Synthesis of Telmisartan Organotin(IV) Complexes and their use as Carbon Dioxide Capture Media
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Organotin(IV) Complexes 1–4
2.2. FTIR Spectroscopy of Organotin(IV) Complexes 1–4
2.3. NMR Spectroscopy of Organotin(IV) Complexes 1–4
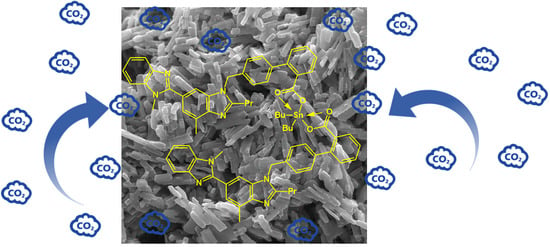
2.4. Field Emission Scanning Electron Microscopy (FESEM) of Organotin(IV) Complexes 1–4
2.5. Pure Gas Adsorption of Organotin(IV) Complexes 1–4
3. Materials and Methods
3.1. General
3.2. Synthesis of Triorganotin(IV) Complexes 1 and 2
3.3. Synthesis of Diorganotin(IV) Complexes 3 and 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fanchi, J.R.; Fanchi, C.J. Energy in the 21st Century, 4th ed.; World Scientific Publishing: Singapore, Malaysia, 2016. [Google Scholar]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.Q.; Zhou, H.-C. Gas storage in porous metal–organic frameworks for clean energy applications. Chem. Commun. 2010, 46, 44–53. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M.; Schneider, M.W.; Oppel, I.M.; Presly, O. A salicylbisimine cage compound with high surface area and selective CO2/CH4 adsorption. Angew. Chem. Int. Ed. 2011, 50, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal−organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef]
- Johnson, J. Putting a lid on carbon dioxide. Carbon sequestration, clean-coal research mark government response to climate-change threat. Chem. Eng. News 2004, 82, 36–42. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, A.E. Adsorption of carbon dioxide at high temperature—A review. Sep. Purif. Technol. 2002, 26, 195–205. [Google Scholar] [CrossRef]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Yang, S.; Blake, A.J.; Schroder, M. Studies on metal–organic frameworks of Cu(II) with isophthalate linkers for hydrogen storage. Acc. Chem. Res. 2014, 47, 296–307. [Google Scholar] [CrossRef]
- Zhao, D.; Timmons, D.J.; Yuan, D.; Zhou, H.-C. Tuning the topology and functionality of metal−organic frameworks by ligand design. Acc. Chem. Res. 2011, 44, 123–133. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Fan, M.; Yang, M.; Cui, S. Dynamic capture of low-concentration CO2 on amine hybrid silsesquioxane aerogel. Chem. Eng. J. 2016, 283, 1059–1068. [Google Scholar] [CrossRef]
- Builes, S.; López-Aranguren, P.; Fraile, J.; Vega, L.F.; Domingo, C. Analysis of CO2 adsorption in amine-functionalized porous silicas by molecular simulations. Energy Fuels 2015, 29, 3855–3862. [Google Scholar] [CrossRef]
- Corvo, M.C.; Sardinha, J.; Casimiro, T.; Marin, G.; Seferin, M.; Einloft, S.; Menezes, S.C.; Dupont, J.; Cabrita, E.J. A rational approach to CO2 capture by imidazolium ionic liquids: Tuning CO2 solubility by cation alkyl branching. ChemSusChem 2015, 8, 1935–1946. [Google Scholar] [CrossRef]
- Japip, S.; Wang, H.; Xiao, Y.; Chung, T.S. Highly permeable zeolitic imidazolate framework (ZIF)-71 nano-particles enhanced polyimide membranes for gas separation. J. Membr. Sci. 2014, 467, 162–174. [Google Scholar] [CrossRef]
