Chemical Composition, Antimicrobial and Antiparasitic Screening of the Essential Oil from Phania matricarioides (Spreng.) Griseb.
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Extraction and Chemical Characterization
4.3. Microorganisms
4.4. Cell Culture
4.5. Antibacterial and Antifungal Assays
4.6. Antiprotozoal Assays
4.7. Cytotoxicity Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials - A review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017, 15, 422–434. [Google Scholar] [CrossRef] [Green Version]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential oils: Extraction techniques, pharmaceutical and therapeutic potential—A review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Orchard, A.; Vuuren, S. Van Commercial essential oils as potential antimicrobials to treat skin diseases. Evidence-Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils — Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Cabrera Suárez, H.R.; Morón Rodríguez, C.F.J.; Victoria Amador, M.d.C.; García Hernández, A.I.; Acosta de la Luz, C.L. Composición fitoquímica de partes aéreas frescas de Phania matricarioides. Rev. Cuba. Plantas Med. 2012, 17, 268–278. [Google Scholar]
- García Hernández, A.I.; Victoria Amador, M.d.C.; Morón Rodríguez, C.F.; López Barreiro, M.; Boucourt Rodríguez, E.; Martínez Guerra, M.J.; Morejón Rodríguez, Z. Disminución de tránsito intestinal y ausencia de toxicología aguda preclínicas de la decocción de partes aéreas frescas de Phania matricarioides (Spreng.) Griseb. Rev. Cuba. Plantas Med. 2013, 18, 71–83. [Google Scholar]
- Jeffrey, C. Compositae: Introduction with key to tribes. In Families and Genera of Vascular Plants, vol. VIII, Flowering Plants, Eudicots, Asterales; Kadereit, J.W., Jeffrey, C., Eds.; Springer: Berlin, Germany, 2007; pp. 61–87. [Google Scholar]
- Singh, R.; Singh, G.; Tiwari, A.K.; Patel, S.; Agrawal, R. Diversity of host plants of aphids (Homoptera: Aphididae) infesting Asteraceae in India. Int. J. Zool. 2015, 1, 137–167. [Google Scholar]
- Tania, P.M.; Del Castilo, B.D.; Serrão, P.C.D.; Lobato, R.A.B.; Da Silva, R.R.; Da Silva, R.E.; De Silva de A, S.S.M. The effect antioxidant aqueous crude extract in Acmella ciliata (Kunth.) (Asteraceae). J. Chem. Pharm. Res. 2016, 8, 417–423. [Google Scholar]
- Panda, S.K.; Luyten, W. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite 2018, 25, 10. [Google Scholar] [CrossRef] [Green Version]
- Hiradeve, S.M.; Rangari, V.D. A review on pharmacology and toxicology of Elephantopus scaber Linn. Nat. Prod. Res. 2014, 28, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, D.; Fan, Z.; Chen, X.; Yu, F.; Hu, X.; Wang, K.; Yuan, L. Blumea balsamifera- A phytochemical and pharmacological review. Molecules 2014, 19, 9453–9477. [Google Scholar] [CrossRef] [PubMed]
- García Hernández, A.I.; Victoria Amador, M.d.C.; Morón Rodríguez, F.; Cabrera Suárez, H.; Frías Vázquez, A.I.; López Barreiro, M.; Boucourt Rodríguez, E.; Morejón Rodríguez, Z.; Martínez Guerra, M.J. Validación preclínica de la actividad analgésica y antiinflamatoria de la decocción de partes aéreas frescas de Phania matricarioides (Spreng.) Griseb. Rev. Cuba. Plantas Med. 2012, 17, 380–392. [Google Scholar]
