Nelumbo nucifera Receptaculum Extract Suppresses Angiotensin II-Induced Cardiomyocyte Hypertrophy
Abstract
1. Introduction
2. Results
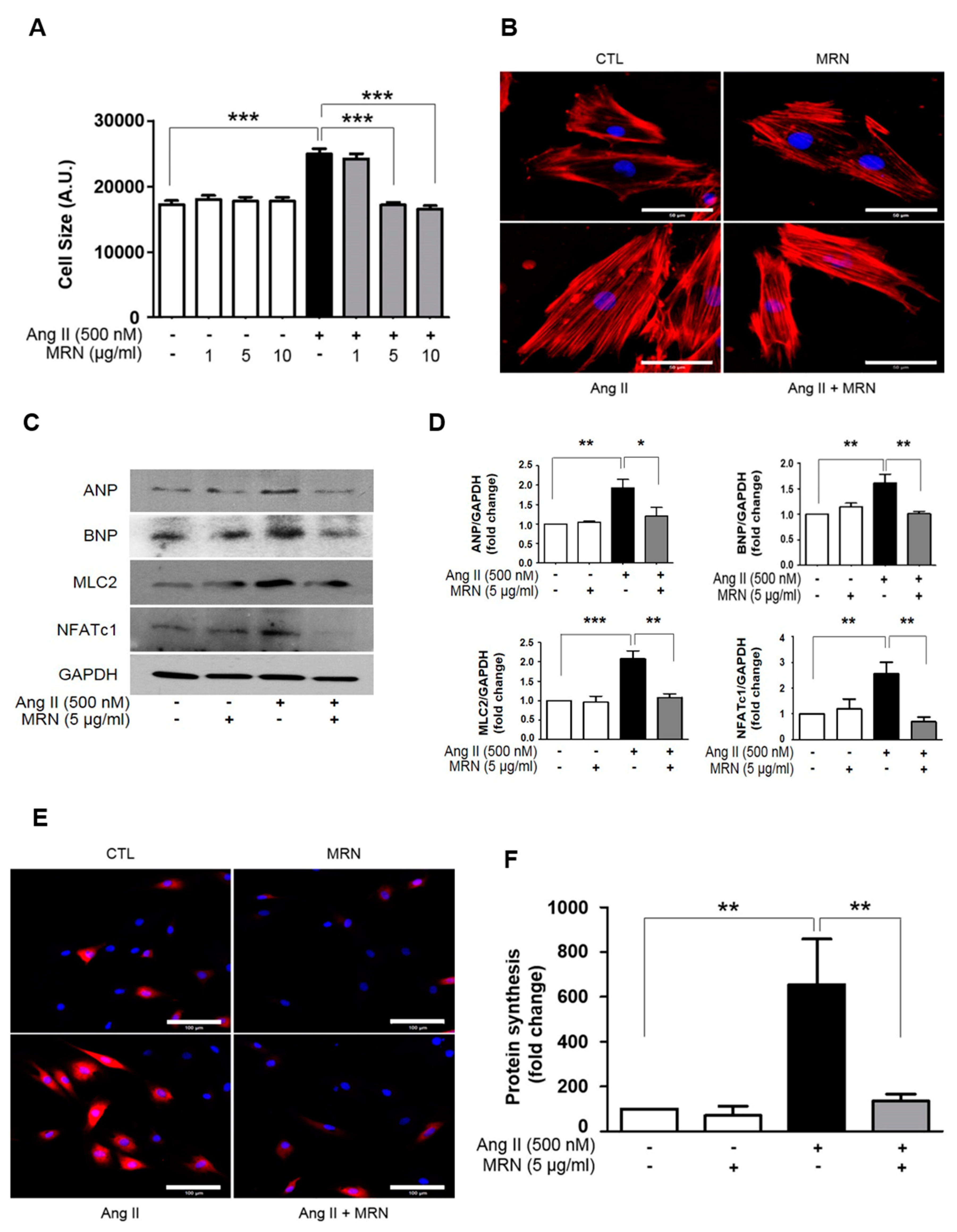
2.1. MRN Attenuated Ang II-Induced Cardiomyocyte Hypertrophy
2.2. MRN Inhibited Intracellular ROS Levels in Ang II-Induced Cardiomyocyte Hypertrophy
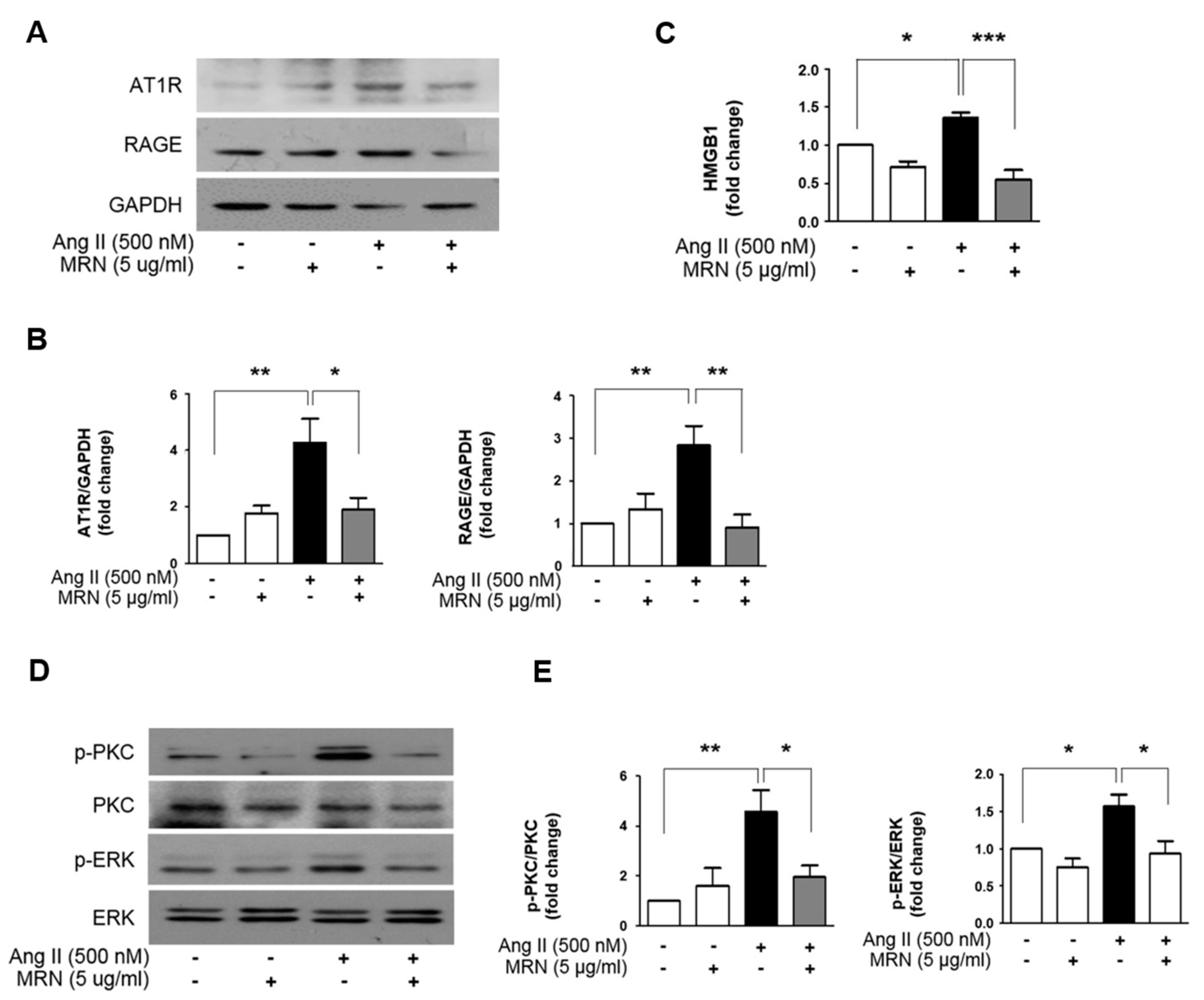
2.3. MRN Modulates Cardiomyocyte Hypertrophy through Regulation of PKC—ERK Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Receptaculum Extracts
4.3. Cell Culture and Treatment
4.4. Measurement of Cell Viability and LDH Assay
4.5. Cell Size Measurement
4.6. Measurement of Total Protein Synthesis
4.7. ROS Detection Assay
4.8. Immunoblotting Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drazner, M.H.; Rame, J.E.; Marino, E.K.; Gottdiener, J.S.; Kitzman, D.W.; Gardin, J.M.; Manolio, T.A.; Dries, D.L.; Siscovick, D.S. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J. Am. Coll Cardiol. 2004, 43, 2207–2215. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef]
- McMullen, J.R.; Jennings, G.L. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin. Exp. Pharmacol Physiol 2007, 34, 255–262. [Google Scholar] [CrossRef]
- Crowley, S.D.; Gurley, S.B.; Herrera, M.J.; Ruiz, P.; Griffiths, R.; Kumar, A.P.; Kim, H.S.; Smithies, O.; Le, T.H.; Coffman, T.M. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. USA 2006, 103, 17985–17990. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Sandberg, K. Ontogeny of angiotensin II receptors. Cell Biol. Int. 1996, 20, 169–176. [Google Scholar] [CrossRef]
- Mazzolai, L.; Pedrazzini, T.; Nicoud, F.; Gabbiani, G.; Brunner, H.R.; Nussberger, J. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension 2000, 35, 985–991. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kojima, M.; Shiojima, I.; Yamazaki, T.; Komuro, I.; Zou, Z.; Wang, Y.; Mizuno, T.; Ueki, K.; Tobe, K.; Kadowaki, T.; et al. Angiotensin II receptor antagonist TCV-116 induces regression of hypertensive left ventricular hypertrophy in vivo and inhibits the intracellular signaling pathway of stretch-mediated cardiomyocyte hypertrophy in vitro. Circulation 1994, 89, 2204–2211. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, M.; Skarda, V.; Leenen, F.H. Effects of ACE inhibitors on circulating versus cardiac angiotensin II in volume overload-induced cardiac hypertrophy in rats. Circulation 1995, 92, 3568–3573. [Google Scholar] [CrossRef] [PubMed]
