Microwave-Assisted Synthesis of Trazodone and Its Derivatives as New 5-HT1A Ligands: Binding and Docking Studies
Abstract
1. Introduction
2. Results and Discussion
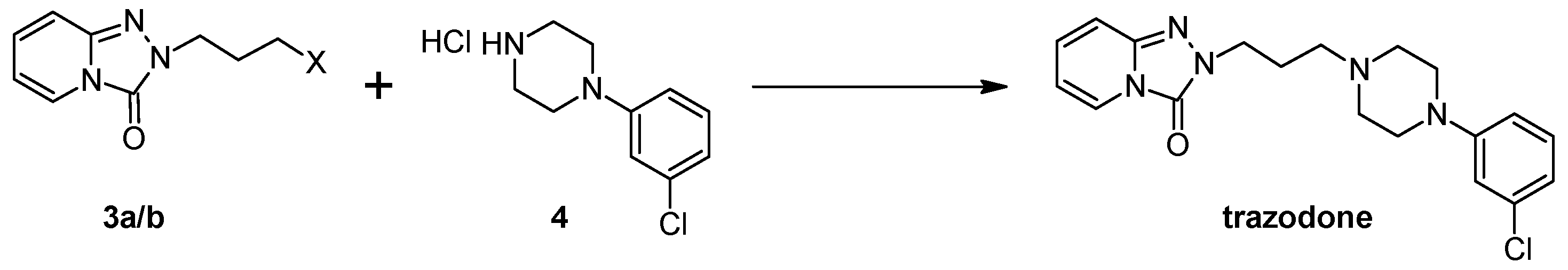
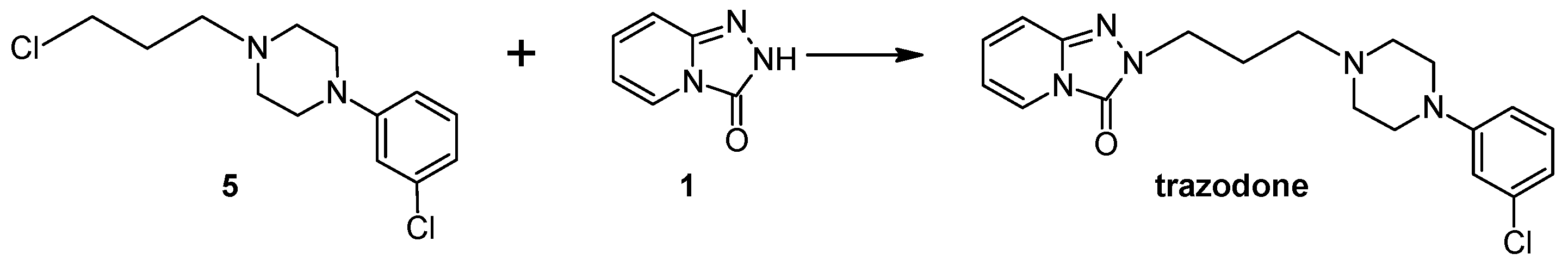
2.1. Synthesis of Trazodone
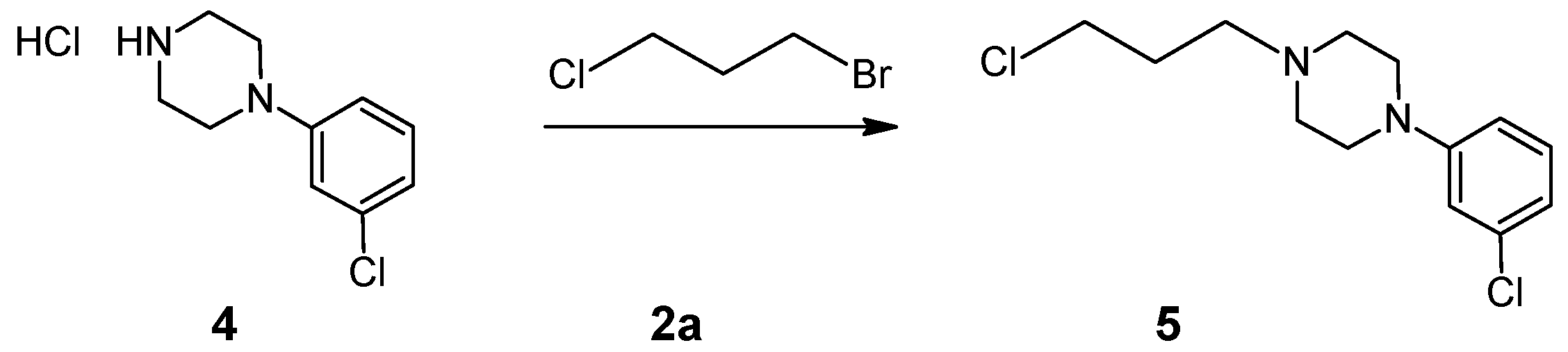
2.1.1. Method I
2.1.2. Method II
2.1.3. Method III—One-Pot Synthesis
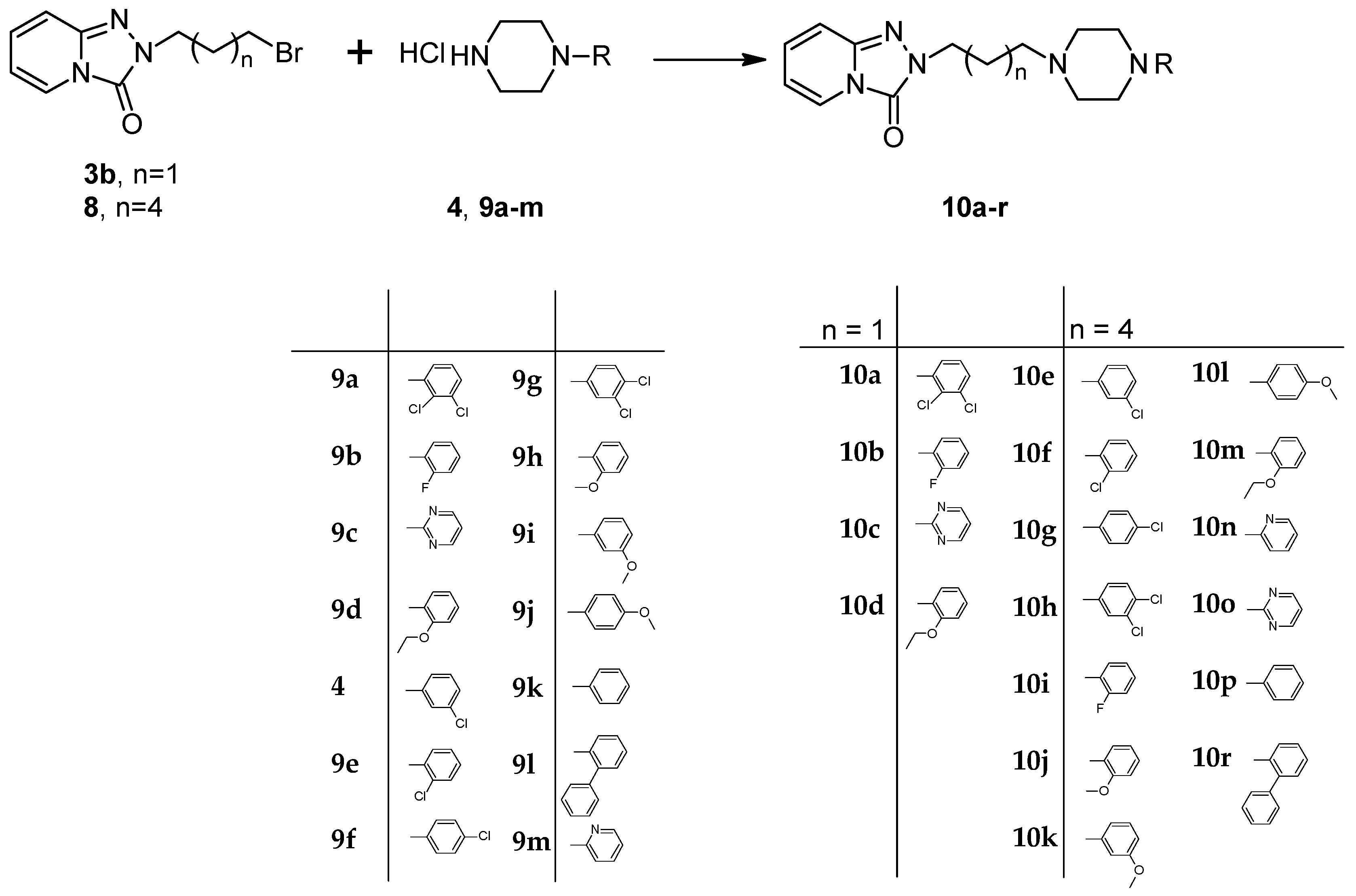
2.2. Synthesis of Trazodone Derivatives
2.3. Biological Evaluation of Trazodone Derivatives
2.4. Molecular Modeling
3. Experimental
3.1. Materials and Methods
3.2. General Procedure for the Preparation of 2-Hydrazinopyridine (7) under Microwave Conditions (Samsung M182DN; 300 W)
3.3. General Procedure for the Preparation of 1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one (1)
3.3.1. Preparation of 1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one (1) from 7 under Microwave Conditions (Samsung M182DN; 300 W)
3.3.2. Preparation of 1,2,4-Triazolo [4,3-a] pyridin-3(2H)-one (1) from 6a/b and Semicarbazide under Microwave Conditions (Samsung M182DN; 300 W)
3.3.3. Preparation of 1,2,4-Triazolo[4,3-a]pyridin-3(2H)-one (1) from 6b and Semicarbazide under Microwave Conditions (Magnum II Reactor; 600 W)
3.4. General Procedures for the Preparation of 2-(3-Halopropyl)[1,2,4]triazolo[4,3-a]pyridin-3 (2H)-one (3a/b)
3.4.1. Synthesis 2-(3-Halopropyl)[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (3a/b) (Samsung M182DN; 300 W)
3.4.2. Synthesis 2-(3-Chloropropyl)[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one (3a) (Magnum II Reactor; 600 W)
3.5. General Procedures for the Preparation of Trazodone According to Method I
