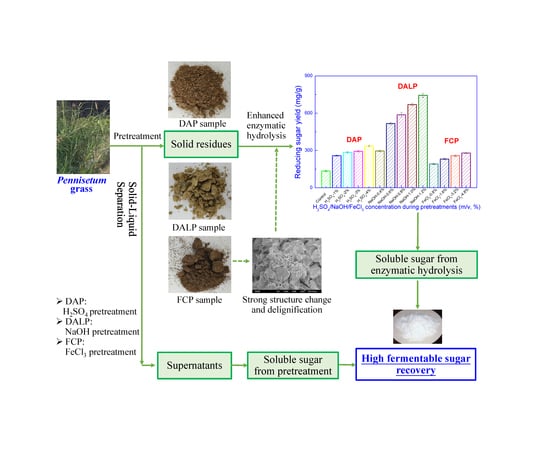
Enhanced Enzymatic Hydrolysis of Pennisetum alopecuroides by Dilute Acid, Alkaline and Ferric Chloride Pretreatments
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Different Pretreatments on Biomass Composition
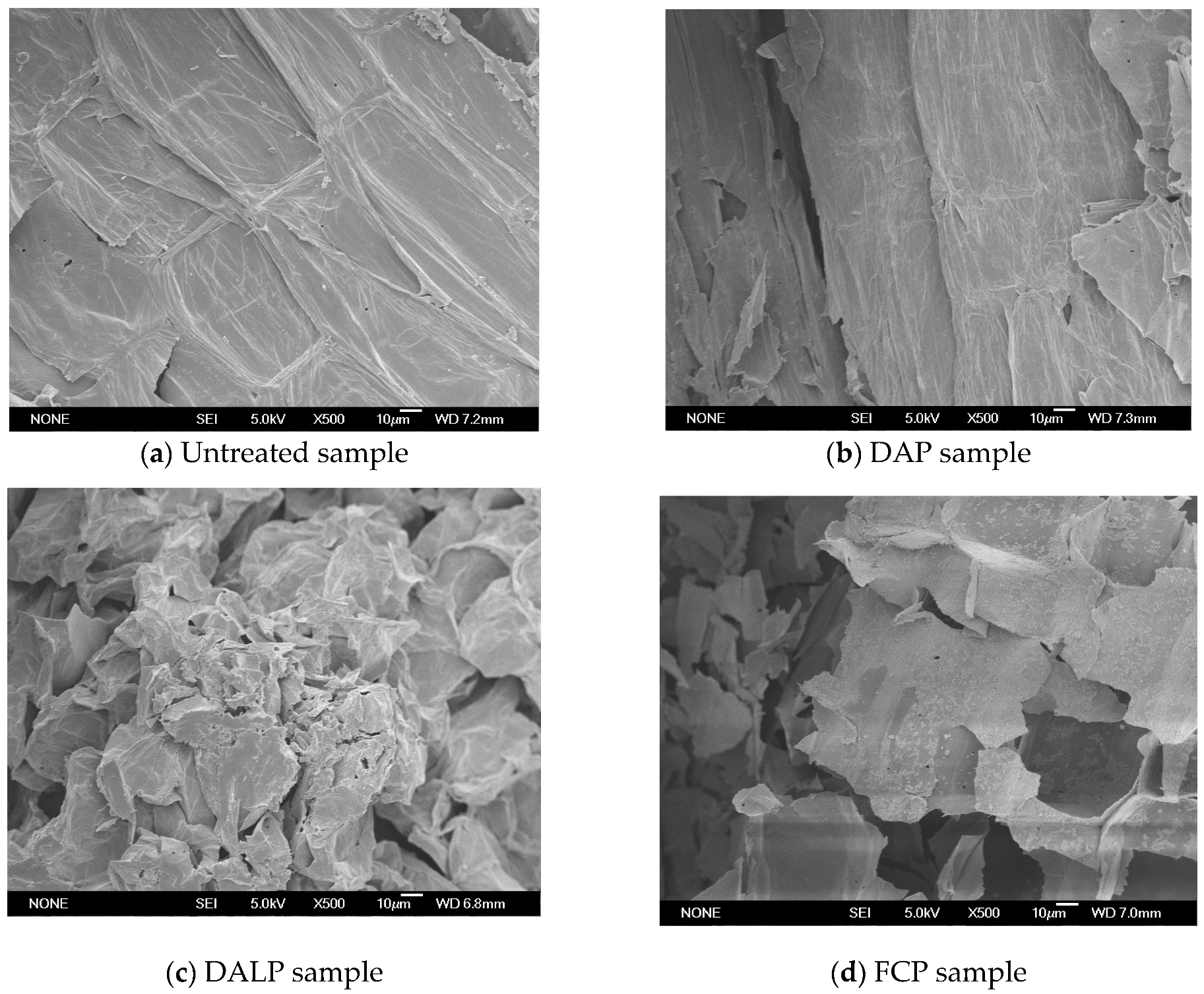
2.2. Effect of Different Pretreatments on Cell Structure
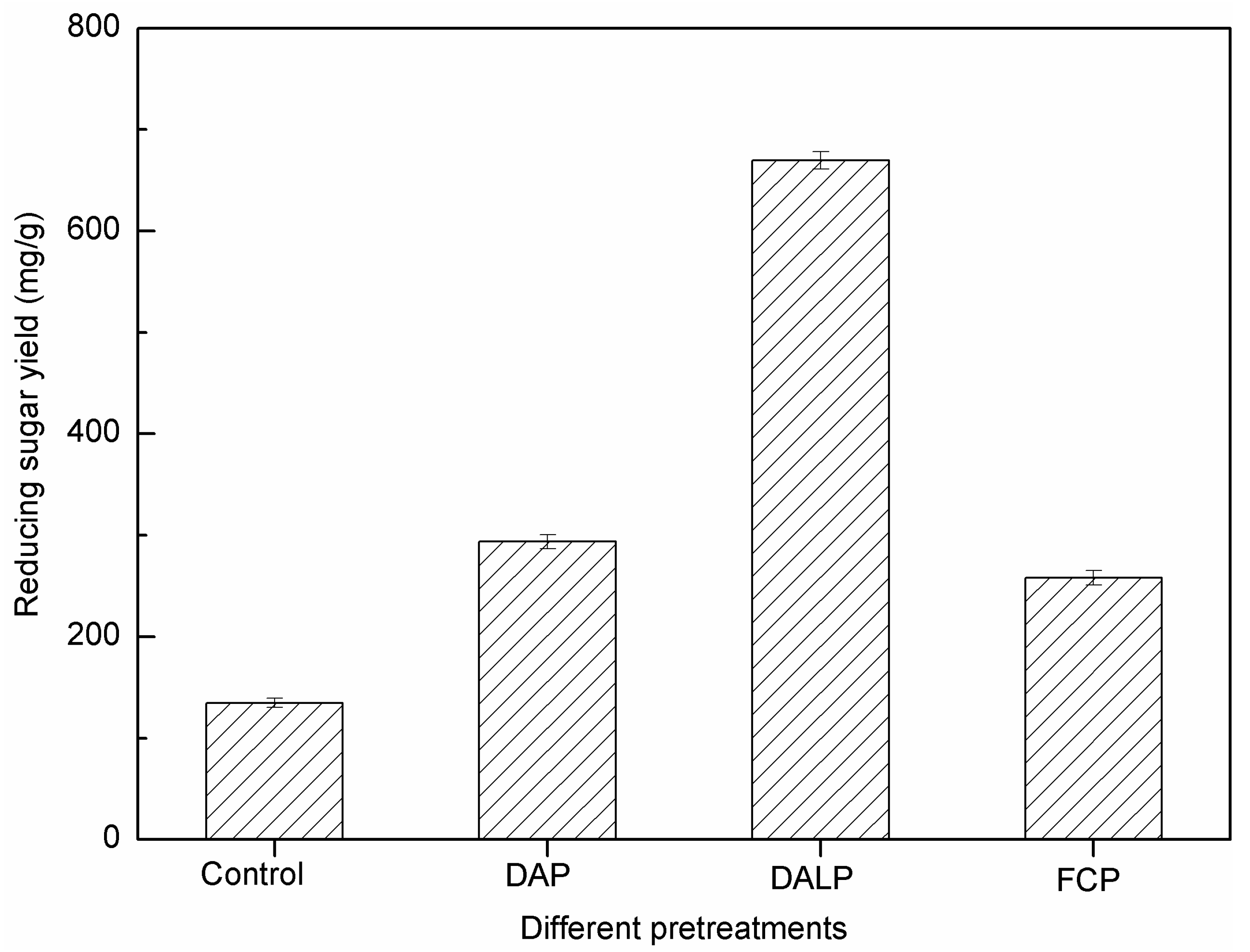
2.3. Effect of Different Pretreatments on Enzymatic Hydrolysis
2.4. Effect of H2SO4/NaOH/FeCl3 Concentrations on Biomass Composition
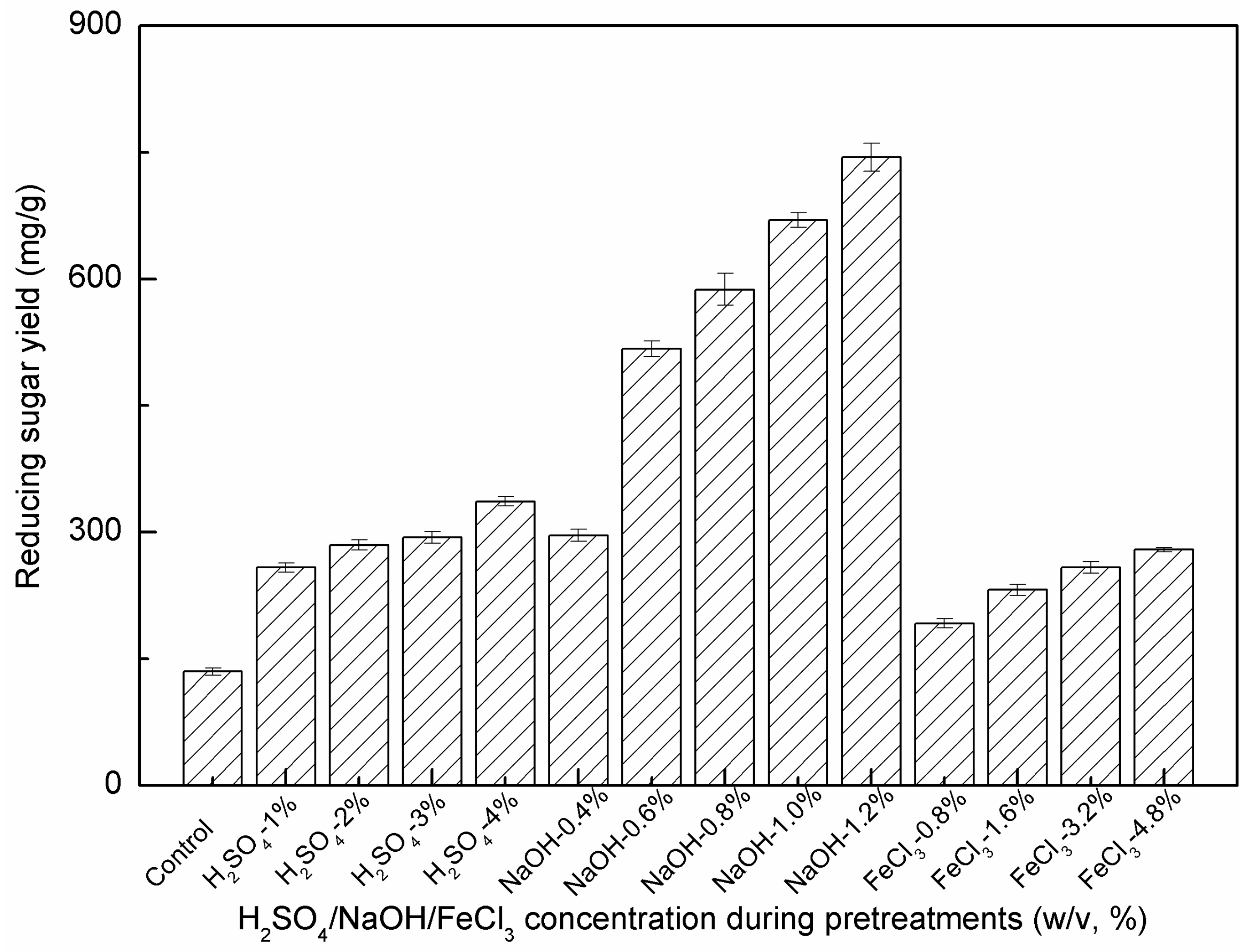
2.5. Effect of H2SO4/NaOH/FeCl3 Concentrations on Enzymatic Hydrolysis
2.6. Analysis of Mass Balance and Prospects for P. alopecuroides
3. Materials and Methods
3.1. Materials
3.2. Pretreatment Process
3.3. Enzymatic Hydrolysis
3.4. Scanning Electron Microscopy (SEM) Observation
3.5. Analytical Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caruso, M.C.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent updates on the use of agro-food waste for biogas production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef]
- You, Z.; Zhang, S.; Kim, H.; Chiang, P.C.; Sun, Y.; Guo, Z.; Xu, H. Effects of corn stover pretreated with NaOH and CaO on anaerobic co-digestion of swine manure and corn stover. Appl. Sci. 2019, 9, 123. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Zhong, C. Effects of feed to inoculum ratio, co-digestion and pretreatment on biogas production from anaerobic digestion of cotton stalk. Energy Fuels 2014, 28, 3157–3166. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, C.Z. Enhanced coproduction of hydrogen and methane from cornstalks by a three-stage anaerobic fermentation process integrated with alkaline hydrolysis. Bioresour. Technol. 2012, 104, 373–379. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Li, Q.; Liu, C.Z. Coproduction of hydrogen and methane via anaerobic fermentation of cornstalk waste in continuous stirred tank reactor integrated with up-flow anaerobic sludge bed. Bioresour. Technol. 