- Hefti, M.A.; Harder, B.A.; Eppenberger, H.M.; Schaub, M.C. Signaling pathways in cardiac myocyte hypertrophy. J. Mol. Cell Cardiol 1997, 29, 2873–2892. [Google Scholar] [CrossRef] [PubMed]
- Bogoyevitch, M.A.; Andersson, M.B.; Gillespie-Brown, J.; Clerk, A.; Glennon, P.E.; Fuller, S.J.; Sugden, P.H. Adrenergic receptor stimulation of the mitogen-activated protein kinase cascade and cardiac hypertrophy. Biochem. J. 1996, 314, 115–121. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid. Redox Signal. 2013, 19, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Hingtgen, S.D.; Tian, X.; Yang, J.; Dunlay, S.M.; Peek, A.S.; Wu, Y.; Sharma, R.V.; Engelhardt, J.F.; Davisson, R.L. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol. Genomics 2006, 26, 180–191. [Google Scholar] [CrossRef]
- Wu, Y.B.; Zheng, L.J.; Wu, J.G.; Chen, T.Q.; Yi, J.; Wu, J.Z. Antioxidant activities of extract and fractions from receptaculum nelumbinis and related flavonol glycosides. Int J. Mol. Sci 2012, 13, 7163–7173. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Panth, N. Phytochemical Profile and Biological Activity of Nelumbo nucifera. Evid. Based. Complement. Alternat. Med. 2015, 2015, 789124. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, X.; Wang, C.; Peng, D.; Zhu, K.; Song, J.L. Anticancer activity of Nelumbo nucifera stamen extract in human colon cancer HCT-116 cells in vitro. Oncol. Lett 2017, 13, 1470–1478. [Google Scholar] [CrossRef]
- Qian, J.Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol Sin. 2002, 23, 1086–1092. [Google Scholar] [PubMed]
- Sohn, D.H.; Kim, Y.C.; Oh, S.H.; Park, E.J.; Li, X.; Lee, B.H. Hepatoprotective and free radical scavenging effects of Nelumbo nucifera. Phytomedicine 2003, 10, 165–169. [Google Scholar] [CrossRef]
- Fukui, T.; Ishizaka, N.; Rajagopalan, S.; Laursen, J.B.; Capers, Q.t.; Taylor, W.R.; Harrison, D.G.; de Leon, H.; Wilcox, J.N.; Griendling, K.K. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ. Res. 1997, 80, 45–51. [Google Scholar] [CrossRef]
- Purcell, N.H.; Tang, G.; Yu, C.; Mercurio, F.; DiDonato, J.A.; Lin, A. Activation of NF-kappa B is required for hypertrophic growth of primary rat neonatal ventricular cardiomyocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 6668–6673. [Google Scholar] [CrossRef]