- Huang, Q.; Eić, M. Commercial adsorbents as benchmark materials for separation of carbon dioxide and nitrogen by vacuum swing adsorption process. Sep. Purif. Technol. 2013, 103, 203–215. [Google Scholar] [CrossRef]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; Yaghi, O.M. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Yaghi, O.M. Storage of hydrogen, methane, and carbon dioxide in highly porous covalent organic frameworks for clean energy applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; de Lasa, H.I. Chemical-looping combustion (CLC) for inherent CO2 separations—A review. Chem. Eng. Sci. 2008, 63, 4433–4451. [Google Scholar] [CrossRef]
- Jones, C.W. CO2 capture from dilute gases as a component of modern global carbon management. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 31–52. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef]
- Alotaibi, M.H.; El-Hiti, G.A.; Hashim, H.; Hameed, A.S.; Ahmed, D.S.; Yousif, E. SEM analysis of the tunable honeycomb structure of irradiated poly(vinyl chloride) films doped with polyphosphate. Heliyon 2018, 4, e01013. [Google Scholar] [CrossRef]
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 2018, 4, e00743. [Google Scholar] [CrossRef]
- Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Alotaibi, M.H.; Hashim, H.; Hameed, A.S.; Ahmed, A. Fabrication of novel ball-like polystyrene films containing Schiff base microspheres as photostabilizers. Polymers 2018, 10, 1185. [Google Scholar] [CrossRef]
- Balakit, A.A.; Smith, K.; El-Hiti, G.A. Synthesis and characterization of a new photochromic alkylene sulfide derivative. J. Sulfur Chem. 2018, 39, 182–192. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus equi using different carbon sources. Arab. J. Sci. Eng. 2017, 42, 2371–2379. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 762. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl)propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef]
- Akram, M.A.; Nazir, T.; Taha, N.; Adil, A.; Sarfraz, M.; Nazir, S.R. Designing, development and formulation of mouth disintegrating telmisartan tablet with extended release profile using response surface methodology. J. Bioequiv. Availab. 2015, 7, 262–266. [Google Scholar] [CrossRef]
- Pejchal, V.; Holeček, J.; Nádvorník, M.; Lyčka, A. 13C and 119Sn-NMR Spectra of some mono-n-butyltin(IV) compounds. Collect. Czech. Chem. Commun. 1995, 60, 1492–1501. [Google Scholar] [CrossRef]
- Shahid, K.; Ali, S.; Shahzadi, S.; Badshah, A.; Khan, K.M.; Maharvi, G.M. Organotin(IV) complexes of aniline derivatives. I. Synthesis, spectral and antibacterial studies of di- and triorganotin(IV) derivatives of 4-bromomaleanilic acid. Synth. React. Inorg. Met. Org. Chem. 2003, 33, 1221–1235. [Google Scholar] [CrossRef]
