Protein Hydrolysate from Pterygoplichthys disjunctivus, Armoured Catfish, with High Antioxidant Activity
Abstract
:1. Introduction
2. Results
2.1. The Degree of Hydrolysis (DH%)
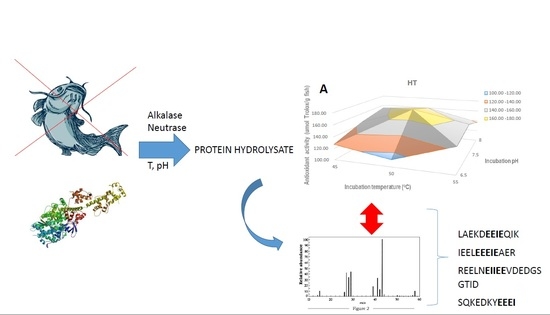
2.2. The Antioxidant Activity of Hydrolysates
2.2.1. The Antioxidant Activity of Hydrolysates by the ABTS Method
2.2.2. The Antioxidant Activity of Hydrolysates by the FRAP Method
2.2.3. The Antioxidant Activity of Hydrolysates by the ORAC Method
2.3. The Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity of Fish Hydrolysates
2.4. The Digestibility of the Hydrolysates
2.5. Bioactive Peptides
3. Discussion
3.1. Bioactivity
3.2. Peptide Structure–Activity Relationship
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. The Preparation and Pre-Treatment of Fish Protein Hydrolysis
4.2.2. Experimental Design
4.2.3. Determination of the Degree of Hydrolysis
4.2.4. Determination of Antioxidant Activity of Hydrolysates
The ABTS Free Radical Scavenging Activity Assay
The Ferric Reducing Antioxidant Power (FRAP) Assay
The Oxygen-Radical Absorbing Capacity (ORAC) Assay
4.2.5. Determination of the Angiotensin-I-Converting Enzyme (ACE) Inhibitory Activity of Fish Hydrolysates
4.2.6. The Digestibility of the Hydrolysates
4.2.7. Identification of Peptides
4.2.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armbruster, J.W. Redescription of Pterygoplichthys punctatus and description of a new species of Pterygoplichthys (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2006, 4, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Mitra, T.; Mahaver, L.; Bhowmick, S.; Paul, S.K.; Das, B.K. Nutritional compositon of the invasive Pterygoplichthys Disjunctivus from East Kolkata wetland. India. J. Inland Fish. Soc. India 2017, 49, 48–54. [Google Scholar]
- Zamora-Sillero, J.; Tavares Kütter, M.; Borges Tesser, M.; Monserrat, J.M.; Prentice, C. Effect of dietary common carp by-product protein hydrolysates on antioxidant status in different organs of zebrafish (Danio rerio). Aquacult. Nutr. 2019, 25, 110–118. [Google Scholar] [CrossRef]
- Nilsang, S.; Lertsiri, S.; Suphantharika, M.; Assavanig, A. Optimization of enzymatic hydrolysis of fish soluble concentrate by commercial proteases. J. Food Eng. 2005, 70, 571–578. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Pliszka, M.; Vegarud, G.E. Antioxidant properties of salmon (Salmo salar L.) protein fraction hydrolysates revealed following their ex vivo digestion and in vitro hydrolysis. J. Sci. Food Agric. 2016, 96, 2764–2772. [Google Scholar] [CrossRef]
- Ovissipour, M.; Kenari, A.A.; Motamedzadegan, A.; Nazari, R.M. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Yellowfin Tuna. Food Bioprocess Technol. 2012, 5, 696–705. [Google Scholar] [CrossRef]
- Chalamaiah, M.; kumar, B.D.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M.; Pegg, R.B.; Synowiecki, J. Chemical composition and nutritional value of processing discards of cod. Food Chem. 1991, 42, 145–151. [Google Scholar] [CrossRef]
- Vieira, G.H.F.; Martin, A.M.; Saker-Sampaiao, S.; Omar, S.; Goncalves, R.C.F. Studies on the enzymatic hydrolysis of Brazilian lobster (Panulirus spp.) processing wastes. J. Food Sci. Agric. 1995, 69, 61–65. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymic Hydrolysis of Food Protein; Elsevier Applied Science: Barking, Essex, UK, 1986. [Google Scholar]