3.5.1. Preparation of Trazodone according to Method I under Microwave Conditions (Samsung M182DN; 300 W)
3.5.2. Preparation of Trazodone According to Method I under Microwave Conditions (Magnum II Reactor; 600 W)
3.6. General Procedures for the Preparation of 1-(3-Chloropropyl)-4-(3-chlorophenyl)piperazine (5)
3.6.1. Preparation of 1-(3-Chloropropyl)-4-(3-chlorophenyl)piperazine (5) under Microwave Radiation (Samsung M182DN; 300 W)
3.6.2. Preparation of 1-(3-Chloropropyl)-4-(3-chlorophenyl)piperazine (5) under Microwave Radiation (Magnum II Reactor; 600 W)
3.7. General Procedures for the Preparation of Trazodone According to Method II
3.7.1. Preparation of Trazodone According to Method II under Microwave Conditions (Samsung M182DN; 300 W)
3.7.2. Preparation of Trazodone According to Method II under Microwave Conditions (Magnum II Reactor; 600 W)
3.8. General Procedure for the Preparation of Trazodone in One-Pot Synthesis
3.9. General Procedure for the Preparation of 2-(3-Bromohexyl)-1,2,4-triazolo[4,3-a]pyridin-3-(2H)-one (8)
3.10. General Procedure for the Preparation Trazodone Derivatives
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Palazzo, G.; Silvestrini, B. Triazole-(4,3-a)-pyridines. U.S. Patent 3,381,009, 30 April 1968. [Google Scholar]
- Pai, N.R.; Pusalkar, D.A. An efficient synthesis of neuroleptic drugs under microwave irradiation. J. Chem. Pharm. Res. 2010, 2, 506–517. [Google Scholar]
- Gant, T.G.; Sarshar, S. Substituted Triazolopyridines. U.S. Patent 2009/209,550 A1, 20 August 2009. [Google Scholar]