- Gutiérrez, Y.I.; Monzote, L.; Rodríguez, K.M.; Bello, A.; Setzer, W.N. Comparative pharmacognosy, chemical profile and antioxidant activity of extracts from Phania matricarioides (Spreng.) Griseb. collected from different localities in Cuba. Plants 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Benelli, G. Ecotoxicology and environmental safety eco-friendly larvicides from Indian plants: Effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicol. Environ. Saf. 2016, 133, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Robu, S.; Aprotosoaie, A.C.; Spac, A.; Cioancă, O.; Hăncianu, M.; Stănescu, U. [Studies regarding chemical composition of lavender volatile oils]. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 584–589. [Google Scholar] [PubMed]
- Nasr, M.; Asgarpanah, J. Volatile constituents of the seeds and fruit of Pycnocycla nodiflora. Nat. Prod. Commun. 2014, 9, 1781–1782. [Google Scholar] [CrossRef]
- Gupta, V.K.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.K.; Rahuja, N.; Luqman, S.; Sisodia, B.S.; Saikia, D.; et al. Antimicrobial potential of Glycyrrhiza glabra roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential oils as natural food antimicrobial agents: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Rao, J.; Chen, B.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef]
- Monzote, L.; García, M.; Pastor, J.; Gil, L.; Scull, R.; Maes, L.; Cos, P.; Gille, L. Essential oil from Chenopodium ambrosioides and main components: Activity against Leishmania, their mitochondria and other microorganisms. Exp. Parasitol. 2014, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W.N. Essential oil from Piper aduncum: Chemical analysis, antimicrobial assessment, and literature review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Sales, V.; Monteiro, Á.B.; de Araújo Delmondes, G.; do Nascimento, E.P.; Nóbrega de Figuêiredo, F.R.S.D.; de Souza Rodrigues, C.K.; de Lacerda, J.F.E.; Fernandes, C.N.; de Oliveira Barbosa, M.; Brasil, A.X.; Tintino, S.R.; et al. Antiparasitic activity and essential oil chemical analysis of the Piper tuberculatum Jacq fruit. Iran. J. Pharm. Res. 2018, 17, 268–275. [Google Scholar] [PubMed]
- Pereira, P.S.; Maia, A.J.; Duarte, A.E.; Oliveira-Tintino, C.D.M.; Tintino, S.R.; Barros, L.M.; Vega-Gomez, M.C.; Rolón, M.; Coronel, C.; Coutinho, H.D.M.; et al. Cytotoxic and anti-kinetoplastid potential of the essential oil of Alpinia speciosa K. Schum. Food Chem. Toxicol. 2018, 119, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Pink, R.; Hudson, A.; Mouriès, M.-As.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–741. [Google Scholar] [CrossRef]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Roth, G. The burden of Chagas disease. Glob. Heart 2015, 10, 139–144. [Google Scholar] [CrossRef]
- Bermudez, J.; Davies, C.; Simonazzi, A.; Pablo, J.; Palma, S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef]
- Rassi, A., Jr.; Rassi, A.; De Rezende, J.M. American trypanosomiasis (Chagas Disease). Infect. Dis. Clin. 2012, 26, 275–291. [Google Scholar] [CrossRef]