- Freund, C.; Schmidt-Ullrich, R.; Baurand, A.; Dunger, S.; Schneider, W.; Loser, P.; El-Jamali, A.; Dietz, R.; Scheidereit, C.; Bergmann, M.W. Requirement of nuclear factor-kappaB in angiotensin II- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation 2005, 111, 2319–2325. [Google Scholar] [CrossRef]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010, 396, 901–907. [Google Scholar] [CrossRef]
- Lim, S.; Lee, M.E.; Jeong, J.; Lee, J.; Cho, S.; Seo, M.; Park, S. sRAGE attenuates angiotensin II-induced cardiomyocyte hypertrophy by inhibiting RAGE-NFkappaB-NLRP3 activation. Inflamm. Res. 2018, 67, 691–701. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol Sin. 2016, 32, 631–639. [Google Scholar]
- Triggle, C.R.; Ding, H. Cardiovascular impact of drugs used in the treatment of diabetes. Ther. Adv. Chronic Dis. 2014, 5, 245–268. [Google Scholar] [CrossRef]
- Montezano, A.C.; Nguyen Dinh Cat, A.; Rios, F.J.; Touyz, R.M. Angiotensin II and vascular injury. Curr. Hypertens. Rep. 2014, 16, 431. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Montezano, A.C.; Burger, D.; Touyz, R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013, 19, 1110–1120. [Google Scholar] [CrossRef]
- Santillo, M.; Colantuoni, A.; Mondola, P.; Guida, B.; Damiano, S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol 2015, 6, 194. [Google Scholar] [CrossRef]
- Zhang, M.; Prosser, B.L.; Bamboye, M.A.; Gondim, A.N.S.; Santos, C.X.; Martin, D.; Ghigo, A.; Perino, A.; Brewer, A.C.; Ward, C.W.; et al. Contractile Function During Angiotensin-II Activation: Increased Nox2 Activity Modulates Cardiac Calcium Handling via Phospholamban Phosphorylation. J. Am. Coll Cardiol 2015, 66, 261–272. [Google Scholar] [CrossRef]
- Polizio, A.H.; Balestrasse, K.B.; Yannarelli, G.G.; Noriega, G.O.; Gorzalczany, S.; Taira, C.; Tomaro, M.L. Angiotensin II regulates cardiac hypertrophy via oxidative stress but not antioxidant enzyme activities in experimental renovascular hypertension. Hypertens Res. 2008, 31, 325–334. [Google Scholar] [CrossRef][Green Version]