2012, 114, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.H.; Sun, Y.M.; Li, L.H.; Kong, X.Y.; Yuan, Z.H. Improving methane production from anaerobic digestion of Pennisetum Hybrid by alkaline pretreatment. Bioresour. Technol. 2018, 255, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Camesasca, L.; Ramı’rez, M.B.; Guigou, M.; Ferrari, M.D.; Lareo, C. Evaluation of dilute acid and alkaline pretreatments, enzymatic hydrolysis and fermentation of napiergrass for fuel ethanol production. Biomass Bioenergy 2015, 74, 193–201. [Google Scholar] [CrossRef]
- Wang, S.D.; Chen, J.H.; Yang, G.H.; Gao, W.H.; Chen, K.F. Efficient conversion of Hybrid Pennisetum to glucose by oxygen-aqueous alkaline ionic liquid media pretreatment under benign conditions. Bioresour. Technol. 2017, 243, 335–338. [Google Scholar] [CrossRef]
- Tsai, M.H.; Lee, W.C.; Kuan, W.C.; Sirisansaneeyakul, S.; Savarajara, A. Evaluation of different pretreatments of Napier grass for enzymatic saccharification and ethanol production. Energy Sci. Eng. 2018, 6, 683–692. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Jeffries, T.W. Effectiveness of dilute oxalic acid pretreatment of Miscanthus×giganteus biomass for ethanol production. Biomass Bioenergy 2013, 59, 540–548. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy. Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Bensah, E.C.; Mensah, M. Chemical pretreatment methods for the production of cellulosic ethanol: Technologies and innovations. Int. J. Chem. Eng. 2013, 2013, 1–21. [Google Scholar] [CrossRef]
- Latika, B.; Sonia, J.; Rumana, A. An economic and ecological perspective of ethanol production from renewable agro waste: A review. AMB Express 2012, 2, 65. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Holowacz, I.; Lukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Fu, S.F.; Chen, K.Q.; Zhu, R.; Sun, W.X.; Zou, H.; Guo, R.B. Improved anaerobic digestion performance of Miscanthus floridulus by different pretreatment methods and preliminary economic analysis. Energ. Convers. Manag. 2018, 159, 120–128. [Google Scholar] [CrossRef]