- Yong, F.-F.; Teo, Y.-C.; Tan, K.-N. Efficient copper-catalyzed cross-coupling of 1-Boc-piperazine with aryl iodides and its application in the synthesis of trazodone. Tetrahedron Letters 2013, 54, 5332–5334. [Google Scholar] [CrossRef]
- Jaśkowska, J. Method for the Preparation of Trazodone. WO2015110883 A1, 14 March 2017. [Google Scholar]
- Savitz, J.; Lucki, I.; Drevets, W.C. 5-HT1A Receptor Function in Major Depressive Disorder. Prog. Neurobiol. 2009, 88, 17–31. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Newman-Tancredi, A.; Leonardo, E.D. 5-HT1A receptors in mood and anxiety: Recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl) 2014, 231, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Graeff, F.G.; Guimarães, F.S.; De Andrade, T.G.C.S.; Deakin, J.F.W. Role of 5-HT in stress, anxiety, and depression. Pharmacol. Biochem. Behav. 1996, 54, 129–141. [Google Scholar] [CrossRef]
- Schreiber, R.; De Vry, J. 5-HT1A Receptor ligands in animal models of anxiety, impulsivity and depression: Multiple mechanisms of action? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1993, 17, 87–104. [Google Scholar] [CrossRef]
- Yohn, C.N.; Gergues, M.M.; Samuels, B.A. The role of 5-HT receptors in depression. Mol. Brain 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Nikiforuk, A. Targeting the Serotonin 5-HT7 Receptor in the Search for Treatments for CNS Disorders: Rationale and Progress to Date. CNS Drugs 2015, 29, 265–275. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Popova, N.K.; Lacivita, E.; Leopoldo, M.; Ponimaskin, E.G. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci Ther. 2014, 20, 582–590. [Google Scholar] [CrossRef]
