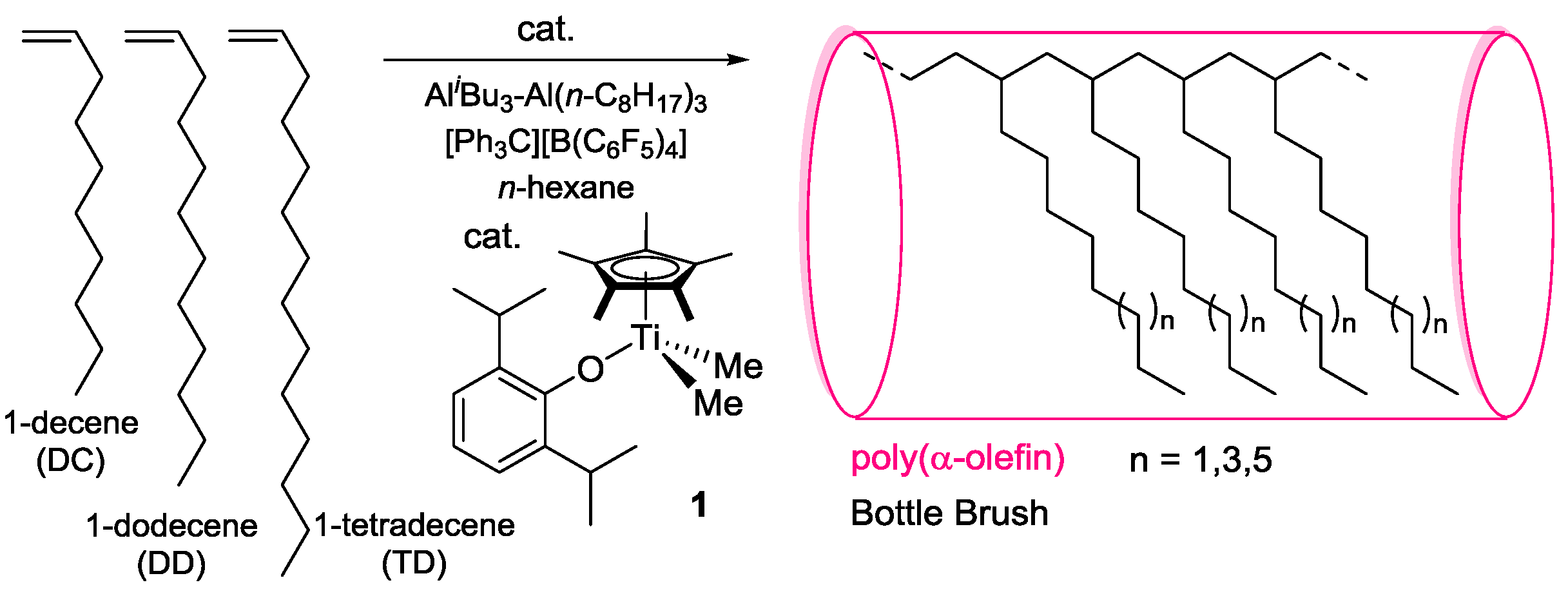
Abstract
Polymerizations of 1-decene (DC), 1-dodecene (DD), and 1-tetradecene (TD) by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–[Ph3C][B(C6F5)4] (borate) catalyst have been explored in the presence of Al cocatalyst. The polymerizations of DC and DD, in n-hexane containing a mixture of AliBu3 and Al(n-C8H17)3, proceeded with high catalytic activities in a quasi-living manner, affording high molecular weight polymers (activity 4120–5860 kg-poly(DC)/mol-Ti·h, Mn for poly(DC) = 7.04–7.82 × 105, after 20 min at −30 °C). The PDI (Mw/Mn) values in the resultant polymers decreased upon increasing the ratio of Al(n-C8H17)3/AliBu3 with decreasing the activities at −30 °C. The PDI values also became low when these polymerizations were conducted at low temperatures (−40 or −50 °C); high molecular weight poly(DD) with low PDI (Mn = 5.26 × 105, Mw/Mn = 1.16) was obtained at −50 °C. The TD polymerization using 1–borate–AliBu3 catalyst (conducted in n-hexane at −30 °C) afforded ultrahigh molecular weight poly(TD) (Mn = 1.02 × 106, Mw/Mn = 1.38), and the PDI values also decreased with increasing the Al(n-C8H17)3/AliBu3 ratio.
1. Introduction
Design of molecular catalysts for olefin polymerization has been considered as an important subject in synthesis of new polymers with specified functions. The recent progress in the catalyst developments provides new possibilities [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Although crystalline isotactic polypropylene has been widely used in our daily life, use of amorphous poly(α-olefin)s (APAOs) showed less attention due to their inherent stickiness and softness. APAOs possess high melt-flow rate with low density, and are used in hot melt applications, these also improve adhesion on wood and polypropylene, and improve the free-flowing ability of their granules. It has been known recently that ultrahigh molecular weight poly(α-olefin)s can be used as drag reducing agents (DRAs) in pipeline transport methods for crude oil and petroleum products, because of their ability to reduce pumping power and increase piping system capacity [18,19,20,21]. Moreover, poly(α-olefin)s with alkyl chain length greater than six have bottlebrush architecture (branched macromolecules with a high graft density along their backbone) [22,23,24,25], and are the simplest bottlebrush polymers, with their backbone and side chains consisting of alkanes.