- Rehman, W.; Baloch, M.K.; Badshah, A.; Ali, S. Synthesis and characterization of biologically potent di-organotin(IV) complexes of mono-methyl glutarate. J. Chin. Chem. Soc. 2005, 52, 231–236. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Rabbani, M.G.; El-Kaderi, H.M. Template-free synthesis of a highly porous benzimidazole-linked polymer for CO2 capture and H2 storage. Chem. Mater. 2011, 23, 1650–1653. [Google Scholar] [CrossRef]
- Jin, Y.; Voss, B.A.; McCaffrey, R.; Baggett, C.T.; Noble, R.D.; Zhang, W. Microwave-assisted syntheses of highly CO2-selective organic cage frameworks (OCFs). Chem. Sci. 2012, 3, 874–877. [Google Scholar] [CrossRef]
- Yu, H.; Tian, M.; Shen, C.; Wang, Z. Facile preparation of porous polybenzimidazole networks and adsorption behavior of CO2 gas, organic and water vapors. Polym. Chem. 2013, 4, 961–968. [Google Scholar] [CrossRef]
- Katsoulidis, A.P.; Dyar, S.M.; Carmieli, R.; Malliakas, C.D.; Wasielewski, M.R.; Kanatzidis, M.G. Copolymerization of terephthalaldehyde with pyrrole, indole and carbazole gives microporous POFs functionalized with unpaired electrons. J. Mater. Chem. A 2013, 1, 10465–10473. [Google Scholar] [CrossRef]
- Zhang, W.; Wojtas, L.; Aguila, B.; Jiang, P.; Ma, S. Metal–metalloporphyrin framework modified with flexible tert-butyl groups for selective gas adsorption. ChemPlusChem 2016, 81, 714–717. [Google Scholar] [CrossRef]
- Meng, L.; Cheng, Q.; Kim, C.; Gao, W.-Y.; Wojtas, L.; Chen, Y.-S.; Zaworotko, M.J.; Zhang, X.P.; Ma, S. Crystal engineering of a microporous, catalytically active fcu topology MOF using a custom-designed metalloporphyrin linker. Angew. Chem. Int. Ed. 2012, 51, 10082–10085. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the telmisartan organotin (IV) complexes are available from the authors. |











| Sn(IV) Complex | R | Color | Yield (%) | Melting Point (°C) | Calcd. (Found; %) | ||
|---|---|---|---|---|---|---|---|
| C | H | N | |||||
| 1 | Ph | pale yellow | 86 | 163–165 | 70.93 (71.12) | 5.14 (5.13) | 6.49 (6.36) |
| 2 | Bu | white | 83 | 243–245 | 67.25 (67.46) | 7.02 (7.11) | 6.97 (7.06) |
| 3 | Ph | off white | 90 | 237–239 | 72.06 (71.97) | 5.27 (5.36) | 8.62 (8.63) |
| 4 | Bu | off white | 89 | 184–186 | 70.53 (70.41) | 6.08 (5.98) | 8.89 (9.00) |
| Sn(IV) Complex | FTIR (ν, cm−1) | ||||
|---|---|---|---|---|---|
| C=O | C=N | C=C | Sn–C | Sn–O | |
| 1 | 1685 | 1541 | 1455 | 526 | 447 |
| 2 | 1697 | 1540 | 1458 | 528 | 447 |
| 3 | 1697 | 1543 | 1456 | 536 | 445 |
| 4 | 1697 | 1536 | 1454 | 536 | 447 |
| Sn(IV) Complex | 1H-NMR | 119Sn-NMR |
|---|---|---|
| 1 | 1.00 (t, J = 7.6 Hz, 3H, Me), 1.83 (quintet, J = 7.6 Hz, 2H, CH2), 2.61 (s, 3H, Me), 3.17 (m, 2H, CH2), 3.81 (s, 3H, Me), 5.62 (s, 2H, CH2), 7.26–7.86 (m, 29H, Ar) | –193.0 |
| 2 | 0.90 (t, J = 7.5 Hz, 9H, 3 Me), 0.99 (t, J = 7.7 Hz, 3H, Me), 1.23 (m, 6H, 3 CH2), 1.46 (m, 6H, 3 CH2), 1.58 (m, 6H, 3 CH2), 1.86 (quintet, J = 7.7 Hz, 2H, CH2), 2.63 (s, 3H, Me), 2.92 (t, J = 7.7 Hz, 2H, CH2), 3.39 (s, 3H, Me), 5.63 (s, 2H, CH2), 7.26–7.74 (m, 14H, Ar) | –185.0 |