- Costa, L.; Sardone, L.M.; Lacivita, E.; Leopoldo, M.; Ciranna, L. Novel agonists for serotonin 5-HT7 receptors reverse metabotropic glutamate receptor-mediated long-term depression in the hippocampus of wild-type and Fmr1 KO mice, a model of Fragile X Syndrome. Front Behav. Neurosci. 2015, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, A. Potential role of the 5-HT6 receptor in depression and anxiety: An overview of preclinical data. Pharmacol. Rep. 2010, 62, 564–577. [Google Scholar] [CrossRef]
- Carr, G.V.; Schechter, L.E.; Lucki, I. Antidepressant and anxiolytic effects of selective 5-HT6 receptor agonists in rats. Psychopharmacology (Berl) 2011, 213, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Staroń, J.; Bugno, R.; Hogendorf, A.S.; Bojarski, A.J. 5-HT1A receptor ligands and their therapeutic applications: Review of new patents. Expert Opin. Ther. Pat. 2018, 28, 679–689. [Google Scholar] [CrossRef]
- Kowalski, P.; Mitka, K.; Jaśkowska, J.; Bojarski, A.J.; Duszyńska, B. New arypiperazines with flexible vs partly constrained linker as serotonin 5-HT1A/5-HT7 receptor ligands. Archiv der Pharmazie 2013, 346, 339–348. [Google Scholar] [CrossRef]
- Kowalski, P.; Jaśkowska, J.; Bojarski, A.J.; Duszyńska, B.; Kołaczkowski, M. Evaluation of 1-arylpiperazine derivative of salicylamides as the 5-HT1A and 5-HT7 serotonin receptor ligands. J. Heterocycl. Chem. 2011, 48, 192–198. [Google Scholar] [CrossRef]
- Jaśkowska, J.; Drabczyk, A.K.; Kułaga, D.; Majka, Z. Solvent-free microwave-assisted synthesis of aripiprazole. Curr. Chem. Letters 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Jarema, M.; Dudek, D.; Landowski, J.; Heitzman, J.; Rabe-Jabłońska, J.; Rybakowski, J. Trazodon-lek przeciwdepresyjny: Mechanizm działania i miejsce w leczeniu depresji. Psychiatria Polska 2011, 750, 611–625. [Google Scholar]