As shown below (Table 1), polymerization of α-olefin (1-hexene, 1-octene, 1-decene, etc.) by ordinary metallocene catalysts gave oligomers [26,27,28]. Several examples [28,29,30,31,32,33] were known for synthesis of (ultra)high molecular weight poly(1-hexene)s by using [2,2-(O-4-Me-6-tBu-C6H3)2S]TiCl2 (in the presence of specified modified MMAO) [31,32], or (C5HMe4)2HfCl2 (under ultrahigh pressure) [29]. One example was known for synthesis of isotactic poly(1-hexene)s with ultrahigh molecular weights by titanium complexes containing diamine bis(phenolate) ligands [30]. Several examples were also known for synthesis of poly(α-olefin)s with rather high molecular weights [27,34,35,36,37,38,39,40,41,42]. However, synthesis of ultrahigh molecular weight polymers by polymerization of higher α-olefins (1-decene, 1-dodecene, 1-tetradecene, etc.) still have been limited [28], probably due to their tendency to undergo β-hydrogen elimination before subsequent/repeated insertion.

Table 1.
Polymerization of 1-octene (OC) and 1-dodecene (DD), using Cp*TiX2(O-2,6-iPr2C6H3) [X = Cl, Me (1)], [Me2Si(C5Me4)(NtBu)]TiCl2, Cp2ZrCl2—cocatalyst systems [28]. a
We recently reported synthesis of high molecular weight poly(α-olefin)s by polymerizations of 1-decene, 1-dodecene, 1-hexadecene, and 1-octadecene by Cp*TiCl2(O-2,6-iPr2C6H3)–MAO catalyst [28], and the Mn values in the resultant polymers were higher than those prepared by [Me2Si(C5Me4)(NtBu)]TiCl2 and Cp2ZrCl2 (Table 1) [28]. Moreover, Cp*TiMe2(O-2,6-iPr2C6H3) (1)–[Ph3C][B(C6F5)4] (borate) catalyst showed the higher catalytic activities, affording ultrahigh molecular weight poly(1-octene)s and poly(1-dodecene)s (Table 1) [28]. On the other hand, we also reported that 1-hexene polymerization by 1–borate catalyst proceeded in a quasi-living manner under certain conditions to afford ultrahigh molecular weight poly(1-hexene)s (Mn ≥ 1.0–1.9 × 106) [33]. Therefore, we herein present synthesis of high molecular weight polymers by polymerizations of 1-decene (DC), 1-dodecene (DD), and 1-tetradecene (TD) with low PDI (Mw/Mn) values using 1-borate catalyst in the presence of Al cocatalyst (Scheme 1).
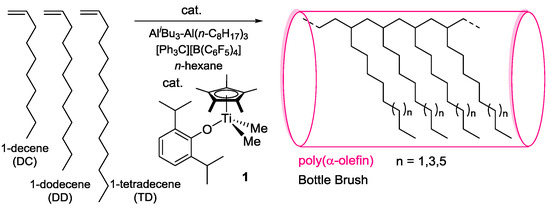
Scheme 1.
Polymerization of 1-decene (DC), 1-dodecene (DD), and 1-tetradecene (TD) using Cp*TiMe2(O-2,6-iPr2C6H3) (1)–[Ph3C][B(C6F5)4] catalyst in the presence of Al cocatalyst.
2. Results and Discussion
2.1. Polymerization of 1-Decene and 1-Dodecene by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–Borate Catalyst
On the basis of our previous report in 1-hexene polymerization [33], polymerizations of 1-decene (DC) using Cp*TiMe2(O-2,6-iPr2C6H3) (1)–[Ph3C][B(C6F5)4] (borate) catalyst were conducted in n-hexane in the presence of Al cocatalyst (Al/Ti = 500, molar ratio) with different Al(n-C8H17)3/AliBu3 ratios at −30 °C. Use of Al(n-C8H17)3, which should be the weak reagent for alkylation and/or chain transfer, but plays a role as a scavenger, was effective in the 1-hexene polymerization to proceed without catalyst deactivation [33]. The similar effect was also observed in the syndiospecific styrene polymerization using (tBuC5H4)TiCl2(O-2,6-iPr2C6H3)–[PhN(H)Me2]-[B(C6F5)4] catalyst [43]. Use of a mixture of Al(n-C8H17)3/AliBu3 cocatalyst was also effective for exclusive obtainment of copolymers with high styrene contents in the ethylene/styrene copolymerization even at high temperature [44,45]. It was assumed that the Al alkyl would also play a role to stabilize the catalytically active species for the subsequent decomposition by reacting with borate [46,47,48].