- Kikuchi, K.; Tancharoen, S.; Ito, T.; Morimoto-Yamashita, Y.; Miura, N.; Kawahara, K.; Maruyama, I.; Murai, Y.; Tanaka, E. Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke. Int. J. Mol. Sci. 2013, 14, 18899–18924. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z. Electrophysiological effects of neferine against ischemic ventricular tachyarrhythmias. Zhonghua Xin Xue Guan Bing Za Zhi 1992, 20, 119–122, 134. [Google Scholar] [PubMed]
- Kim, A.R.; Jeong, S.M.; Kang, M.J.; Jang, Y.H.; Choi, H.N.; Kim, J.I. Lotus leaf alleviates hyperglycemia and dyslipidemia in animal model of diabetes mellitus. Nutr Res. Pract 2013, 7, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Jung, W.S.; An, B.G.; Cho, J.H.; Jung, S.Y. Isolation of Compounds having Inhibitory Activity toward Tyrosinase from Receptaculum Nelumbinis. Kor. J. Pharmacogn 2013, 44, 1–5. [Google Scholar]
- Gupta, R.; Sharma, A.K.; Dobhal, M.P.; Sharma, M.C.; Gupta, R.S. Antidiabetic and antioxidant potential of beta-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes 2011, 3, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kang, H.J.; Kim, S.Z.; Kwon, T.O.; Jeong, S.I.; Jang, S.I. Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. Arch. Pharm Res. 2013, 36, 912–917. [Google Scholar] [CrossRef]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. Beta-sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Cho, H.W.; Woo, K.W.; Jeong, J.; Lim, J.; Park, S.; Seo, M.; Lim, S. Nelumbo nucifera Receptaculum Extract Suppresses Angiotensin II-Induced Cardiomyocyte Hypertrophy. Molecules 2019, 24, 1647. https://doi.org/10.3390/molecules24091647
Cho S, Cho HW, Woo KW, Jeong J, Lim J, Park S, Seo M, Lim S. Nelumbo nucifera Receptaculum Extract Suppresses Angiotensin II-Induced Cardiomyocyte Hypertrophy. Molecules. 2019; 24(9):1647. https://doi.org/10.3390/molecules24091647
Chicago/Turabian StyleCho, Soyoung, Hyun Woo Cho, Kyeong Wan Woo, Jisu Jeong, Juyeon Lim, Sungha Park, Miran Seo, and Soyeon Lim. 2019. "Nelumbo nucifera Receptaculum Extract Suppresses Angiotensin II-Induced Cardiomyocyte Hypertrophy" Molecules 24, no. 9: 1647. https://doi.org/10.3390/molecules24091647
APA StyleCho, S., Cho, H. W., Woo, K. W., Jeong, J., Lim, J., Park, S., Seo, M., & Lim, S. (2019). Nelumbo nucifera Receptaculum Extract Suppresses Angiotensin II-Induced Cardiomyocyte Hypertrophy. Molecules, 24(9), 1647. https://doi.org/10.3390/molecules24091647




