Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Drying Kinetics
2.2. Water Activity Analysis
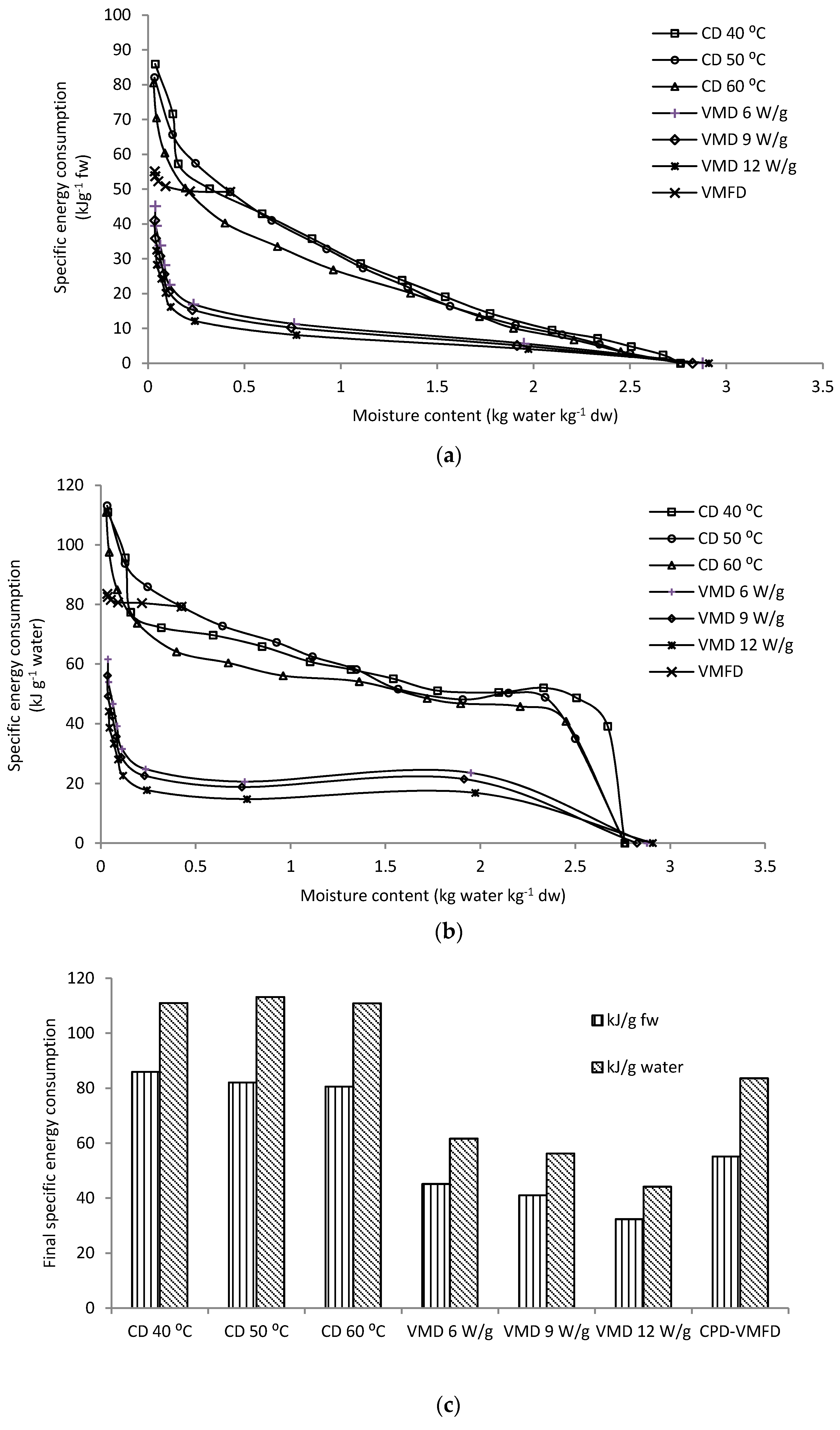
2.3. Specific Energy Consumption
2.4. Colour Analysis
2.5. Antioxidant Activity and Total Phenolic Content (TPC) Analysis
2.6. Analysis of Volatile Content
2.7. Phytosterol Analysis
2.8. Fatty Acid Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material Preparation
3.3. Drying Methods
3.3.1. CD
3.3.2. VMD
3.3.3. Two-Stage Drying: CPD-VMFD
3.3.4. FD
3.4. Modelling of Drying Kinetics
3.5. Water Activity Analysis
3.6. Determination of Energy Consumption
3.6.1. Energy Consumption
3.6.2. Specific Energy Consumption
3.7. Colour Analysis
3.8. Extraction of Polyphenol Compounds
3.9. Antioxidant Activity Analysis
3.9.1. ABTS●+ Radical-Scavenging Assay
3.9.2. FRAP Assay
3.10. Total Phenolic Content Analysis
3.11. Analysis of Volatile Compounds
3.11.1. Headspace Solid-Phase Microextraction (HS-SPME)
3.11.2. GC–MS Analysis of Volatile Compounds
3.12. Phytosterol and Fatty Acid Analysis
3.12.1. Lipid Extraction
3.12.2. GC–MS Analysis of Phytosterols
3.12.3. GC–MS Analysis of Fatty Acids
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sagnia, B.; Fedeli, D.; Casetti, R.; Montesano, C.; Falcioni, G.; Colizzi, V. Antioxidant and anti-inflammatory activities of extracts from Cassia alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva medicinal plants collected in Cameroon. Plos ONE 2014, 9, e103999. [Google Scholar] [CrossRef] [PubMed]
- Somchit, M.N.; Reezal, I.; Elysha Nur, I.; Mutalib, A.R. In vitro antimicrobial activity of ethanol and water extracts of Cassia alata. J. Ethnopharmacol. 2003, 84, 1–4. [Google Scholar] [CrossRef]
- Chatterjee, S. Cassia alata-An useful antimicrobial agent. Med. Aromat. Plants 2013, 2, e143. [Google Scholar] [CrossRef]
- Ross, I.A. Cassia alata L. In Medicinal Plants of the World; Springer Science & Business Media: New York, NY, USA, 2003; Volume 1, pp. 165–174. [Google Scholar]
- Hazni, H.; Ahmad, N.; Hitotsuyanagi, Y.; Takeya, K.; Choo, C.Y. Phytochemical constituents from Cassia alata with inhibition against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Study of antioxidant activity and immune stimulating potency of the ethnomedicinal plant, Cassia alata (L.) Roxb. Med. Aromat. Plants 2013, 2, 2–7. [Google Scholar] [CrossRef]
