 2.44 38.70 ± 3.82 ** 69.57 ± 1.56 ** 86.58 ± 5.21 ** | 1.58 ± 0.15 |
| (DAC)2Mn (5) | 0.1 0.33 1 3.22 10.19 | 8.79 ± 0.68 7.87 ± 0.69 * 5.71 ± 0.31 ** 1.61 ± 0.48 ** 0.29 ± 0.01 ** | 14.18 ± 2.76 23.20 ± 2.76 * 44.10 ± 0.55 ** 83.69 ± 5.37 ** 97.18 ± 0.14 ** | 1.24 ± 0.10 |
| Compounds | HCT-15 | MCF-7 | SKLU-1 |
|---|---|---|---|
| DAC (1) | 22.32 ± 3.0 | 21.79 ± 5.0 | 14.07 ± 3.3 |
| (DAC)2Mg (2) | 11.59± 1.97 * | 5.72 ± 0.31 * | 8.87 ± 1.56 * |
| (DAC)2Zn (3) | 7.21 ± 0.61 * | 4.92 ± 0.071 * | 4.74 ± 0.31 * |
| (DAC)2Mn (5) | 15.54 ± 1.94 * | 13.46 ± 1.63 * | 9.23 ± 1.22 * |
| Cisplatin | 10.0 ± 0.9 * | 9.4 ± 1.0 * | 4.3 ± 0.5 * |
| Complexes | Doses (mg/kg) | No. of Animals | Sex | Body Weight at Day | % Body Weight Gain | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 15 | 1–8 | 8–15 | ||||
| (DAC)2Mg | 1000 | 3 | Male | 25.3 ± 0.3 | 27.0 ± 0.5 | 30.0 ± 0.4 | 6.7 ± 0.3 | 10.7 ± 0.6 |
| (DAC)2Mg | 3000 | 3 | Male | 26.5 ± 0.1 | 28.6 ± 0.2 | 32.0 ± 1.5 | 7.5 ± 0.3 | 13.3 ± 1.5 |
| (DAC)2Zn | 1000 | 3 | Male | 27.1 ± 0.5 | 28.6 ± 0.2 | 31.3 ± 0.5 | 5.5 ± 0.7 | 9.8 ± 0.4 |
| (DAC)2Zn | 3000 | 3 | Male | 28.4 ± 0.4 | 30.0 ± 0.3 | 33.7 ± 0.3 | 5.6 ± 0.1 | 12.3 ± 0.0 |
| (DAC)2Cu | 1000 | 3 | Male | 29.3 ± 0.6 | 31.4 ± 1.1 | 33.6 ± 0.3 | 8.5 ± 0.7 | 8.0 ± 1.1 |
| (DAC)2Cu | 3000 | 3 | Male | 27.5 ± 0.9 | 29.4 ± 1.5 | 30.5 ± 0.8 | 6.5 ± 0.6 | 4.1 ± 0.8 |
| (DAC)2Mn | 1000 | 3 | Male | 25.8 ± 0.2 | 26.9 ± 0.5 | 29.0 ± 0.4 | 6.5 ± 0.6 | 4.1 ± 0.8 |
| (DAC)2Mn | 3000 | 3 | Male | 26.1 ± 1.8 | 27.6 ± 0.8 | 29.4 ± 1.3 | 4.2 ± 1.0 | 5.8 ± 0.5 |
| Complexes | Doses (mg/kg) | No. of Animals | Sex | Clinical Signs | Mortality |
|---|---|---|---|---|---|
| (DAC)2Mg | 1000 | 3 | male | normal | 0 |
| (DAC)2Mg | 3000 | 3 | male | normal | 0 |
| (DAC)2Zn | 1000 | 3 | male | normal | 0 |
| (DAC)2Zn | 3000 | 3 | male | normal | 0 |
| (DAC)2Cu | 1000 | 3 | male | bristly fur | 0 |
| (DAC)2Cu | 3000 | 3 | male | bristly fur | 0 |
| (DAC)2Mn | 1000 | 3 | male | bristly fur | 0 |
| (DAC)2Mn | 3000 | 3 | male | bristly fur | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Morales, W.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules 2019, 24, 1598. https://doi.org/10.3390/molecules24081598
Meza-Morales W, Estévez-Carmona MM, Alvarez-Ricardo Y, Obregón-Mendoza MA, Cassani J, Ramírez-Apan MT, Escobedo-Martínez C, Soriano-García M, Reynolds WF, Enríquez RG. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules. 2019; 24(8):1598. https://doi.org/10.3390/molecules24081598
Chicago/Turabian StyleMeza-Morales, William, M. Mirian Estévez-Carmona, Yair Alvarez-Ricardo, Marco A. Obregón-Mendoza, Julia Cassani, María Teresa Ramírez-Apan, Carolina Escobedo-Martínez, Manuel Soriano-García, William F. Reynolds, and Raúl G. Enríquez. 2019. "Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo" Molecules 24, no. 8: 1598. https://doi.org/10.3390/molecules24081598