- Eliana, C.; Jorge, R.; Juan, P.; Luis, R. Effects of the pretreatment method on enzymatic hydrolysis and ethanol fermentability of the cellulosic fraction from elephant grass. Fuel 2014, 118, 41–47. [Google Scholar] [CrossRef]
- Sabanci, K.; Buyukkileci, A.Q. Comparison of liquid hot water, very dilute acid and alkali treatments for enhancing enzymatic digestibility of hazelnut tree pruning residues. Bioresour. Technol. 2018, 261, 158–165. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Negi, S. Impact of surfactant assisted acid and alkali pretreatment on lignocellulosic structure of pine foliage and optimization of its saccharification parameters using response surface methodology. Bioresour. Technol. 2015, 192, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Li, K.N.; Wan, J.M.; Wang, X.; Wang, J.F.; Zhang, J.H. Comparison of dilute acid and alkali pretreatments in production of fermentable sugars from bamboo: Effect of Tween 80. Ind. Crops Products 2016, 83, 414–422. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, C.Z. Enhanced biogas production from herbal-extraction process residues by microwave-assisted alkaline pretreatment. J. Chem. Technol. Biotechnol. 2010, 85, 127–131. [Google Scholar] [CrossRef]
- Liu, C.Z.; Cheng, X.Y. Improved hydrogen production via thermophilic fermentation of corn stover by microwave-assisted acid pretreatment. Int. J. Hydrogen Energy 2010, 35, 8945–8952. [Google Scholar] [CrossRef]
- Menegol, D.; Schol, A.L.; Dillon, A.J.; Camassola, M. Influence of different chemical pretreatments of elephant grass (Pennisetum purpureum, Schum.) used as a substrate for cellulase and xylanase production in submerged cultivation. Bioprocess Biosyst. Eng. 2016, 39, 1455–1464. [Google Scholar] [CrossRef]
- Kamireddy, S.R.; Li, J.B.; Tucker, M.; Degenstein, J.; Ji, Y. Effects and mechanism of metal chloride salts on pretreatment and enzymatic digestibility of corn stover. Ind. Eng. Chem. Res. 2013, 52, 1775–1782. [Google Scholar] [CrossRef]
- Kang, K.E.; Park, D.H.; Jeong, G.T. Effects of inorganic salts on pretreatment of Miscanthus straw. Bioresour. Technol. 2013, 132, 160–165. [Google Scholar] [CrossRef]