- Saidi, S.; Belleville, M.-P.; Deratani, A.; Amar, R.B. Optimization of peptide production by enzymatic hydrolysis of tuna dark muscle by-product using commercial proteases. Afr. J. Biotechnol. 2013, 12, 1533–1547. [Google Scholar]
- Lompong, V.K.; Benjakul, S.; Yachai, M.; Visessanguan, W.; Shahidi, F.; Hayes, K.D. Amino Acid Composition and AntioxidativePeptides from Protein Hydrolysates of Yellow Stripe Trevally (Selaroides leptolepis). J. Food Sci. 2009, 74, C126–C133. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S.; Kishimura, H. Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brownstripe red snapper prepared using pyloric caeca and commercial proteases. Process Biochem. 2011, 46, 318–327. [Google Scholar] [CrossRef]
- Prior, R.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2011, 1, 1–10. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Jordán, M.; Bañón, S. Relationship between antioxidant status and oxidative stability in lamb meat reinforced with dietary rosemary diterpenes. Food Chem. 2016, 190, 1056–1063. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Jung, W.-K.; Kim, S.-K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bullfrog skin, Rana catesbeiana Shaw. Bioresour. Technol. 2008, 99, 1690–1698. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Nasri, R.; Aydi, M.; Toldra, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization, antioxidative and ACE inhibitory properties of hydrolysates obtained from thornback ray (Raja clavata) muscle. J. Proteomics 2015, 128, 458–468. [Google Scholar] [CrossRef]
- Sai-Ut, S.; Benjakul, S.; Sumpavapol, P.; Kishimura, H. Effect of Drying Methods on Odorous Compounds and Antioxidative Activity of Gelatin Hydrolysate Produced by Protease from B. amyloliquefaciens H11. Drying Technol. 2014, 32, 1552–1559. [Google Scholar] [CrossRef]
- Noriega, D.; ElviraZuñiga, M.; Soto, C.; Maidin, N.M.; Michael, N.; Jauregi, P. Colloidal Gas Aphrons separation to obtain polyphenol rich fractions from artichoke agro-industrial discards. Food Bioprod. Process. 2018, 110, 50–59. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; Souza Schmidt Gonçalves, A.E.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Theodore, A.E.; Raghavan, S.; Kristinsson, H.G. Antioxidative Activity of Protein Hydrolysates Prepared from Alkaline-Aided Channel Catfish Protein Isolates. J. Agric. Food Chem. 2008, 56, 7459–7466. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular Antioxidant Activity of Common Fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Hsieh, Y.H.P.; Hsieh, Y.P. Kinetics of Fe(III) reduction by ascorbic acid in aqueous solutions. J. Agric. Food Chem. 2000, 48, 1569–1573. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambamann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. Food Chem. Toxicol. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Tecan Group Ltd. ORAC Assay for the determination of antioxidant capacity in foods. In Application Note; Tecan Group Ltd.: Männedorf, Switzerlan, 2013. [Google Scholar]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.A.; Walsh, D.J.; FitzGerald, R.J. Modification of the furanacryloyl-l-phenylalanylglycylglycine assay for determination of angiotensin-I-converting enzyme inhibitory activity. J. Biochem. Biophys. Methods 2004, 59, 127–137. [Google Scholar] [CrossRef]