- Rodríguez-Morales, O.; Monteón-Padilla, V.; Carrillo-Sánchez, S.C.; Rios-Castro, M.; Martínez-Cruz, M.; Carabarin-Lima, A.; Arce-Fonseca, M. Experimental vaccines against Chagas disease: A journey through history. J. Immunol. Res. 2015, 2015, 489758. [Google Scholar] [CrossRef]
- Urbina, J.A. Specific chemotherapy of Chagas disease: Relevance, current limitations and new approaches. Acta Trop. 2010, 115, 55–68. [Google Scholar] [CrossRef]
- Chatelain, E. Chagas disease drug discovery: Toward a new era. J. Biomol. Screen. 2015, 20, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.T.; Fernandes, J.P.S. Natural products: Key prototypes to drug discovery against neglected diseases caused by trypanosomatids. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef]
- Moreno, É.M.; Leal, S.M.; Stashenko, E.E.; García, L.T. Induction of programmed cell death in Trypanosoma cruzi by Lippia alba essential oils and their major and synergistic terpenes (citral, limonene and caryophyllene oxide). BMC Complement. Altern. Med. 2018, 18, 225. [Google Scholar] [CrossRef]
- Estevam, E.B.B.; De Deus, I.P.B.; da Silva, V.P.; Da Silva, E.A.J.; Alves, C.C.F.; Alves, J.M.; Cazal, C.M.; Magalhaes, L.G.; Pagotti, M.C.; Esperandim, V.R.; et al. In vitro antiparasitic activity and chemical composition of the essential oil from Protium ovatum leaves (Burceraceae). An. Acad. Bras. Cienc. 2017, 89, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evidence-Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Kumaresan, J.; Sachs, S.E.; Sachs, J.D.; Savioli, L. Control of neglected tropical diseases. N. Engl. J. Med. 2007, 357, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Kovats, E. Gas-chromatographische charakterisierung organischer verbindungen. Teil 1: Retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; 4th ed.; Allured: Carol Stream, IL, USA, 2007. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Thesis, University of Alabama, Huntsville, AL, USA, 2015. [Google Scholar]
- Cos, P.; Vlietinck, A.J.; Berghe, D. Vanden; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue® assay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. J. Parasitol. 2005, 91, 484–486. [Google Scholar] [CrossRef]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Buckner, F.S.; Verlinde, C.L.M.J.; La Flamme, A.C.; van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [PubMed]
- Torres-Santos, E.C.; Moreira, D.L.; Kaplan, M.A.; Meirelles, M.N.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar] [CrossRef]
- Sladowski, D.; Steer, S.J.; Clothier, R.H.; Balls, M. An improved MTT assay. J. Immunol. Methods 1993, 157, 203–207. [Google Scholar] [CrossRef]
Sample Availability: The essential oil is no longer available from the authors. |

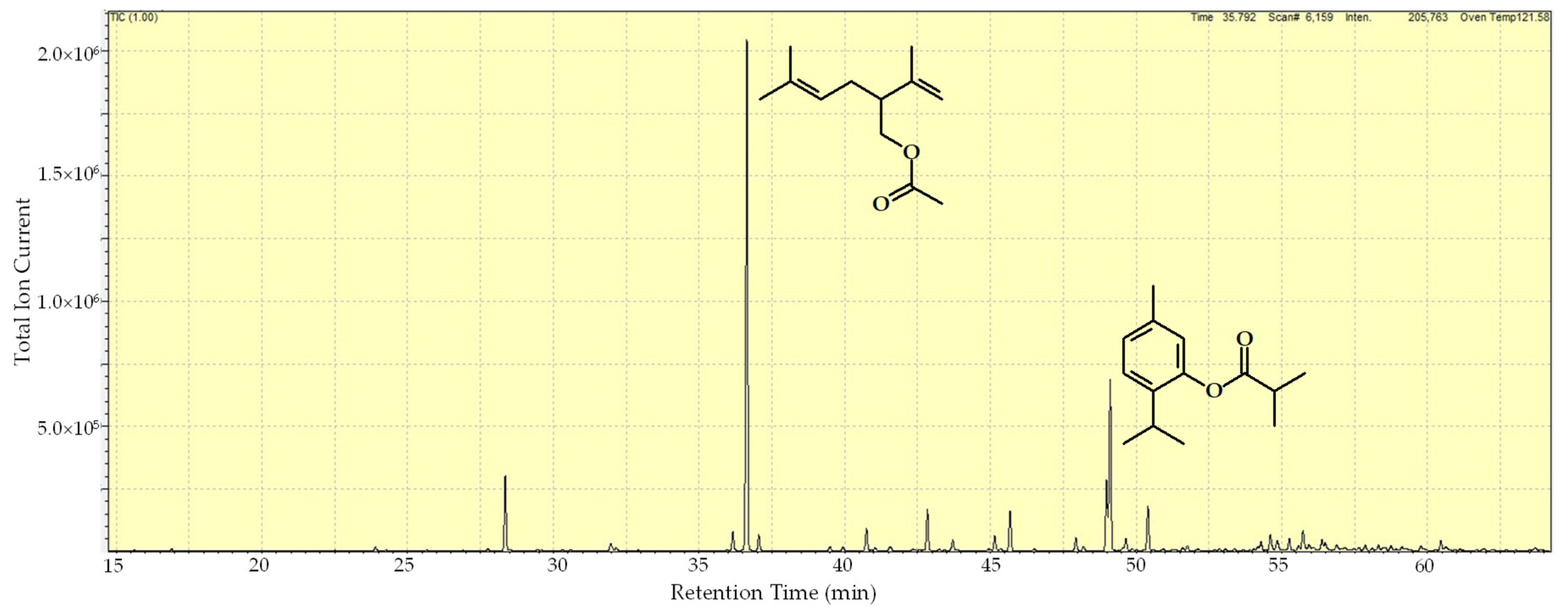
| RI a | Compound | Area (%) |
|---|---|---|
| 994 | Yomogi alcohol | 0.1 |
| 1099 | Linalool | 0.3 |
| 1154 | (Z)-chrysenthemol | 0.2 |
| 1163 | Lavandulol | 5.5 |
| 1215 | Coahuilensol methyl ether | 0.7 |
| 1217 | 3-isopropylbenzaldehyde | 0.2 |
| 1276 | Chrysanthemyl acetate | 1.4 |
| 1283 | Lavandulyl acetate | 40.1 |
| 1289 | Thymol | 1.2 |
| 1325 | Silphiperfol-5-ene | 0.3 |