- Gupta, D.; Singh, J. Flavonoid glycosides from Cassia alata. Phytochemistry 1991, 30, 2761–2763. [Google Scholar] [CrossRef]
- Okpuzor, J.; Ogbunugafo, H.; Kareem, G.K.; Igwo-Ezikp, M.N. In vitro investigation of antioxidant phenolic compounds in extracts of Senna alata. Res. J. Phytochem. 2010, 3, 68–76. [Google Scholar] [CrossRef]
- Chan, J.W.R.; Chong, C.H.; Ng, D.K.S. Process synthesis and design for extraction of bioactive compounds from Strobilanthes crispus. J. Eng. Sci. Technol. 2015, 113–137. [Google Scholar]
- Sarker, S.D.; Latif, Z.; Gray, A.I. Natural product isolation. In Natural Products Isolation, 2nd ed.; Humana Press: New Jersey, NJ, USA, 2005; pp. 1–25. [Google Scholar]
- Wray, D.; Ramaswamy, H.S. Novel concepts in microwave drying of foods. Dry. Technol. 2015, 33, 769–783. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K. Recent advances in hybrid drying technologies. In Emerging Technologies for Food Processing, 2nd ed.; Sun, D.W., Ed.; Academic Press: London, UK, 2014; pp. 447–457. [Google Scholar]
- Feng, H.; Yin, Y.; Tang, J. Microwave drying of food and agricultural materials: Basics and heat and mass transfer modeling. Food Eng. Rev. 2012, 4, 89–106. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Physicochemical properties of whole fruit plum powders obtained using different drying technologies. Food Chem. 2016, 207, 223–232. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Lech, K.; Szumny, A.; Carbonell-Barrachina, Á. Effects of drying methods on the composition of thyme ( Thymus vulgaris L.) essential oil. Int. J. 2013, 31, 224–235. [Google Scholar]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Hu, Q.-g.; Zhang, M.; Mujumdar, A.S.; Xiao, G.-n.; Jin-Cai, S. Drying of edamames by hot air and vacuum microwave combination. J. Food Eng. 2006, 77, 977–982. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Wojdyło, A.; Szarycz, M.; Carbonell-Barrachina, Á.A. Drying of garlic slices using convective pre-drying and vacuum-microwave finishing drying: Kinetics, energy consumption, and quality studies. Food Bioprocess Technol. 2014, 7, 398–408. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water content, water activity, water structure and the stability of foodstuffs. Food Control 2001, 12, 409–417. [Google Scholar] [CrossRef]