- Zhang, H.D.; Lyu, G.J.; Zhang, A.P.; Li, X.; Xie, J. Effects of ferric chloride pretreatment and surfactants on the sugar production from sugarcane bagasse. Bioresour. Technol. 2018, 265, 93–101. [Google Scholar] [CrossRef]
- Meng, X.; Foston, M.; Leisen, J.; Demartini, J.; Wyman, C.E.; Ragauskas, A.J. Determination of porosity of lignocellulosic biomass before and after pretreatment by using Simons’stain and NMR techniques. Bioresour. Technol. 2013, 144, 467–476. [Google Scholar] [CrossRef]
- Brienzo, M.; Fikizolo, S.; Benjamin, Y.; Tyhoda, L.; Görgens, J. Influence of pretreatment severity on structural changes, lignin content and enzymatic hydrolysis of sugarcane bagasse samples. Renew. Energy 2017, 104, 271–280. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhang, C.Y.; Tong, S.S.; Cui, Z.Y.; Liu, P. Enhanced enzymatic hydrolysis and structural features of corn stover by NaOH and ozone combined pretreatment. Molecules 2018, 23, 1300. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Wang, C.; Sun, S.; Sun, R. Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: Chemical and anatomical changes. Biotechnol. Biofuels 2016, 9, 166. [Google Scholar] [CrossRef]
- Yan, Y.H.; Zhang, C.H.; Lin, Q.X.; Wang, X.H.; Cheng, B.G.; Li, H.L.; Ren, J.L. Microwave-assisted oxalic acid pretreatment for the enhancing of enzyme hydrolysis in the production of xylose and arabinose from bagasse. Molecules 2018, 23, 862. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, G.; Zhang, P.; Li, F.; Wang, S.; Fan, S.; Zhou, S. Microwave assisted alkaline pretreatment to enhance enzymatic saccharification of catalpa sawdust. Bioresour. Technol. 2016, 221, 26–30. [Google Scholar] [CrossRef]
- Wang, Z.N.; Hou, X.F.; Sun, J.; Li, M.; Chen, Z.Y.; Gao, Z.Z. Comparison of ultrasound-assisted ionic liquid and alkaline pretreatment of Eucalyptus for enhancing enzymatic saccharification. Bioresour. Technol. 2018, 254, 145–150. [Google Scholar] [CrossRef]
- Shimizu, F.L.; Monteiro, P.Q.; Ghiraldi, P.H.C.; Melati, R.B.; Pagnocca, F.C.; Souza, W.; Sant’Anna, C.; Brienzo, M. Acid, alkali and peroxide pretreatments increase the cellulose accessibility and glucose yield of banana pseudostem. Ind. Crops Prod. 2018, 115, 62–68. [Google Scholar] [CrossRef]