- Temple, D.L., Jr.; Lobeck, W.G., Jr. Phenoxyethyl-1,2,4,-triazol-3-one Antidepressants. U.S. Patent 4,338,317, 6 July 1982. [Google Scholar]
- Pindelska, E.; Madura, I.D.; Szeleszczuk, Ł.; Żeszko, A.; Jaśkowska, J.; Marek, P.H.; Kolodziejski, W. Alkyl spacer length and protonation induced changes in crystalline psychoactive arylpiperazine derivatives: Single-crystal X-ray, solid-state NMR, and computational studies. Cryst. Growth Des. 2016, 16, 6371–6380. [Google Scholar] [CrossRef]
- Richelson, E.; Nelson, A. Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J. Pharmacol. Exp. Ther. 1984, 230, 94–102. [Google Scholar]
- Pai, N.R.; Pusalkar, D.A. Development and validation of liquid chromatogrphic method for Trazodone hydrochloride. J. Chem. Pharm. Res. 2010, 2, 478–488. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Rataj, K.; Witek, J.; Mordalski, S.; Kosciolek, T.; Bojarski, A.J. Impact of template choice on homology model efficiency in virtual screening. J. Chem. Inf. Model. 2014, 54, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

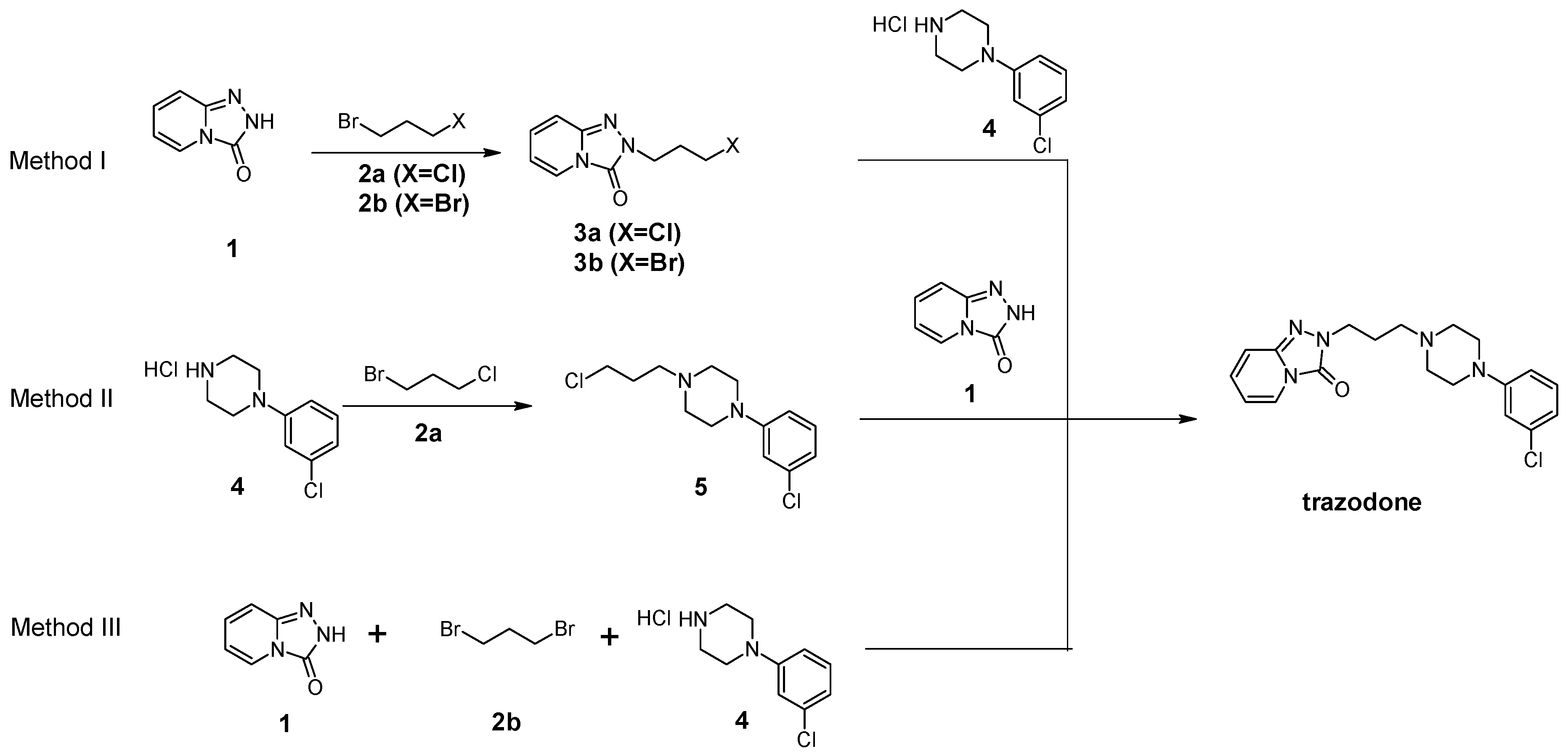

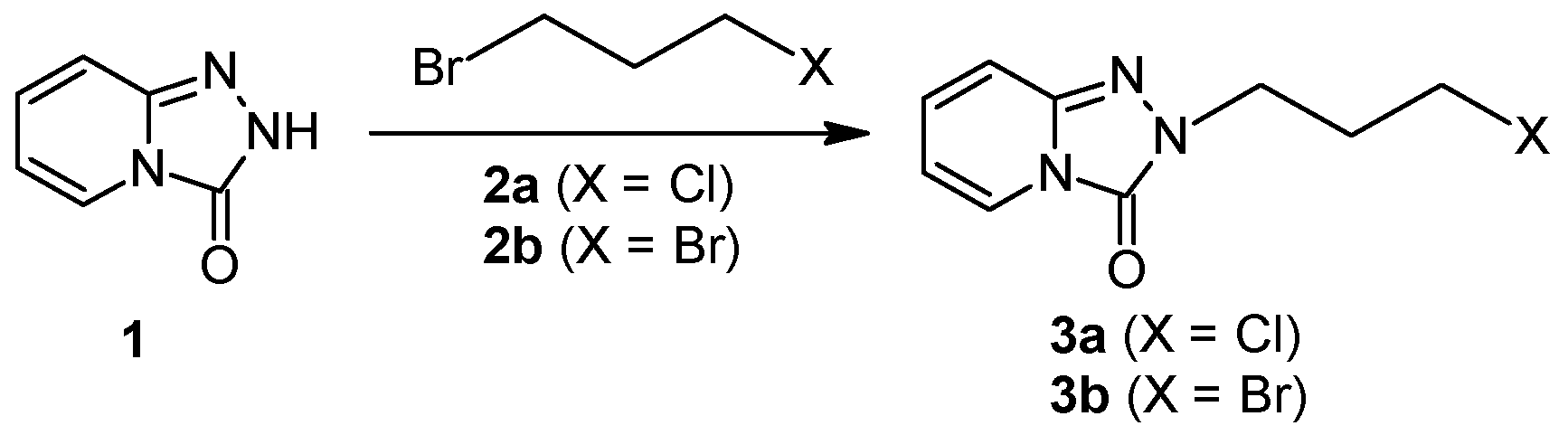

| No. | Solvent | Amount of Solvent (cm3) | X | Time (s) | Yield (%) |
|---|---|---|---|---|---|
| 1 | DMF | 5 | Cl | 50 | 24 |
| 2 | ACN | 6 | Cl | 50 | 83 |
| 3 | ACN | 3 | Cl | 80 | 92 |
| 4 | ACN | 0.75 | Cl | 50 | 90 |
| 5 * | ACN | 0.075 | Br | 120 | 81 |
| 6 | ACN | 0.75 | Br | 60 | 73 |
| 7 | ACN | 0.4 | Cl | 60 | 82 |