- MacDougall, D.B. Colour in Food. Improving quality. Colour Food 2002, 388. [Google Scholar]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Effect of drying method on the antioxidant capacity of six Lamiaceae herbs. Food Chem. 2010, 123, 85–91. [Google Scholar] [CrossRef]
- Li, R.; Shang, H.; Wu, H.; Wang, M.; Duan, M.; Yang, J. Thermal inactivation kinetics and effects of drying methods on the phenolic profile and antioxidant activities of chicory (Cichorium intybus L.) leaves. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Jaloszynski, K.; Figiel, A.; Wojdylo, A. Drying kinetics and antioxidant activity of oregano. Acta Agrophysica 2009, 11, 81–90. [Google Scholar]
- Sun, Y.; Shen, Y.; Liu, D.; Ye, X. Effects of drying methods on phytochemical compounds and antioxidant activity of physiologically dropped un-matured citrus fruits. Lwt-Food Sci. Technol. 2015, 60, 1269–1275. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Wang, K.; Lei, Y.; Yao, S.; Lu, B.; Huang, W. Antioxidant synergistic effects of Osmanthus fragrans flowers with green tea and their major contributed antioxidant compounds. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hugo, P.C.; Gil-Chávez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; González-Aguilar, G.A. Antioxidant interactions between major phenolic compounds found in “Ataulfo” mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules 2012, 17, 12657–12664. [Google Scholar]
- Kowalczyk, D.; Świeca, M.; Cichocka, J.; Gawlik-Dziki, U. The phenolic content and antioxidant activity of the aqueous and hydroalcoholic extracts of hops and their pellets. J. Inst. Brew. 2013, 119, 103–110. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Szumny, A.; Figiel, A.; Jałoszyński, K.; Adamski, M.; Carbonell-Barrachina, Á.A. Effects of vacuum level and microwave power on rosemary volatile composition during vacuum-microwave drying. J. Food Eng. 2011, 103, 219–227. [Google Scholar] [CrossRef]
- Soupas, L.; Juntunen, L.; Lampi, A.M.; Piironen, V. Effects of sterol structure, temperature, and lipid medium on phytosterol oxidation. J. Agric. Food Chem. 2004, 52, 6485–6491. [Google Scholar] [CrossRef]