- Wu, S.; Feng, X.; Lan, X.; Xu, Y.; Liao, D. Purification and identification of Angiotensin-I Converting Enzyme (ACE) inhibitory peptide from lizard fish (Saurida elongata) hydrolysate. J. Funct. Foods 2015, 13, 295–299. [Google Scholar] [CrossRef]
- Qiu, C.; Sun, W.Z.; Cui, C.; Zhao, M. Effect of citric acid deamidation on in vitro digestibility and antioxidant properties of wheat gluten. Food Chem. 2013, 141, 2772–2778. [Google Scholar] [CrossRef]
- Sullivan, A.C.; Pangloli, P.; Dia, V.P. Impact of ultrasonication on the physicochemical properties of sorghum kafirin and in vitro pepsin-pancreatin digestibility of sorghum gluten-like flour. Food Chem. 2018, 240, 1121–1130. [Google Scholar] [CrossRef]
Sample Availability: Samples of the fish are available from the authors. |








| Enzyme | Peptide | Mr(expt) | Mr(calc) | Score | Expect | Protein |
|---|---|---|---|---|---|---|
| PAL | REELNEIIEEVDEDGSGT | 2032.9123 | 2032.9073 | 100 | 2.7 × 10−5 | Troponin C |
| REELNEIIEEVDEDGSGTID | 2261.0018 | 2261.0183 | 85 | 7.3 × 10−4 | ||
| IAEKDEEIEQLK | 1443.7383 | 1443.7456 | 79 | 4.9 × 10−3 | Embryonic myosin heavy chain | |
| IEELEEEIEAER | 1487.6950 | 1487.6991 | 88 | 4.8 × 10−4 | ||
| KKAEPAPAPAPAPE | 1372.7291 | 1372.7350 | 76 | 0.009 | Embryonic myosin light chain | |
| HT | LAEKDEEIEQI | 1315.64 | 1315.6507 | 71 | 0.03 | Myosin heavy chain |
| LAEKDEEIEQIK | 1443.7384 | 1443.7456 | 78 | 0.0056 | ||
| IEELEEEIEAER | 1487.6917 | 1487.6991 | 90 | 2.5 × 10−4 | ||
| NSYEEALDHLETL | 1532.6970 | 1532.6994 | 68 | 0.045 | ||
| MDLENDKQQSEEK | 1592.6925 | 1592.6988 | 68 | 0.03 | ||
| IMDLENDKQQSEEK | 1705.7735 | 1705.7828 | 80 | 0.0028 | ||
| TERLEDEEEINAE | 1575.6856 1575.6900 | 71 | 0.03 | |||
| LESEVEGEQR | 1174.5428 1174.5466 | 70 | 0.03 | |||
| FDMFDTDGGGDISTK | 1604.6567 1604.6665 | 109 | 9.5 × 10−7 | |||
| REELNEIIEEVDEDGSGTID | 2261.0113 2261.0183 | 73 | 0.012 | |||
| SQKEDKYEEEI | 1396.6302 1396.6358 | 82 | 0.015 | |||
| LEKTIDDLEDELYSQ | 1809.8489 1809.8520 | 98 | 6.2 × 10−5 | |||
| GQKDSYVGDEAQSK | 1510.6838 | 150.6900 | 97 | 2.3 × 10−5 | Mutant beta actin (homo sapiens) | |
| SIIDQDKSGFIEEDELKL | 2078.0318 | 2078.0419 | 91 | 4 × 10−4 | Parvalbumin | |
| GDTDGDGKIGVDEF | 1423.6112 | 1423.6104 | 88 | 2.2 × 10−4 | Parvalbumin beta | |
| KQFLEELLTTQ | 1348.7228 | 1348.7238 | 74 | 0.012 | Myosin light chain 2 | |
| IVGDDLTVTNPK | 1270.6723 | 1270.6769 | 74 | 0.011 | Enolase | |
| PF | DDLQAEEDKVNT | 1375.6122 | 1375.6103 | 71 | 0.016 | Myosin heavy chain fast skeletal muscle-like [Ictalurus punctatus] |
| TEEMASQDESIAK | 1437.6256 | 1437.6293 | 97 | 3.3 × 10−5 | ||
| AQRLQEAEESIEAV | 1571.7776 | 1571.7791 | 71 | 0.029 | ||
| QGEVEDLMIDVERA | 1602.7473 | 1602.7559 | 86 | 7 × 10−4 | ||
| RNAEEKAKKAITDAA | 1614.8767 | 1614.8689 | 74 | 0.012 | ||
| LEEAEGTLEHEESKI | 1712.8086 | 1712.8104 | 87 | 7 × 10−4 | ||
| EELKKEQDTSAHLER | 1811.8991 | 1811.9013 | 80 | 0.0047 | ||
| LEEAEGTLEHEESKIL | 1825.8829 | 1825.8945 | 72 | 0.026 | ||
| KRQAEEAEEQANTHLS | 1839.8700 | 1839.8711 | 71 | 0.026 | ||