- Thomas, H.L.; Seira, J.; Escudi, R.; Carrère, H. Lime pretreatment of miscanthus: Impact on bmp and batch dry co-digestion with cattle manure. Molecules 2018, 23, 1608. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; González, A.; Ballesteros, M. Study of the application of alkaline extrusion to the pretreatment of Eucalyptus biomass as first step in a bioethanol production process. Energies 2018, 11, 2961. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzyme. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzyme. Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Michelin, M.; Ximenes, E.; de Lourdes Teixeira de Moraes Polizeli, M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures: A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef]
- Imman, S.; Arnthong, J.; Burapatana, V.; Champred, V.; Laosiripojana, N. Effects of acid and alkali promoters on compressed liquid hot water pretreatment of rice straw. Bioresour. Technol. 2014, 171, 29–36. [Google Scholar] [CrossRef]
- Rivera, O.M.P.; Moldes, A.B.; Torrado, A.M.; Domínguez, J.M. Lactic acid and biosurfactants production from hydrolyzed distilled grape marc. Process Biochem. 2007, 42, 1010–1020. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 2011, 35, 3094–3103. [Google Scholar] [CrossRef]
- Meng, X.Z.; Wells, T.; Sun, Q.N.; Huang, F.; Ragauskas, A. Insights into the effect of dilute acid, hot water or alkaline pretreatment on the cellulose accessible surface area and the overall porosity of Populus. Green Chem. 2015, 17, 4239–4246. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Fermentable sugar production from the peels of two durian (Durio zibethinus Murr.) cultivars by phosphoric acid pretreatment. Resources 2018, 7, 60. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Sakka, K.; Ratanakhanokchai, K. Structural changes and enzymatic response of Napier grass (Pennisetum purpureum) stem induced by alkaline pretreatment. Bioresour. Technol. 2016, 218, 247–256. [Google Scholar] [CrossRef]