- Rudzińska, M.; Przybylski, R.; Wa̧sowicz, E. Products formed during thermo-oxidative degradation of phytosterols. J. Am. Oil Chem. Soc. 2009, 86, 651–662. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Lim, S.-M.; Bae, S.-Y.; Oh, S.C. Thermal decomposition and stability of fatty acid methyl esters in supercritical methanol. J. Anal. Appl. Pyrolysis 2011, 92, 332–338. [Google Scholar] [CrossRef]
- Charuwat, P.; Boardman, G.; Bott, C.; Novak, J.T. Thermal degradation of long chain fatty acids. Water Environ. Res. 2018, 90, 278–287. [Google Scholar] [CrossRef]
- Bourourou, M.; Duncan, M.W.; Lipsky, R.H.; Gorelick, P.B.; Marini, A.M.; Blondeau, N. Alpha-linolenic acid: An omega-3 fatty acid with neuroprotective properties—Ready for use in the stroke clinic? Biomed. Res. Int. 2015, 2015, 1–8. [Google Scholar]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Choong, T.S.Y. Antioxidant activity, and volatile and phytosterol contents of Strobilanthes crispus dehydrated using conventional and vacuum microwave drying methods. Molecules 2019, 24, 1397. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Politowicz, J.; Lech, K.; Sánchez-Rodríguez, L.; Szumny, A.; Carbonell-Barrachina, Á.A. Volatile composition and sensory profile of Cantharellus cibarius Fr. as affected by drying method. J. Sci. Food Agric. 2017, 97, 5223–5232. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publ. Corp. Carol Stream 2007, 1–804. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Drying Method | Final Moisture Content (kg water/ kg dw) | Water Activity aw |
|---|---|---|
| FD | 0.0575 ± 0.001 | 0.2836 ± 0.000 b |
| CD (40 °C) | 0.0372 ± 0.000 | 0.2258 ± 0.010 c |
| CD (50 °C) | 0.0336 ± 0.001 | 0.1558 ± 0.004 f |
| CD (60 °C) | 0.0289 ± 0.001 | 0.1291 ± 0.004 g |
| VMD (6 W/g) | 0.0372 ± 0.003 | 0.1905 ± 0.002 d |
| VMD (9 W/g) | 0.0347 ± 0.002 | 0.1697 ± 0.005 e,f |
| VMD (12 W/g) | 0.0423 ± 0.002 | 0.2200 ± 0.003 c |
| CPD-VMFD | 0.0343 ± 0.002 | 0.1726 ± 0.004 e |
| Drying Conditions | Colour Parameters | ||
|---|---|---|---|
| L* | a* | b* | |
| Fresh | 36.94 ± 0.093 a | −2.75 ± 0.451 a | 9.04 ± 0.440 a |
| FD | 44.71 ± 0.322 b | −2.17 ± 0.064 b | 17.00 ± 0.320 b |
| CD (40 °C) | 38.55 ± 0.095 b | −0.20 ± 0.118 d,e | 10.65 ± 0.109 c |
| CD (50 °C) | 38.75 ± 0.070 b | 0.00 ± 0.056 e,f | 10.53 ± 0.101 c |
| CD (60 °C) | 38.31 ± 0.207 e | 0.39 ± 0.025 g | 10.04 ± 0.186 d |