| 1332 | δ-elemene | 0.3 |
| 1344 | 7-epi-silphiperfol-5-ene | 1.8 |
| 1349 | Neryl acetate | 0.2 |
| 1357 | Silphiperfol-4,7(14)-diene | 0.4 |
| 1376 | α-copaene | 3.2 |
| 1389 | β-elemene | 0.9 |
| 1411 | Thymohydroquinone dimethyl ether | 1.2 |
| 1415 | Lavandulyl isobutyrate | 0.1 |
| 1420 | β-caryophyllene | 3.3 |
| 1456 | α-humulene | 1.1 |
| 1460 | allo-aromadendrene | 0.3 |
| 1473 | 8,9-dehydrothymyl isobutyrate | 5.9 |
| 1475 | Thymyl isobutyrate | 13.9 |
| 1483 | Neryl isobutyrate | 1.0 |
| 1495 | Bicyclogermacrene | 3.7 |
| 1515 | Cubebol | 0.3 |
| 1518 | β-guaiene | 0.4 |
| 1536 | Silphiperfol-5-en-3-ol B | 0.2 |
| 1545 | Silphiperfol-5-en-3-one B | 0.1 |
| 1558 | Silphiperfol-5-en-3-ol A | 0.3 |
| 1560 | (E)-nerolidol | 0.7 |
| 1565 | Thymyl 2-methylbutanoate | 1.6 |
| 1570 | Neryl 2-methylbutanoate | 1.1 |
| 1576 | Spathulenol | 0.9 |
| 1582 | Caryophyllene oxide | 0.4 |
| 1584 | allo-spathulenol | 1.7 |
| 1588 | β-copaen-4α-ol | 0.3 |
| 1595 | Fokienol | 0.8 |
| 1604 | Ledol | 0.4 |
| 1618 | Silphiperfol-6-en-5-one | 0.2 |
| 1621 | Unidentified b | 0.5 |
| 1629 | Unidentified c | 0.4 |
| 1632 | Caryophylla-4(12),8(13)-dien-5α-ol | 0.5 |
| 1637 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.4 |
| 1644 | τ-murrolol | 0.2 |
| 1655 | α-cadinol | 0.3 |
| 1667 | 6-methoxythymyl isobutyrate | 0.9 |
| 1726 | Unidentified d | 0.2 |
| TOTAL IDENTIFIED = 98.9% |
| IC50 (µg/mL) a | |||||
|---|---|---|---|---|---|
| P. falciparum | T. brucei | T. cruzi | L. amazonensis | L. infantum | |
| Essential oil | 20.7 | 8.0 | 2.2 | 56.6 | 7.5 |
| Reference drug c | 0.02 | 0.04 | 3.2 | 0.02 | 3.7 |
| CC50 (µg/mL) b | |||||
| Peritoneal Macrophage | |||||
| Essential oil | 28.0 | ||||
| Reference drug c | 5.8 | ||||
| Selectivity Index d | |||||
| P. falciparum | T. brucei | T. cruzi | L. amazonensis | L. infantum | |
| Essential oil | 1 | 4 | 13 | 0 | 4 |
| Reference drug c | 290 | 145 | 2 | 290 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, Y.I.; Scull, R.; Villa, A.; Satyal, P.; Cos, P.; Monzote, L.; Setzer, W.N. Chemical Composition, Antimicrobial and Antiparasitic Screening of the Essential Oil from Phania matricarioides (Spreng.) Griseb. Molecules 2019, 24, 1615. https://doi.org/10.3390/molecules24081615
Gutiérrez YI, Scull R, Villa A, Satyal P, Cos P, Monzote L, Setzer WN. Chemical Composition, Antimicrobial and Antiparasitic Screening of the Essential Oil from Phania matricarioides (Spreng.) Griseb. Molecules. 2019; 24(8):1615. https://doi.org/10.3390/molecules24081615
Chicago/Turabian StyleGutiérrez, Yamilet I., Ramón Scull, Anabel Villa, Prabodh Satyal, Paul Cos, Lianet Monzote, and William N. Setzer. 2019. "Chemical Composition, Antimicrobial and Antiparasitic Screening of the Essential Oil from Phania matricarioides (Spreng.) Griseb." Molecules 24, no. 8: 1615. https://doi.org/10.3390/molecules24081615
APA StyleGutiérrez, Y. I., Scull, R., Villa, A., Satyal, P., Cos, P., Monzote, L., & Setzer, W. N. (2019). Chemical Composition, Antimicrobial and Antiparasitic Screening of the Essential Oil from Phania matricarioides (Spreng.) Griseb. Molecules, 24(8), 1615. https://doi.org/10.3390/molecules24081615