- Jiao, X.X.; Jin, H.Y.; Wang, M.M. Research progress of straw pretreatment for anaerobic fermentation produc ing biogas in china. China Biogas 2011, 29, 29–39. [Google Scholar]
- Yu, Y.; Zheng, P.; Chen, X.G.; Cai, J. Three typical fermentation utilization modes of biomass energy. Bull. Sci. Technol. 2009, 25, 854–859. (In Chinese) [Google Scholar] [CrossRef]
- Moreno, J.; Dufour, J. Life cycle assessment of lignocellusosic bioethanol: Environmental impacts and energy balance. Renew. Sustain. Energy. Rev. 2015, 42, 1349–1361. [Google Scholar] [CrossRef]
- Du, J. Novozymes accelerates cellulosic ethanol commercialized. China WTO Tribune 2010, 10, 81. (In Chinese) [Google Scholar] [CrossRef]
- Duque, S.H.; Cardona, C.A.; Moncada, J. Techno-economic and environmental analysis of ethanol production from agroindustrial residues in Colombia. Energy Fuels 2015, 29, 775–783. [Google Scholar] [CrossRef]
- Chovau, S.; Degrauwe, D.; Van der Bruggen, B. Critical analysis of techno-economic estimates for the production cost of lignocellulosic bio-ethanol. Renew. Sustain. Energy Rev. 2013, 26, 307–321. [Google Scholar] [CrossRef]
- Gubicza, K.; Nieves, I.U.; Sagues, W.J.; Barta, Z.; Shanmugam, K.T.; Ingram, L.O. Techno-economic analysis of ethanol production from sugarcane bagasse using a liquefaction plus simultaneous saccharification and co-fermentation process. Bioresour. Technol. 2016, 208, 42–48. [Google Scholar] [CrossRef]
- Denis, B.; Mark, M.W.; Robert, B. More than ethanol: A techno-economic analysis of a corn stover-ethanol biorefinery integrated with a hydrothermal liquefaction process to convert lignin into biochemicals. Biofuel. Bioprod. Bior. 2018, 12, 497–509. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Guo, X.D.; Liu, J.J.; Zhang, Z.J. Assessment of marginal land potential for energy plants in China. Land Develop. Eng. Res. 2017, 2, 1–7. (In Chinese) [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- State Environmental Protection Administration of China (SEPAC). The Methods for Water and Wastewater Monitoring and Analysis, 4th ed.; China Environmental Science Press: Beijing, China, 2002.