| 8 | - | - | Cl | 80 | 81 |
| No. | Solvent | Amount of Solvent (cm3) | PTC | X | Time (s) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | DMF | 4 | TBAB | Br | 60 | 91 |
| 2 | DMF | 2 | TBAB | Br | 60 | 98 |
| 3 | DMF | 2 | TEAC | Br | 120 | 90 |
| 4 | DMF | 2 | DABCO | Br | 120 | 83 |
| 5 | - | - | TBAB | Br | 100 | 69 |
| 6 | - | - | TBAB | Br | 300 | 82 |
| 7 | ACN | 3 | TBAB | Br | 60 | 78 |
| 8 | ACN | 8 | TBAB | Cl | 60 | 90 |
| 9 | ACN | 3 | TBAB | Cl | 60 | 86 |
| 10 | ACN | 1 | TBAB | Cl | 80 | 89 |
| 11 | - | - | TBAB | Cl | 100 | 76 |
| 12 | DMF | 6 | TBAB | Cl | 60 | 50 |
| 13 | H2O | 50 | TBAB | Cl | 60 | 51 |
| 14 * | ACN | 0.28 | TBAB | Cl | 120 (1 bar) | 86 |
| 15 * | ACN | 0.28 | TBAB | Cl | 120 (5 bar) | 86 |
| 16 * | ACN | 0.28 | TBAB | Cl | 120 (10 bar) | 65 |
| No. | Solvent | Amount of Solvent (cm3) | Time (s) | Yield (%) |
|---|---|---|---|---|
| 1 | DMF | 3 | 120 | 62 |
| 2 | ACN | 3 | 40 | 88 |
| 3 * | ACN | 0.3 | 120 | 83 |
| No. | Solvent | Amount of Solvent (cm3) | Time (s) | Yield (%) |
|---|---|---|---|---|
| 1 | ACN | 8 | 80 | 92 |
| 2 | ACN | 6 | 60 | 73 |
| 3 | ACN | 6 | 80 | 82 |
| 4 | ACN | 2 | 80 | 92 |
| 5 * | ACN | 0.2 | 120 | 77 |
| Entry | No. | n | R | Yield (%) | Purity* (%) | M.P. (°C) |
|---|---|---|---|---|---|---|
| 1 | 10a | 1 |  | 25 | 97 | 225–230 |
| 2 | 10b | 1 | 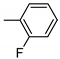 | 30 | 99 | 146–150 |
| 3 | 10c | 1 | 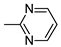 | 34 | 99 | 235–240 |
| 4 | 10d | 1 | 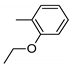 | 33 | 98 | 104–111 |
| 5 | 10e | 4 | 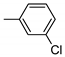 | 44 | 97 | 178–183 |