| VMD (6 W/g) | 40.27 ± 0.036 d | 0.45 ± 0.044 g | 13.81 ± 0.125 e |
| VMD (9 W/g) | 39.42 ± 0.388 c | −0.47 ± 0.051 c,d | 11.89 ± 0.143 f |
| VMD (12 W/g) | 39.43 ± 0.272 c | −0.54 ± 0.042 c | 12.25 ± 0.115 f |
| CPD-VMFD | 39.30 ± 0.110 c | 0.18 ± 0.055 f,g | 11.82 ± 0.149 f |
| Drying Method | Antioxidant Activity (µM Trolox/100 g dw) | Total Phenolic Content (mg/100 g dw) | |
|---|---|---|---|
| ABTS | FRAP | ||
| Fresh | 65.53 ± 4.97 a | 30.69 ± 6.91 a | 9368.59 ± 1460.98 a |
| FD | 11.49 ± 0.25 b,c | 5.34 ± 0.17 b | 2883.05 ± 146.45 b |
| VMD 6 W/g | 9.04 ± 1.19 b,c | 3.28 ± 0.08 b | 1982.44 ± 53.17 b |
| VMD 9 W/g | 11.29 ± 1.03 b,c | 4.70 ± 0.25 b | 2379.87 ± 216.92 b |
| VMD 12 W/g | 9.15 ± 1.70 b,c | 5.39 ± 0.38 b | 2441.91 ± 82.86 b |
| CPD-VMFD | 11.19 ± 0.33 b,c | 5.01 ± 0.18 b | 2413.36 ± 277.94 b |
| CD at 40 °C | 13.54 ± 0.02 c | 5.55 ± 0.24 b | 2965.12 ± 54.97 b |
| CD at 50 °C | 8.65 ± 0.27 b,c | 3.88 ± 0.58 b | 2342.75 ± 74.18 b |
| CD at 60 °C | 7.43 ± 0.63 b | 2.58 ± 0.09 b | 1821.54 ± 41.18 b |
| Compound | RT | Retention Indexes | Concentration (mg 100 g−1 db) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Lit | Fresh | FD | CD (40 °C) | CD (50 °C) | CD (60 °C) | VMD (6 W/g) | VMD (9 W/g) | VMD (12 W/g) | CPD-VMFD | ||
| 2-Hexenal | 4.770 | 843 | 851 | 60.28 | 2.98 | 7. 19 | 5.57 | 6.71 | 0.45 | 1.07 | 4.89 | 2.13 |
| 2-Hexen-1-ol, (E)- | 5.030 | 858 | 862 | 10.11 | 0.65 | 0.82 | 0.50 | 0.38 | 0.00 | 0.18 | 1.66 | 0.31 |
| 1-Hexanol | 5.070 | 861 | 868 | 18.70 | 1.85 | 0.78 | 0.62 | 0.80 | 0.02 | 0.95 | 6.37 | 0.43 |
| 2-Heptanol | 5.810 | 899 | 901 | 1.62 | 0.33 | 1.37 | 0.58 | 0.80 | 0.16 | 0.20 | 1.11 | 0.37 |
| Anisole | 6.330 | 917 | 920 | 1.29 | 0.47 | 0.38 | 0.17 | 0.11 | 0.01 | 0.03 | 0.24 | 0.09 |
| Benzaldehyde | 7.500 | 959 | 962 | 8.37 | 0.91 | 1.99 | 0.71 | 0.65 | 0.10 | 0.21 | 1.10 | 0.31 |
| 1-Octen-3-ol | 8.180 | 883 | 880 | 2.75 | 0.24 | 1.67 | 1.02 | 0.07 | 0.00 | 0.18 | 1.15 | 0.04 |
| Phenol | 8.100 | 980 | 980 | 5.02 | 1.02 | 0.96 | 0.32 | 0.09 | 0.08 | 0.38 | 1.00 | 0.24 |
| 5-Hepten-2-one, 6-methyl- | 8.260 | 986 | 986 | 4.62 | 1.08 | 3.46 | 2.40 | 2.12 | 0.48 | 1.34 | 7.50 | 1.18 |
| 3-Octanol | 8.430 | 992 | 994 | 3.68 | 0.46 | 1.01 | 0.76 | 1.10 | 0.19 | 0.23 | 1.60 | 0.34 |