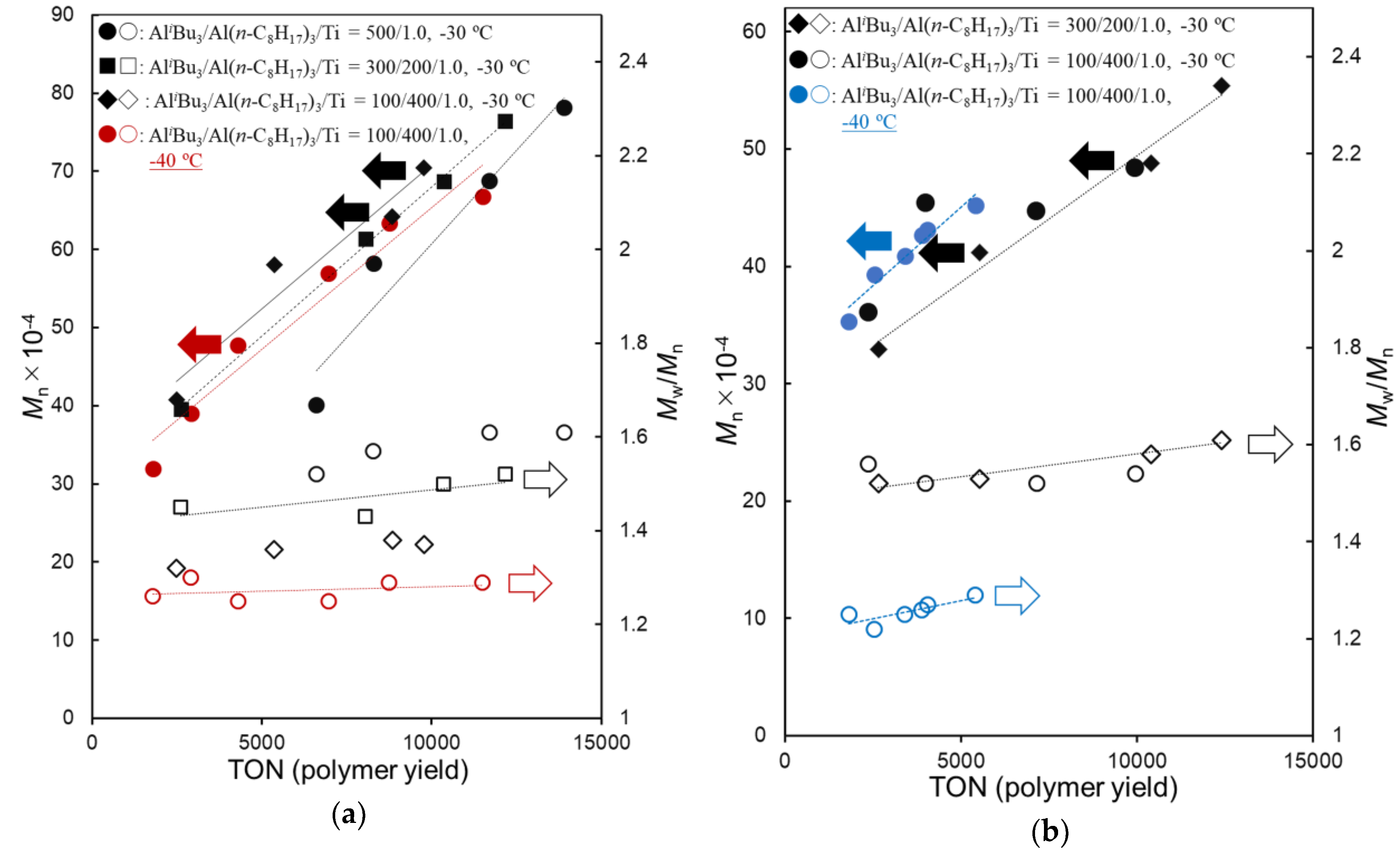
As shown in Table 2, the polymerization of DC at −30 °C (runs 1–3) proceeded with high catalytic activities (4120–5860 kg-polymer/mol-Ti·h after 20 min) without significant catalyst deactivation to afford high molecular weight poly(DC)s (Mn = 7.04–7.82 × 105), and the Mn values increased over time course without increasing the PDI (Mw/Mn) values. It turned out that the PDI values decreased upon increasing the Al(n-C8H17)3/AliBu3 molar ratio, whereas the catalytic activity decreased with increasing the ratio (runs 1–3). As shown in Figure 1a (shown below), rather linear relationships between the Mn values and the polymer yields (turnover numbers, TON) were observed, suggesting that these polymerizations proceeded in a (quasi) living manner.

Table 2.
1-Decene polymerization by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–borate catalyst. a
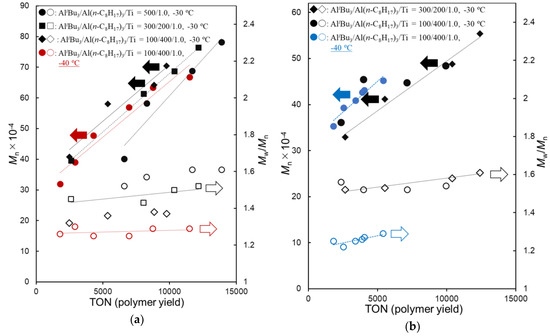
It also turned out that the PDI values became low when the polymerization was conducted at −40 °C (run 4), with decreasing the activity [activity after 20 min: 4120 kg-polymer/mol-Ti·h (run 3, at −30 °C) vs. 1810 (run 4, at −40 °C)]. As shown in Figure 1a, a relatively good linear relationship between the Mn values and the polymer yields consistent with rather low PDI values (Mw/Mn = 1.25–1.30) clearly suggest that the polymerization proceeded in a (quasi) living manner. Moreover, the PDI value became low (Mw/Mn = 1.13–1.21) when the polymerization was conducted at −50 °C. The Mn value in the resultant polymers increased over time course consistent with low PDI values—high molecular weight poly(DC) with low PDI (Mn = 4.65 × 105, Mw/Mn = 1.15, run 5) was thus obtained after 2 h.