| 3 | 1.02 (t, J = 7.5 Hz, 6H, 2 Me), 1.83 (quintet, J = 7.5 Hz, 4H, 2 CH2), 2.63 (s, 6H, 2 Me), 2.93 (t, J = 7.5 Hz, 4H, 2 CH2), 3.40 (s, 6H, 2 Me), 5.63 (s, 4H, 2 CH2), 7.28–7.73 (m, 38H, Ar) | –267.0 |
| 4 | 0.94–1.01 (m, 12H, 4 Me), 1.21–1.31 (m, 8H, 4 CH2), 1.79–1.83 (m, 8H, 4 CH2), 2.63 (s, 6H, 2 Me), 2.91 (t, J = 7.7 Hz, 4H, 2 CH2), 3.80 (s, 6H, 2 Me), 5.61 (s, 4H, 2 CH2), 7.21–7.86 (m, 28H, Ar) | –242.5 |
| Sn(IV) Complex | 13C-NMR |
|---|---|
| 1 | 168.3 (C=O), 156.7, 154.4, 143.1, 142.6, 141.3, 140.6, 137.0, 136.6, 136.4, 136.3, 135.2, 130.9, 129.8, 129.4, 128.6, 127.8, 126.8, 123.7, 122.7, 122.3, 119.1, 110.8, 109.7, 46.6 (CH2), 32.2 (Me), 29.2 (CH2), 21.2 (CH2), 16.9 (Me), 14.3 (Me) |
| 2 | 171.0 (C=O), 156.6, 154.5, 143.2, 142.5, 137.0, 136.4, 135.2, 130.8, 129.6, 129.2, 128.7, 128.1, 127.8, 126.8, 123.7, 122.6, 119.1, 110.4, 109.8, 46.0 (CH2), 32.2 (Me), 29.2 (CH2), 28.4 (CH2), 28.2 (CH2), 26.7 (CH2), 21.2 (CH2), 16.9 (Me), 14.3 (Me), 14.1 (Me) |
| 3 | 170.0 (C=O), 156.7, 154.4, 142.5, 141.5, 140.9, 140.7, 136.9, 136.4, 135.2, 132.7, 131.3, 130.8, 129.6, 129.2, 128.8, 127.7, 126.9, 123.7, 122.7, 122. 5, 119.0, 111.0, 109.9, 46.6 (CH2), 32.3 (Me), 29.2 (CH2), 21.2 (CH2), 16.9 (Me), 14.3 (Me) |
| 4 | 169.6 (C=O), 156.6, 154.5, 143.2, 142.9, 141.0, 140.8, 137.1, 136.3, 135.2, 133.1, 130.1, 129.8, 129.4, 128.6, 127.8, 126.8, 126.8, 123.7, 122.7, 119.1, 110.8, 109.4, 46.6 (CH2), 32.2 (Me), 30.8 (CH2), 29.2 (CH2), 27.3 (CH2), 26.1 (CH2), 21.2 (CH2), 16.9 (Me), 14.3 (Me), 13.9 (Me) |
| Sn(IV) Complex | SBET (m2·g−1) a | Vtotal (cm3·g−1) b | Pore Size (nm) c |
|---|---|---|---|
| 1 | 46.338 | 0.062 | 2.433 |
| 2 | 68.434 | 0.097 | 2.428 |
| 3 | 32.374 | 0.046 | 2.432 |
| 4 | 130.357 | 0.162 | 2.429 |
| Sn(IV) Complex | CO2 Uptake (cm3·g−1) | CO2 Uptake (wt%) | H2 Uptake (cm3·g−1) | H2 Uptake (wt%) |
|---|---|---|---|---|
| 1 | 18.2 | 3.6 | 1.1 | 0.009 |
| 2 | 20.5 | 4.0 | 0.7 | 0.006 |
| 3 | 16.5 | 3.3 | 0.5 | 0.006 |
| 4 | 35.0 | 7.1 | 1.1 | 0.013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, A.G.; Jawad, K.; Yousif, E.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S. Synthesis of Telmisartan Organotin(IV) Complexes and their use as Carbon Dioxide Capture Media. Molecules 2019, 24, 1631. https://doi.org/10.3390/molecules24081631
Hadi AG, Jawad K, Yousif E, El-Hiti GA, Alotaibi MH, Ahmed DS. Synthesis of Telmisartan Organotin(IV) Complexes and their use as Carbon Dioxide Capture Media. Molecules. 2019; 24(8):1631. https://doi.org/10.3390/molecules24081631
Chicago/Turabian StyleHadi, Angham G., Khudheyer Jawad, Emad Yousif, Gamal A. El-Hiti, Mohammad Hayal Alotaibi, and Dina S. Ahmed. 2019. "Synthesis of Telmisartan Organotin(IV) Complexes and their use as Carbon Dioxide Capture Media" Molecules 24, no. 8: 1631. https://doi.org/10.3390/molecules24081631
APA StyleHadi, A. G., Jawad, K., Yousif, E., El-Hiti, G. A., Alotaibi, M. H., & Ahmed, D. S. (2019). Synthesis of Telmisartan Organotin(IV) Complexes and their use as Carbon Dioxide Capture Media. Molecules, 24(8), 1631. https://doi.org/10.3390/molecules24081631