| 5-Hepten-2-ol, 6-methyl- | 8.570 | 997 | 994 | 1.19 | 0.01 | 0.07 | 0.02 | 0.07 | 0.00 | 0.10 | 0.91 | 0.00 |
| Hexanoic acid, ethyl ester | 8.670 | 1000 | 1000 | 2.21 | 1.33 | 2.47 | 0.51 | 1.27 | 0.16 | 0.42 | 2.63 | 0.16 |
| Octanal | 8.750 | 1002 | 1003 | 0.50 | 0.53 | 0.23 | 0.27 | 0.41 | 0.12 | 0.06 | 0.24 | 0.02 |
| Anisole, o-methyl- | 9.000 | 1010 | 1009 | 0.95 | 0.05 | 0.28 | 0.12 | 0.16 | 0.02 | 0.06 | 0.31 | 0.05 |
| Acetic acid, hexyl ester | 9.100 | 1013 | 1011 | 0.36 | 0.06 | 0.02 | 0.00 | 0.00 | 0.01 | 0.01 | 0.07 | 0.00 |
| 4-Hepten-1-ol, 6-methyl- | 9.230 | 1017 | 1020 | 3.36 | 0.14 | 0.35 | 0.36 | 0.29 | 0.01 | 0.04 | 0.16 | 0.18 |
| 3-Ethyl-4-methylpentan-1-ol | 9.320 | 1020 | 1023 | 8.47 | 0.50 | 2.11 | 0.78 | 0.87 | 0.06 | 0.15 | 0.59 | 0.35 |
| p-Cymene | 9.470 | 1024 | 1025 | 1.18 | 0.04 | 0.68 | 0.14 | 0.17 | 0.09 | 0.10 | 0.37 | 0.07 |
| Limonene | 9.610 | 1029 | 1030 | 2.18 | 0.05 | 0.22 | 0.15 | 0.13 | 0.05 | 0.17 | 1.25 | 0.41 |
| Eucalyptol | 9.700 | 1031 | 1032 | 1.92 | 0.00 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.02 | 1.88 |
| Benzyl alcohol | 9.800 | 1035 | 1036 | 13.42 | 2.92 | 4.67 | 2.81 | 2.86 | 0.49 | 1.22 | 4.36 | 0.01 |
| Benzeneacetaldehyde | 10.070 | 1042 | 1045 | 3.28 | 0.96 | 0.89 | 0.48 | 0.63 | 0.06 | 0.37 | 0.98 | 0.28 |
| Ether, benzyl ethyl | 10.500 | 1056 | 1046 | 2.51 | 0.24 | 0.17 | 0.08 | 0.12 | 0.02 | 0.13 | 0.14 | 0.02 |
| Fenchone | 11.580 | 1088 | 1096 | 7.99 | 0.22 | 0.57 | 0.16 | 0.02 | 0.02 | 0.04 | 1.18 | 0.05 |
| 2-Nonanone | 11.660 | 1091 | 1092 | 1.01 | 0.08 | 0.40 | 0.02 | 0.10 | 0.00 | 0.04 | 0.32 | 0.04 |
| Ethyl (4E)-4-heptenoate | 11.740 | 1094 | 1090 | 3.63 | 0.64 | 1.03 | 0.53 | 0.67 | 0.04 | 0.06 | 0.26 | 0.01 |
| Linalool | 11.960 | 1100 | 1099 | 10.59 | 0.75 | 2.95 | 1.26 | 1.20 | 0.13 | 0.33 | 1.80 | 0.46 |
| Nonanal | 12.080 | 1104 | 1104 | 2.81 | 0.16 | 0.91 | 0.45 | 0.86 | 0.09 | 0.27 | 0.97 | 0.15 |
| β-Thujone | 12.180 | 1107 | 1114 | 1.12 | 0.70 | 0.66 | 0.44 | 0.59 | 0.07 | 0.39 | 1.11 | 0.20 |
| Phenylethyl alcohol | 12.440 | 1114 | 1116 | 1.82 | 0.42 | 3.90 | 0.80 | 0.83 | 0.00 | 0.20 | 1.60 | 0.08 |
| 3-Thujanone | 12.560 | 1118 | 1119 | 1.01 | 0.69 | 0.66 | 1.01 | 1.17 | 0.09 | 0.67 | 2.20 | 0.15 |
| Veratrol | 13.540 | 1147 | 1148 | 6.49 | 0.47 | 1.65 | 0.44 | 0.54 | 0.10 | 0.14 | 0.62 | 0.23 |