Similarly, as shown in Table 3, the polymerization of DD at −30 °C (runs 6–8) proceeded with high catalytic activities (5020–8950 kg-polymer/mol-Ti·h after 20 min) without significant catalyst deactivation to afford high molecular weight poly(DD)s (Mn = 4.84–6.74 × 105), and the Mn values increased over time course without increasing the PDI (Mw/Mn) values. The PDI values decreased upon increasing the Al(n-C8H17)3/AliBu3 molar ratio, whereas the catalytic activity decreased with increasing the ratio. As also shown in Figure 1b (shown below), rather linear relationships between the Mn values and the polymer yields (turnover numbers, TON) suggest that these polymerizations also proceeded in a (quasi) living manner.

Table 3.
1-Dodecene polymerization by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–borate catalyst. a
It was also revealed that the PDI values became low when the polymerization was conducted at −40 °C (run 9), although the catalytic activity decreased at −40 °C [activity after 20 min: 5020 kg-polymer/mol-Ti·h (run 8, at −30 °C) vs. 1720 (run 9, at −40 °C)]. As shown in Figure 1b, a relatively good linear relationship between Mn value and the polymer yield consistent with rather low PDI values (Mw/Mn = 1.22–1.29) clearly suggests that the polymerization proceeded in a (quasi) living manner. Moreover, the PDI value became low (Mw/Mn = 1.11–1.18) when the polymerization was conducted at −50 °C. The Mn value in the resultant polymers increased over time course consistent with low PDI values; high molecular weight poly(DD) with low PDI (Mn = 5.26 × 105, Mw/Mn = 1.14) could be thus obtained after 2 h.
As shown in Figure 1, good linear relationships between the Mn values and the polymer yields (TONs) were observed without increasing the PDI values in all cases, suggesting that these polymerizations proceeded in a (quasi) living manner. In particular, the polymerizations at −40 °C afforded polymers with low PDI values, the results thus strongly indicate a possibility of living polymerization under these conditions. The resultant poly(DD)s showed similar thermal property (melting temperature (Tm) = −24 °C) to those prepared by Cp*TiCl2(O-2,6-iPr2C6H3)–MAO catalyst [28], whereas Tm values increased upon increasing methylene units in the alkyl side chain (poly(1-hexadecane) = 26 °C, poly(1-octadecene = 42 °C) due to called side chain crystallization [28].
2.2. Polymerization of 1-Tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–Borate Catalyst
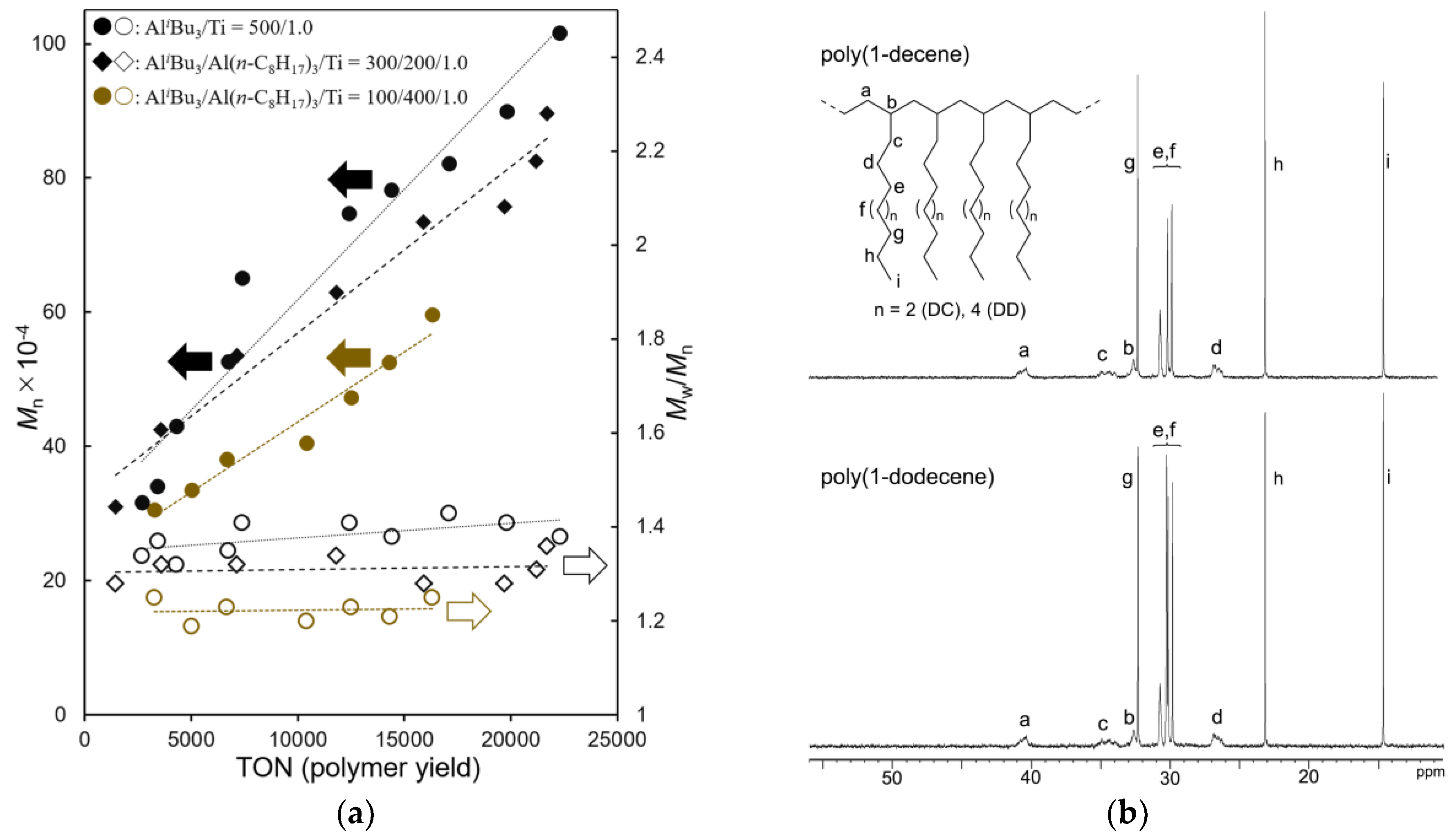