| REQFEEEQEAKAELQ | 1862.8581 | 1862.8646 | 86 | 7.6 × 10−4 | ||
| EQQVDDLEGSLEQEKK | 1873.8915 | 1873.8905 | 71 | 0.034 | ||
| AEELKKEQDTSAHLER | 1882.9359 | 1882.9384 | 92 | 0.00031 | ||
| QARIEELEEEIEAERAA. + Gln->pyro-Glu (N-term Q) | 1967.9345 | 1967.9435 | 78 | 0.0063 | ||
| KQKYEEGQAELEGAQKEA | 2034.9739 | 2034.9857 | 92 | 2.4 × 10−4 | ||
| EMEEAQERADIAESQVNK | 2075.9393 | 2075.9429 | 81 | 0.0021 | ||
| KRENKNLQQEISDLTEQI | 2185.1241 | 2185.1338 | 72 | 0.034 | ||
| KLEQQVDDLEGSLEQEKKL | 2228.1563 | 2228.1536 | 89 | 0.00059 | ||
| HELEKAKKTVETEKSEIQTA | 2298.2000 | 2298.2067 | 84 | 0.002 | ||
| RKVQHEMEEAQERADIAESQVNK | 2724.3189 | 2724.3249 | 104 | 2 × 10−5 | ||
| EEGQAELEGAQKEARS | 1730.8073 | 1730.8071 | 86 | 7.5 × 10−4 | Myosin heavy chain, fast skeletal muscle isoform X1 [Danio rerio] | |
| KMEIDDLSSNMEAVAKS | 1866.8692 | 1866.8703 | 86 | 8.2 × 10−4 | Myosin heavy chain (Seriola demirili) | |
| SYKRQAEEAEEQANTHLS | 2089.9620 | 2089.9664 | 72 | 0.02 | Myosin heavy chain-2 [Thunnus orientalis] | |
| AEQELLDASERVGL | 1528.7728 | 1528.7733 | 71 | 0.027 | Myosin heavy chain, fast skeletal muscle-like [Clupea harengus] | |
| EADLVQIQGEVDDTVQEA | 1957.9077 | 1957.9117 | 96 | 8.8 × 10−5 | Myosin heavy chain [Pennahia argentata] | |
| KAISEELDHALNDMTSI | 1885.9049 | 1885.9091 | 84 | 0.0017 | Tropomyosin alpha-1 chain-like isoform X2 [Nothobranchius furzeri] | |
| EKTIDDLEDELYSQKLK | 2066.0377 | 2066.0419 | 82 | 0.0028 | ||
| KLEKTIDDLEDELYSQKL | 2179.1241 | 2179.1259 | 87 | 9.1 × 10−4 | ||
| KATEDELDKYSEALKDAQEKL | 2423.1937 | 2423.2067 | 88 | 8.4 × 10−4 | ||
| RALGQNPTNKDVAK | 1510.8211 | 1510.8216 | 78 | 0.0039 | Myosin light chain 1 [Thunnus thynnus] | |
| KKAEPAPAPAPAPE | 1372.7315 | 1372.7350 | 76 | 0.0087 | ||
| SSSSLEKSYELPDGQVI | 1837.8938 | 1837.8945 | 71 | 0.032 | Alpha-smooth muscle actin-rabbit (fragment) | |
| SSSSLEKSYELPDGQVIT | 1938.9372 | 1938.9422 | 71 | 0.038 | ||
| AVFDISNADRLGSSEVDQV | 2020.9632 | 2020.9702 | 82 | 0.0027 | Creatine kinase M-type [Gekko japonicus] | |
| GDFSADQIEDFKEA | 1612.6844 | 1612.6893 | 76 | 0.0031 | Myosin light chain 1/3, skeletal muscle isoform [Cynoglossus semilaevis] |
| Enzyme | Temperature (°C) | pH | #%(w/v) | Time (min) | ||||
|---|---|---|---|---|---|---|---|---|
| HT PROTEOLITIC®L 200 | 45 | 50 | 55 | 6.5 | 7.5 | 8.0 | 0.2% | 120 |
| PAL®660 | 55 | 60 | 65 | 9.0 | 9.5 | 10.0 | 0.2% | 120 |
| Proteasa Fungal | 45 | 50 | 55 | 6.0 | 7.0 | 8.0 | 0.2% | 120 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Michael, N.; Fonseca Madrigal, J.; Sosa Aguirre, C.; Jauregi, P. Protein Hydrolysate from Pterygoplichthys disjunctivus, Armoured Catfish, with High Antioxidant Activity. Molecules 2019, 24, 1628. https://doi.org/10.3390/molecules24081628
Guo Y, Michael N, Fonseca Madrigal J, Sosa Aguirre C, Jauregi P. Protein Hydrolysate from Pterygoplichthys disjunctivus, Armoured Catfish, with High Antioxidant Activity. Molecules. 2019; 24(8):1628. https://doi.org/10.3390/molecules24081628
Chicago/Turabian StyleGuo, Yuchen, Nicholas Michael, Jorge Fonseca Madrigal, Carlos Sosa Aguirre, and Paula Jauregi. 2019. "Protein Hydrolysate from Pterygoplichthys disjunctivus, Armoured Catfish, with High Antioxidant Activity" Molecules 24, no. 8: 1628. https://doi.org/10.3390/molecules24081628