| 2,6-Nonadienal, (E,Z)- | 13.770 | 1153 | 1155 | 5.75 | 0.21 | 0.72 | 0.24 | 0.32 | 0.01 | 0.08 | 0.13 | 0.12 |
| 2-Nonenal, (E)- | 14.000 | 1160 | 1162 | 0.39 | 0.34 | 1.48 | 0.68 | 0.71 | 0.09 | 0.20 | 0.00 | 0.36 |
| endo-Borneol | 14.260 | 1167 | 1167 | 0.32 | 0.31 | 1.13 | 0.39 | 0.66 | 0.10 | 0.06 | 0.25 | 0.29 |
| Hexanoic acid, butyl ester | 15.070 | 1191 | 1189 | 1.20 | 0.03 | 0.06 | 0.04 | 0.08 | 0.01 | 0.02 | 0.15 | 0.03 |
| Methyl salicylate | 15.200 | 1195 | 1192 | 5.18 | 0.27 | 1.18 | 0.06 | 0.78 | 0.17 | 0.02 | 0.23 | 0.38 |
| 2-Octynoic acid, methyl ester | 15.550 | 1210 | 1212 | 2.91 | 0.16 | 0.73 | 0.49 | 0.50 | 0.04 | 0.15 | 0.53 | 0.25 |
| Citronellol | 16.330 | 1228 | 1228 | 13.26 | 0.93 | 2.96 | 1.28 | 1.91 | 0.02 | 0.20 | 0.06 | 0.81 |
| Butanoic acid, 2-methyl-, hexyl ester | 16.650 | 1238 | 1236 | 1.56 | 0.21 | 1.19 | 0.38 | 0.49 | 0.07 | 0.21 | 0.70 | 0.23 |
| D-Carvone | 16.900 | 1245 | 1246 | 0.65 | 0.22 | 0.90 | 0.27 | 0.32 | 0.06 | 0.13 | 0.80 | 0.19 |
| Salicylic acid, ethyl ester | 17.850 | 1274 | 1270 | 15.05 | 0.58 | 1.32 | 0.61 | 0.60 | 0.07 | 0.15 | 0.70 | 0.27 |
| 2-Undecanone | 18.480 | 1293 | 1294 | 2.62 | 0.01 | 2.13 | 0.17 | 0.48 | 0.06 | 0.03 | 0.51 | 0.01 |
| Nonanoic acid, ethyl ester | 18.620 | 1296 | 1296 | 0.55 | 0.01 | 0.15 | 0.01 | 0.05 | 0.00 | 0.00 | 0.04 | 0.00 |
| Methyl 4-methylsalicylate | 19.270 | 1319 | - | 1.86 | 0.17 | 0.28 | 0.15 | 0.09 | 0.02 | 0.15 | 0.18 | 0.08 |
| β-Cubebene | 21.400 | 1391 | 1389 | 2.54 | 0.08 | 1.38 | 0.56 | 0.87 | 0.04 | 0.28 | 0.84 | 0.35 |
| Geranyl acetone | 22.800 | 1457 | 1453 | 0.86 | 0.10 | 0.33 | 0.18 | 0.25 | 0.04 | 0.12 | 0.35 | 0.13 |
| trans-β-Ionone | 23.440 | 1489 | 1486 | 2.59 | 0.24 | 0.80 | 0.48 | 0.51 | 0.07 | 0.27 | 0.78 | 0.23 |
| Widdrol | 25.450 | 1618 | 1610 | 1.92 | 0.02 | 0.08 | 0.03 | 0.00 | 0.00 | 0.01 | 0.04 | 0.01 |
| Isopropyl myristate | 27.400 | 1825 | 1827 | 1.10 | 0.05 | 0.53 | 0.22 | 0.13 | 0.07 | 0.11 | 0.68 | 0.06 |
| TOTAL | 254.79 a | 24.91 c | 61.85 b | 29.73 c | 34.55 c | 11.91 d | 11.93 d | 55.67 b | 14.04 c,d | |||
| Compound | Retention time | ANOVA | Fresh | CPD-VMFD | CD at 50 °C |
|---|---|---|---|---|---|
| Concentration (mg 100 g−1 db) | |||||
| α-tocopherol | 25.945 | n.s.a | 106.08 a | 106.32 a | 85.85 a,b |