Polymerizations of 1-tetradecene (TD) polymerizations using 1–[Ph3C][B(C6F5)4] (borate) catalyst were conducted in n-hexane in the presence of Al cocatalyst with different AliBu3/Al(n-C8H17)3 molar ratios at −30 °C. In order to avoid freezing of the reaction mixture at −30 °C (due to melting temperature of TD, ca. −12 °C), rather diluted conditions compared to those for DC, DD polymerizations (TD 20 mL in n-hexane total 60 mL) were chosen. The results conducted under various AliBu3/Al(n-C8H17)3 molar ratios are summarized in Table 4. Plots of Mn, Mw/Mn vs. polymer yields (turnover numbers, TON) in the polymerization are also shown in Figure 2a.

Table 4.
Polymerization of 1-tetradecene by Cp*TiMe2(O-2,6-iPr2C6H3) (1)–borate catalyst (−30 °C). a
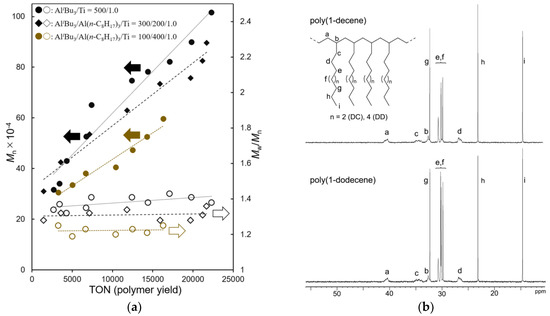
Figure 2.
(a) Plots of Mn, Mw/Mn vs. polymer yields (turnover numbers, TON) in 1-tetradecene (TD) polymerization using Cp*TiMe2(O-2,6-iPr2C6H3) (1)–borate catalyst. Detailed data are shown in Table 4. (b) 13C NMR spectrum (in CDCl3 at 25 °C) for poly(1-decene) (top, sample, run 1) and poly(1-dodecene) (bottom, sample, run 10).
It turned out that, as observed in polymerizations of DC and DD, these polymerizations proceeded without significant decreases in the catalytic activities (based on polymer yields), whereas the observed activity decreased with decreasing the AliBu3/Al(n-C8H17)3 molar ratios. The Mn values in the resultant polymers increased upon increasing the polymer yields (over time course) with consistent PDI values, and good linear relationships between the Mn values and the polymer yields were thus obtained, as shown in Figure 2a. These results clearly suggest that these polymerizations proceed in a (quasi) living manner. It also turned out that the Mn values after certain turnovers increased upon decreasing the AliBu3/Al(n-C8H17)3 molar ratios (eg. Mn = 7.83 × 105 (14,400 turnovers, run 11) vs. Mn = 7.34 × 105 (15,900 turnovers, run 12) vs. Mn = 5.26 × 105 (14,300 turnovers, run 13)) along with decreasing the PDIs. The results thus suggest an increase of percentage of catalytically active species in situ upon increasing the ratio of Al(n-C8H17)3, although we could not estimate the exact number of catalytically active species at this moment (because the Mn values were estimated on gel-permeation chromatography (GPC) trace vs. polystyrene standards). Poly(TD) with ultrahigh molecular weight (Mn = 1.02 × 106, Mw/Mn = 1.38) could be thus obtained in the polymerization using 1–borate-AliBu3 catalyst (run 11, after 2 h).
Figure 2b shows selected 13C NMR spectra (in CDCl3 at 25 °C) for poly(DC) and poly(DD). It is clear that the resultant polymers do not have stereo-regularity (atactic polymers) [49], and as observed in the spectra in poly(1-hexene) [50], resonances ascribed to 2,1- or other insertion units could not be found. The results strongly suggest that these polymerizations proceeded with (in high certainty) 1,2-insertion manner.