| 6 | 10f | 4 | 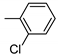 | 45 | 92 | 145–148 |
| 7 | 10g | 4 |  | 44 | 98 | 163–167 |
| 8 | 10h | 4 | 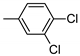 | 45 | 98 | 116–120 |
| 9 | 10i | 4 |  | 45 | 94 | 183–185 |
| 10 | 10j | 4 | 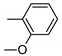 | 32 | 95 | 140–141 |
| 11 | 10k | 4 | 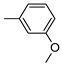 | 30 | 100 | 137–138 |
| 12 | 10l | 4 | 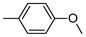 | 27 | 92 | 144–146 |
| 14 | 10m | 4 |  | 31 | 97 | 143–145 |
| 15 | 10n | 4 | 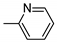 | 70 | 98 | 175–178 |
| 16 | 10o | 4 | 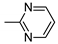 | 45 | 90 | 146–148 |
| 17 | 10p | 4 | 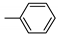 | 55 | 90 | 133–137 |
| 18 | 10r | 4 |  | 61 | 95 | oil |
| Entry | No. | D2 | 5-HT1A | 5-HT2A | 5-HT6 | 5-HT7 |
|---|---|---|---|---|---|---|
| 1 | trazodone | 3650 * | 78 * | 16* | >10,000 ** | 1782 ** |
| 2 | 10a | 116 ± 19 | 285 ± 42 | 181 ± 22 | 1430 ± 294 | 157 ± 13 |
| 3 | 10b | 1652 ± 203 | 1547 ± 314 | 969 ± 114 | 36,380 ± 5817 | 7415 ± 625 |
| 4 | 10c | 122 ± 8 | 459 ± 36 | 178 ± 25 | 3249 ± 401 | 174 ± 34 |
| 5 | 10d | 152 ± 17 | 593 ± 81 | 6713 ± 952 | 9459 ± 1138 | 539 ± 68 |
| 6 | 10e | 137 ± 11 | 16 ± 2 | 342 ± 48 | 1945 ± 281 | 278 ± 19 |
| 7 | 10f | 522 ± 71 | 49 ± 4 | 2600 ± 318 | 3573 ± 563 | 2595 ± 359 |
| 8 | 10g | 362 ± 17 | 27 ± 3 | 234 ± 46 | 2062 ± 173 | 435 ± 61 |
| 9 | 10h | 310 ± 42 | 19 ± 3 | 77 ± 14 | 576 ± 82 | 196 ± 29 |
| 10 | 10i | 195 ± 11 | 7 ± 2 | 417 ± 52 | 4736 ± 687 | 993 ± 214 |
| 11 | 10j | 57 ± 6 | 4 ± 1 | 841 ± 105 | 11,530 ± 2114 | 219 ± 36 |