| Desmosterol | 26.630 | *** | 218.08 a | 37.74 b | 42.23 b |
| Lanosterol | 26.875 | *** | 152.20 a | 19.84 b | 22.33 b |
| Campesterol | 27.575 | *** | 498.76 a | 128.45 b | 132.60 b |
| Stigmasterol | 28.035 | *** | 1001.48 a | 221.47 b | 193.81 b |
| β-sitosterol | 28.950 | *** | 1162.24 a | 282.31 b | 264.62 b |
| β-amyrin | 29.195 | *** | 268.82 a | 2.78 b | 2.31 b |
| Cycloartenol | 30.045 | *** | 67.20 a | 0.00 b | 0.93 b |
| Betulin | 31.250 | *** | 176.62 a | 10.64 b | 12.38 b |
| TOTAL | 3647.48 a | 809.56 b | 757.07 b | ||
| Compound | Retention Time | Total Area % |
|---|---|---|
| Lauric acid | 23.505 | 0.16 ± 0.11 |
| Myristic acid | 27.805 | 1.34 ± 0.31 |
| Pentadecanoic acid | 29.820 | 0.24 ± 0.08 |
| Palmitic acid | 31.745 | 20.59 ± 5.7 |
| Palmitoleic acid | 32.135 | 2.03 ± 0.37 |
| Hexadecenoic acid, methyl ester, (11Z)- | 32.655 | 0.91 ± 0.15 |
| Heptadecanoic acid | 33.590 | 0.49 ± 0.09 |
| cis-10-Heptadecenoic acid | 33.925 | 0.18 ± 0.05 |
| Stearic acid | 35.365 | 4.47 ± 0.8 |
| Oleic acid | 35.640 | 8.28 ± 0.21 |
| Elaidic acid | 35.760 | 0.40 ± 0.14 |
| Linoleic acid | 36.375 | 23.07 ± 3.12 |
| α-Linolenic acid | 37.410 | 34.78 ± 5.17 |
| Arachidic acid | 38.675 | 0.97 ± 0.31 |
| Behenic acid | 41.160 | 1.86 ± 0.65 |
| cis-4,7,10,13,16,19- Docosahexaenoic acid | 42.345 | 0.23 ± 0.19 |
| Model Name | Model Equation |
|---|---|
| Lewis | MR = exp(−k·t) |
| Modified Page | |
| Henderson and Pabis |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K. Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies. Molecules 2019, 24, 1625. https://doi.org/10.3390/molecules24081625
Chua LYW, Chua BL, Figiel A, Chong CH, Wojdyło A, Szumny A, Lech K. Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies. Molecules. 2019; 24(8):1625. https://doi.org/10.3390/molecules24081625
Chicago/Turabian StyleChua, Lisa Yen Wen, Bee Lin Chua, Adam Figiel, Chien Hwa Chong, Aneta Wojdyło, Antoni Szumny, and Krzysztof Lech. 2019. "Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies" Molecules 24, no. 8: 1625. https://doi.org/10.3390/molecules24081625
APA StyleChua, L. Y. W., Chua, B. L., Figiel, A., Chong, C. H., Wojdyło, A., Szumny, A., & Lech, K. (2019). Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies. Molecules, 24(8), 1625. https://doi.org/10.3390/molecules24081625