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Goering, H.K.; Van-Soest, P.J. Agricultural handbook No. 379. Forage fiber analyses, apparatus, reagents, procedures and some applications; U.S. Department of Agriculture: Washington, DC, USA, 1970.
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam-aqueous pretreatments [and discussion]. Philos. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity-relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
Sample Availability: Samples of the chemical compounds used in this study are available from the authors. |
| Different Pretreatment | Solid Yield (%) | Hemicellulose Content (%) | Cellulose Content (%) | Lignin Content (%) | Soluble Sugar from Pretreatment (mg/g RS) 1 |
|---|---|---|---|---|---|
| Control | -- | 28.7 ± 0.4 | 41.8 ± 0.9 | 17.5 ± 0.6 | 20.0 ± 0.4 |
| DAP, 3% H2SO4 | 53.3 ± 1.7 | 15.0 ± 1.7 | 55.9 ± 2.3 | 17.1 ± 1.5 | 112.2 ± 2.0 |
| DALP, 1.0%NaOH | 58.2 ± 1.2 | 15.3 ± 0.1 | 64.1 ± 1.7 | 11.7 ± 0.6 | 86.7 ± 0.2 |
| FCP, 3.2% FeCl3 | 55.9 ± 1.8 | 11.9 ± 1.3 | 60.8 ± 1.3 | 16.4 ± 0.7 | 193.4 ± 8.7 |
| Different Pretreatment | H2SO4/NaOH/FeCl3 Concentrations (%) | Solid Yield (%) | Hemicellulose Content (%) | Cellulose Content (%) | Lignin Content (%) | Soluble Sugar from Pretreatment (mg/g RS) 1 |
|---|---|---|---|---|---|---|
| Control | -- | -- | 28.7 ± 0.4 | 41.8 ± 0.9 | 17.5 ± 0.6 | 20.0 ± 0.4 |
| DAP with H2SO4 | 1% | 59.4 ± 1.0 | 16.9 ± 1.6 | 51.8 ± 1.9 | 15.7 ± 1.1 | 40.0 ± 1.3 |
| 2% | 55.0 ± 0.9 | 15.5 ± 1.3 | 55.0 ± 0.5 | 16.8 ± 1.2 | 69.5 ± 1.2 | |
| 3% | 53.3 ± 1.7 | 15.0 ± 1.7 | 55.9 ± 2.3 | 17.1 ± 1.5 | 112.2 ± 2.0 | |
| 4% | 52.6 ± 2.1 | 12.2 ± 0.2 | 56.5 ± 1.6 | 17.3 ± 1.4 | 119.3 ± 0.7 | |
| DALP with NaOH | 0.4% | 74.6 ± 2.5 | 22.0 ± 0.9 | 53.3 ± 0.3 | 14.5 ± 0.4 | 50.5 ± 0.2 |
| 0.6% | 64.7 ± 1.7 | 17.4 ± 0.9 | 59.0 ± 1.4 | 14.0 ± 0.1 | 63.4 ± 2.0 | |
| 0.8% | 59.4 ± 2.1 | 16.0 ± 0.2 | 63.2 ± 0.6 | 12.6 ± 0.1 | 83.5 ± 0.7 | |
| 1.0% | 58.2 ± 1.2 | 15.3 ± 0.1 | 64.1 ± 1.7 | 11.7 ± 0.6 | 86.7 ± 0.2 | |
| 1.2% | 53.9 ± 1.7 | 14.8 ± 0.3 | 68.0 ± 0.7 | 10.2 ± 0.8 | 107.3 ± 0.6 | |
| FCP with FeCl3 | 0.8% | 68.8 ± 3.1 | 15.2 ± 1.7 | 56.9 ± 1.6 | 18.0 ± 1.6 | 90.2 ± 2.2 |
| 1.6% | 60.8 ± 1.7 | 13.5 ± 1.5 | 60.7 ± 1.1 | 17.3 ± 0.1 | 163.6 ± 3.2 | |
| 3.2% | 55.9 ± 1.8 | 11.9 ± 1.3 | 60.8 ± 1.3 | 16.4 ± 0.7 | 193.4 ± 8.7 | |
| 4.8% | 53.1 ± 2.3 | 9.7 ± 1.7 | 62.7 ± 1.5 | 15.3 ± 1.5 | 200.2 ± 6.7 |
| Different Methods | Solid Yield (%) | Soluble Sugar from Pretreatment (mg/g RS) | Soluble Sugar from Enzymatic Hydrolysis (mg/g PS) | Soluble Sugar from Enzymatic Hydrolysis (mg/g RS) | Total Soluble Sugar Yield (mg/g RS) 1 |