3. Materials and Methods
All experiments were carried out under a nitrogen atmosphere in a Vacuum Atmospheres drybox unless otherwise specified. All chemicals used were of reagent grade and were purified by the standard purification procedures. Anhydrous grade of toluene (Kanto Kagaku Co. Ltd., Tokyo, Japan) was transferred into a bottle containing molecular sieves (mixture of 3A and 4A 1/16, and 13X) in the drybox, and was used without further purification. Reagent grades 1-decene (TCI Co., Ltd., Tokyo, Japan), 1-dodecene (TCI Co., Ltd.), 1-tetradecene (TCI Co., Ltd.) were stored in bottles in the drybox and were passed through an alumina short column prior to use. Syntheses of Cp*TiMe2(O-2,6-iPr2C6H3) (1) was according to our previous report [50]. Ph3CB(C6F5)4 was purchased from Asahi Glass Co. Ltd., and was used as received in the drybox.
All 1H and 13C NMR spectra were recorded on a Bruker AV 500 spectrometer (500.13 MHz for 1H; 125.77 MHz for 13C), and all chemical shifts are given in ppm and are referred to SiMe4. 13C NMR spectra for the resultant polymers were recorded with proton decoupling, and the pulse interval was 5.2 s, the acquisition time was 0.8 s, the pulse angle was 90°, and the number of transients accumulated was about 6000. The polymer samples for analysis were prepared by dissolving the polymers in CDCl3 solution, and the spectra was measured at 25 °C. Molecular weights and the molecular weight distributions of the resultant polymers were measured by gel-permeation chromatography (GPC). HPLC grade THF was used for GPC and was degassed prior to use. GPC was performed at 40 °C on a Shimadzu SCL-10A using a RID-10A detector (Shimadzu Co. Ltd.) in THF (containing 0.03 wt. % of 2,6-di-tert-butyl-p-cresol, flow rate 1.0 mL/min). GPC columns (ShimPAC GPC-806, 804 and 802, 30 cm × 8.0 mm diameter, spherical porous gel made of styrene/divinylbenzene copolymer, ranging from <102 to 2 × 107 MW) were calibrated versus polystyrene standard samples. The molecular weight was calculated by a standard procedure based on the calibration with standard polystyrene samples.
Typical polymerization procedures were as follows: 1-decene (30 mL), n-hexane (30 mL) and a prescribed amount of AliBu3 [and Al(n-C8H17)3] was added into a 100 mL round-bottom flask connected to three-way valves under N2, the solution was then cooled to −30 °C. A toluene solution containing 1 (2.0 µmol/mL) [pre-treated with 2.0 eq. of AliBu3 at −30 °C] was added into the mixture, and the polymerization was then started by the addition of a prescribed amount of toluene solution containing Ph3CB(C6F5)4 (2.0 μmol/mL). A prescribed amount (3.0 mL) of the reaction mixture was removed via a syringe from the polymerization solution to monitor the time course, and the sample solution was then quickly poured into iPrOH (150 mL) containing HCl (10 mL). The resultant polymer was collected and was adequately washed with iPrOH and then dried in vacuo.
4. Conclusions
We have shown that polymerizations of 1-decene (DC), 1-dodecene (DD), and 1-tetradecene (TD) using Cp*TiMe2(O-2,6-iPr2C6H3) (1)–[Ph3C][B(C6F5)4] (borate) catalyst proceeded with high catalytic activities, affording (ultra)high molecular weight polymers. The polymerizations of DC and DD, in n-hexane containing a mixture of AliBu3 and Al(n-C8H17)3 at −30 °C, proceeded with high catalytic activities (4120–5860 kg-poly(DC)/mol-Ti·h) without catalyst deactivation, affording high molecular weight polymers (Mn for poly(DC) = 7.04–7.82 × 105 after 20 min). The PDI (Mw/Mn) values were affected by the ratio of Al(n-C8H17)3/AliBu3 as well as the polymerization temperature. Synthesis of high molecular weight poly(DD) with low PDI (Mn = 5.26 × 105, Mw/Mn = 1.16) could be attained at −50 °C. The TD polymerization using 1–borate–AliBu3 catalyst (conducted in n-hexane at −30 °C) also afforded ultrahigh molecular weight poly(TD) (Mn = 1.02 × 106, Mw/Mn = 1.38). The results presented here are rare demonstrations for successful synthesis of (ultra)high molecular weight bottlebrush poly(α-olefin)s with narrow molecular weight distributions by polymerization of higher α-olefins, which proceeded in a (quasi) living manner. The fact should be important for synthesis of new polyolefins as well as design of efficient molecular catalysts for olefin polymerization.
Author Contributions
Data collection and writing reports, S.P.; project support, technical teaching, and supervision, W.A.; Project administration, funding acquisition, conceptualization, supervision, and original draft preparation, K.N.
Funding
This project was partly supported by Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS, No. 18H01982).