| 12 | 10k | 21 ± 3 | 9 ± 2 | 343 ± 21 | 3497 ± 537 | 1024 ± 184 |
| 13 | 10l | 350 ± 54 | 826 ± 91 | 3903 ± 432 | 5617 ± 743 | 8297 ± 1351 |
| 14 | 10m | 1643 ± 219 | 9 ± 2 | 1540 ± 225 | 4816 ± 581 | 251 ± 52 |
| 15 | 10n | 10 ± 2 | 18 ± 3 | 1718 ± 193 | 4637 ± 341 | 1049 ± 91 |
| 16 | 10o | 202 ± 31 | 104 ± 12 | 10,620 ± 1954 | 7224 ± 827 | 5569 ± 438 |
| 17 | 10p | 1526 ± 116 | 28 ± 4 | 391 ± 27 | 3328 ± 197 | 404 ± 67 |
| 18 | 10r | 191 ± 14 | 20 ± 3 | 328 ± 49 | 1188 ± 165 | 19 ± 3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśkowska, J.; Zaręba, P.; Śliwa, P.; Pindelska, E.; Satała, G.; Majka, Z. Microwave-Assisted Synthesis of Trazodone and Its Derivatives as New 5-HT1A Ligands: Binding and Docking Studies. Molecules 2019, 24, 1609. https://doi.org/10.3390/molecules24081609
Jaśkowska J, Zaręba P, Śliwa P, Pindelska E, Satała G, Majka Z. Microwave-Assisted Synthesis of Trazodone and Its Derivatives as New 5-HT1A Ligands: Binding and Docking Studies. Molecules. 2019; 24(8):1609. https://doi.org/10.3390/molecules24081609
Chicago/Turabian StyleJaśkowska, Jolanta, Przemysław Zaręba, Paweł Śliwa, Edyta Pindelska, Grzegorz Satała, and Zbigniew Majka. 2019. "Microwave-Assisted Synthesis of Trazodone and Its Derivatives as New 5-HT1A Ligands: Binding and Docking Studies" Molecules 24, no. 8: 1609. https://doi.org/10.3390/molecules24081609
APA StyleJaśkowska, J., Zaręba, P., Śliwa, P., Pindelska, E., Satała, G., & Majka, Z. (2019). Microwave-Assisted Synthesis of Trazodone and Its Derivatives as New 5-HT1A Ligands: Binding and Docking Studies. Molecules, 24(8), 1609. https://doi.org/10.3390/molecules24081609