|---|---|---|---|---|---|
| Control | -- | -- | -- | 134.8 | 134.8 |
| DAP | 52.6 | 119.3 | 336.4 | 176.9 | 296.2 |
| DALP | 53.9 | 107.3 | 744.4 | 401.2 | 508.5 |
| FCP | 53.1 | 200.2 | 279.3 | 148.4 | 348.6 |
| Biomass | Pretreatment Conditions | Sugar Yield (mg/g PS) 1 | Conversion Ratio (%) | Ref. |
|---|---|---|---|---|
| P. alopecuroides | 1.2% NaOH, 121 °C, 30 min | 744.4 | 85.4 | This study |
| Wild rice grass | 2% H2SO4, 121 °C, 60 min | 457 | 93.2 | [19] |
| Bamboo | 1% NaOH, 3% Tween 80,121 °C, 60 min | 629 | 75.4 | [46] |
| Bamboo | 1% H2SO4, 3% Tween 80,121 °C, 60 min | 153 | 24.7 | [46] |
| Pine foliage | 1% C-TAB, 1% H2SO4, 121 °C, 60 min | 588 | 98.1 | [20] |
| Pine foliage | 1% PEG-6000, 1% NaOH, 121 °C, 60 min | 477 | 88.4 | [20] |
| Eucalyptus | 12.5% [TBA][OH], ultrasound irradiation (at a power of 360 W for 60 min) | 426.6 | 51.5 | [34] |
| Eucalyptus | 2% NaOH, ultrasound irradiation (at a power of 360 W for 60 min) | 362.3 | 56.6 | [34] |
| P. purpureum Schum | 0.5% NaOH, 90 °C, 60 min | (glucose yield: 245 mg/g RS) | NA | [9] |
| P. purpureum | 1.5% NaOH, 121 °C, 60 min | 146.9 | 24.7 | [17] |
| P. purpureum | 2% Ca(OH)2 or NaOH, 121 °C, 60 min | 324~5372 | 65.5~88.7 | [47] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Xu, C.; Vu, L.T.K.; Liu, S.; Ye, P.; Li, L.; Wu, Y.; Chen, M.; Xiao, Y.; Wu, Y.; et al. Enhanced Enzymatic Hydrolysis of Pennisetum alopecuroides by Dilute Acid, Alkaline and Ferric Chloride Pretreatments. Molecules 2019, 24, 1715. https://doi.org/10.3390/molecules24091715
Tang S, Xu C, Vu LTK, Liu S, Ye P, Li L, Wu Y, Chen M, Xiao Y, Wu Y, et al. Enhanced Enzymatic Hydrolysis of Pennisetum alopecuroides by Dilute Acid, Alkaline and Ferric Chloride Pretreatments. Molecules. 2019; 24(9):1715. https://doi.org/10.3390/molecules24091715
Chicago/Turabian StyleTang, Shangyuan, Chunming Xu, Linh Tran Khanh Vu, Sicheng Liu, Peng Ye, Lingci Li, Yuxuan Wu, Mengyu Chen, Yao Xiao, Yue Wu, and et al. 2019. "Enhanced Enzymatic Hydrolysis of Pennisetum alopecuroides by Dilute Acid, Alkaline and Ferric Chloride Pretreatments" Molecules 24, no. 9: 1715. https://doi.org/10.3390/molecules24091715
APA StyleTang, S., Xu, C., Vu, L. T. K., Liu, S., Ye, P., Li, L., Wu, Y., Chen, M., Xiao, Y., Wu, Y., Wang, Y., Yan, Q., & Cheng, X. (2019). Enhanced Enzymatic Hydrolysis of Pennisetum alopecuroides by Dilute Acid, Alkaline and Ferric Chloride Pretreatments. Molecules, 24(9), 1715. https://doi.org/10.3390/molecules24091715