Acknowledgments
Authors express their thanks to A. Inagaki, Ken Tsutsumi, and S. Komiya (Tokyo Metropolitan University) for fruitful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. A tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 8811–8823. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Liu, J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef] [PubMed]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. Engl. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef] [PubMed]
- Bolton, P.D.; Mountford, P. Transition metal imido compounds as Ziegler—Natta olefin polymerisation catalysts. Adv. Synth. Catal. 2005, 347, 355–366. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI Catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef]
- Delferro, M.; Marks, T.J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative chain transfer polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef]
- Gladysz, J.A. Frontiers in Metal-Catalyzed Polymerization: Designer Metallocenes, Designs on New Monomers, Demystifying MAO, Metathesis Déshabillé. Chem. Rev. 2000, 100, 1167–1168. [Google Scholar] [CrossRef]
- Milani, B.; Claver, C. (Eds.) Metal-Catalysed Polymerisation (special issue). Dalton Trans. 2009, 41, 8769–9076. [Google Scholar]
- Liu, B.; Terano, M.; Busico, V.; Wong, W.-Y.; Tang, Y. (Eds.) Metal-Catalyzed Polymerization of Olefins (special issue). J. Organomet. Chem. 2015, 798, 291–436. [Google Scholar]
- Osakada, K. (Ed.) Organometallic Reactions and Polymerization; Lecture Notes in Chemistry 85; Springer: Berlin, Germany, 2014. [Google Scholar]
- Hoff, R. (Ed.) Handbook of Transition Metal Polymerization Catalysts, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Choi, H.J.; Jhon, M.S. Polymer-induced turbulent drag reduction. Ind. Eng. Chem. Res. 1996, 35, 2993–2998. [Google Scholar] [CrossRef]
- Ivchenko, P.V.; Nifant’ev, I.E.; Tavtorkin, A.V. Polyolefin drag reducing agents. Petrol. Chem. 2016, 56, 775–787. [Google Scholar] [CrossRef]
- Brostow, W. Drag reduction in flow: Review of applications, mechanism and prediction. J. Ind. Eng. Chem. 2008, 14, 409–416. [Google Scholar] [CrossRef]
- Cuenca, F.G.; Marín, M.G.; Folgueras Díaz, M.B. Energy-savings modeling of oil pipelines that use drag-reducing additives. Energy Fuels 2008, 22, 3293–3298. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Tsou, A.H.; Younker, J.M.; Norman, A.I.; Schaefer, J.J.; Hagadorn, J.R.; Throckmorton, J.A. Microstructure of crystallizable α-olefin molecular bottlebrushes: Isotactic and atactic poly(1-octadecene). Macromolecules 2018, 51, 872–883. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Tsou, A.H.; Hagadorn, J.R.; Throckmorton, J.A. Highly entangled α-olefin molecular bottlebrushes: Melt structure, linear rheology, and interchain friction mechanism. Macromolecules 2018, 51, 6958–6966. [Google Scholar] [CrossRef]
- Paturej, J.; Sheiko, S.S.; Panyukov, S.; Rubinstein, M. Molecular structure of bottlebrush polymers in melts. Sci. Adv. 2016, 2, e1601478. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Wang, Z.; Sheiko, S.S.; Dobrynin, A.V. Combs and Bottlebrushes in a Melt. Macromolecules 2017, 50, 3430–3437. [Google Scholar] [CrossRef]
- Grumel, V.; Brüll, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.; Wahner, U.M. Homopolymerization of higher α-olefins with metallocene/MAO catalysts. Macromol. Mater. Eng. 2001, 286, 480–487. [Google Scholar] [CrossRef]
- Saito, J.; Suzuki, Y.; Fujita, T. Higher α-olefin polymerization behavior of a bis(phenoxy-imine)titanium complex/i-Bu3Al/Ph3CB(C6F5)4 catalyst system. Chem. Lett. 2003, 32, 236–237. [Google Scholar] [CrossRef]
- Nomura, K.; Pengoubol, S.; Apisuk, W. Synthesis of ultrahigh molecular weight polymers by homopolymerisation of higher a-olefins catalysed by aryloxo-modified half-titanocenes. RSC Adv. 2016, 6, 16203–16207. [Google Scholar] [CrossRef]
- Fries, A.; Mise, T.; Matsumoto, A.; Ohmori, H.; Wakatsuki, Y. Polymerization of hex-1-ene by homogeneous zirconocene and hafnocene catalysts in compressed solution. Chem. Commun. 1996, 783–784. [Google Scholar] [CrossRef] Synthesis of ultrahigh molecular weight poly(1-hexene) by (C5HMe4)2HfCl2–MAO catalyst under ultrahigh pressure (ex. 250 MPa).
- Segal, S.; Goldberg, I.; Kol, M. Zirconium and titanium diamine bis(phenolate) catalysts for α-olefin polymerization: From atactic oligo(1-hexene) to ultrahigh-molecular-weight isotactic poly(1-hexene). Organometallics 2005, 24, 200–202. [Google Scholar] [CrossRef]
- Fujita, M.; Seki, Y.; Miyatake, T. Enhancement of productivity, molecular weight and stereoregularity of 1-butene polymerization by MAO modification. Macromol. Chem. Phys. 2004, 205, 884–887. [Google Scholar] [CrossRef]
- Fujita, M.; Seki, Y.; Miyatake, T. Synthesis of ultra-high-molecular-weight poly(α-olefin)s by thiobis(phenoxy)titanium/modified methylaluminoxane system. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1107–1111. [Google Scholar] [CrossRef]
- Nomura, K.; Fudo, A. Efficient living polymerization of 1-hexene by Cp*TiMe2(O-2,6-iPr2C6H3)-borate catalyst systems at low temperature. J. Mol. Catal. A Chem. 2004, 209, 9–17. [Google Scholar] [CrossRef]
- Jayaratne, K.C.; Sita, L.R. Stereospecific living Ziegler-Natta polymerization of 1-hexene. J. Am. Chem. Soc. 2000, 122, 958–959. [Google Scholar] [CrossRef]
- Domski, G.J.; Lobkovsky, E.B.; Coates, G.W. Polymerization of α-olefins with pridylamidohafnium ctalysts: Living bhavior and uexpected ioselectivity from a Cs-smmetric ctalyst pecursor. Macromolecules 2007, 40, 3510–3513. [Google Scholar] [CrossRef]
- Cai, Z.; Ohmagari, M.; Nakayama, Y.; Shiono, T. Highly active syndiospecific living polymerization of higher 1-alkene with ansa-fluorenylamidodimethyltitanium complex. Macromol. Rapid Commun. 2009, 30, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Kotzabasakis, V.; Mourmouris, S.; Pitsikalis, M.; Hadjichristidis, N.; Lohse, D.J. Synthesis and characterization of complex macromolecular architectures based on poly(α-olefins) utilizing a Cs-symmetry hafnium metallocene catalyst in combination with atom transfer radical polymerization (ATRP). Macromolecules 2011, 44, 1952–1968. [Google Scholar] [CrossRef]
- Kiesewetter, E.T.; Waymouth, R.M. Octahedral group IV bis(phenolate) catalysts for 1-hexene homopolymerization and ethylene/1-hexene copolymerization. Macromolecules 2013, 46, 2569–2575. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled isospecific polymerization of α-olefins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis(phenolate) ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, S.; Zhang, W.; Chen, C. Systematic investigations of ligand steric effects on α-diimine palladium catalyzed olefinpolymerization and copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Nomura, K.; Fujita, K.; Fujiki, M. Effects of cyclopentadienyl fragment in ethylene, 1-hexene, and styrene polymerizations catalyzed by half-titanocenes containing ketimide ligand of the type, Cp’TiCl2(N=CtBu2). Catal. Commun. 2004, 5, 413–417. [Google Scholar] [CrossRef]
- Nomura, K.; Komatsu, T.; Imanishi, Y. Ligand effect in olefin polymerization catalyzed by (cyclopentadienyl)(aryloxy)titanium(IV) complexes, Cp’TiCl2(OAr)–MAO system. Ethylene/1-hexene copolymerization by (1,3-tBu2C5H3)TiCl2(O-2,6-iPr2C6H3)–MAO catalyst system. J. Mol. Catal. A Chem. 2000, 159, 127–137. [Google Scholar] [CrossRef]
- Nomura, K.; Fudo, A. Syndiospecific styrene polymerization by (tert-BuC5H4)TiCl2(O-2,6-iPr2C6H3)–borate catalyst system. Catal. Commun. 2003, 4, 269–274. [Google Scholar] [CrossRef]
- Nomura, K.; Suzuki, N.; Kim, D.-H.; Kim, H.J. Effect of cocatalyst in ethylene/styrene copolymerization by aryloxo-modified half-titanocene–cocatalyst systems for exclusive synthesis of copolymers at high styrene concentrations. Macromol. React. Eng. 2012, 6, 351–356. [Google Scholar] [CrossRef]
- Nomura, K.; Pracha, S.; Phomphrai, K.; Katao, S.; Kim, D.-H.; Kim, H.J.; Suzuki, N. Synthesis and structural analysis of phenoxy-substituted half-titanocenes with different anionic ligands, Cp*TiX(Y)(O-2,6-iPr2C6H3): Effect of anionic ligands (X,Y) in ethylene/styrene copolymerization. J. Mol. Catal. A Chem. 2012, 365, 136–145. [Google Scholar] [CrossRef]
- Hagihara, H.; Shiono, T.; Ikeda, T. Living polymerization of propene and 1-hexene with the [t-BuNSiMe2Flu]TiMe2/B(C6F5)3 catalyst. Macromolecules 1998, 31, 3184–3188. [Google Scholar] [CrossRef]They proposed that Al(n-C8H17)3 interacts with the counteranion of the cationic active Ti species to improve coordinative unsaturation of the Ti species.
- Fukui, Y.; Murata, M.; Soga, K. Living polymerization of propylene and 1-hexene using bis-Cp type metallocene catalysts. Macromol. Rapid Commun. 1999, 20, 637–640. [Google Scholar] [CrossRef]
- Beckerle, K.; Manivannan, R.; Spaniol, T.P.; Okuda, J. Living isospecific styrene polymerization by chiral benzyl titanium complexes that contain a tetradentate [OSSO]-type bis(phenolato) ligand. Organometallics 2006, 25, 3019–3026. [Google Scholar] [CrossRef]
- Asakura, T.; Demura, M.; Nishiyama, Y. Carbon-13 NMR spectral assignment of five polyolefins determined from the chemical shift calculation and the polymerization mechanism. Macromolecules 1991, 24, 2334–2340. [Google Scholar] [CrossRef]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Olefin polymerization by (cyclopentadienyl)(aryloxy)- titanium(IV) complexes-cocatalyst systems. Macromolecules 1998, 31, 7588–7597. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).