Phytochemical Study of Aerial Parts of Leea asiatica
Abstract
:1. Introduction
2. Results and Discussion
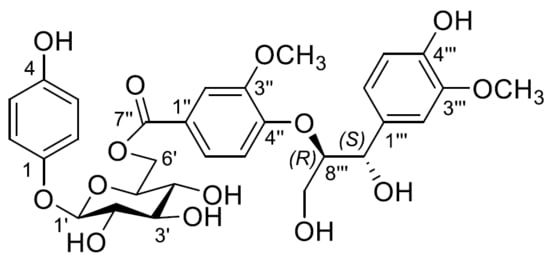
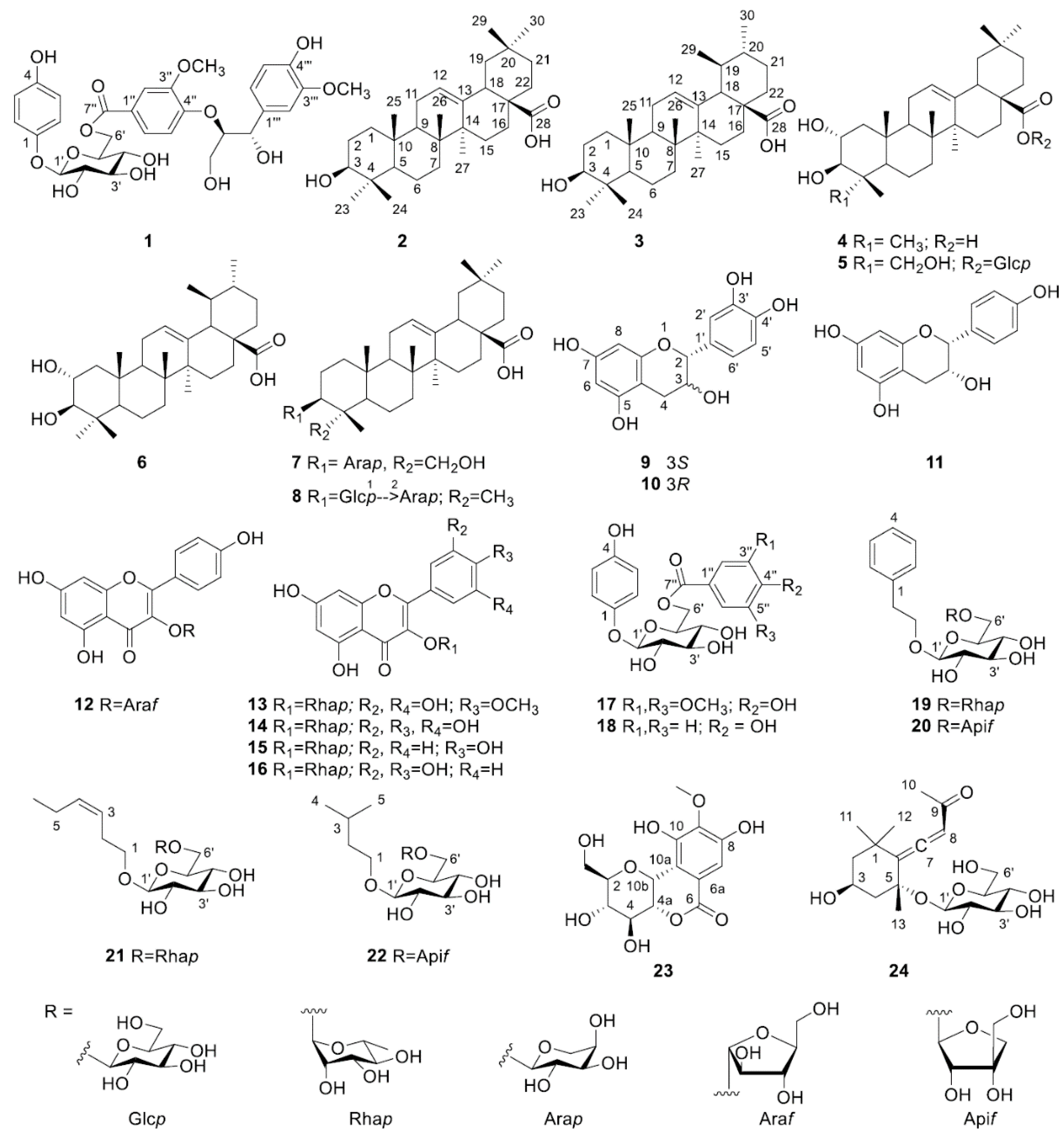
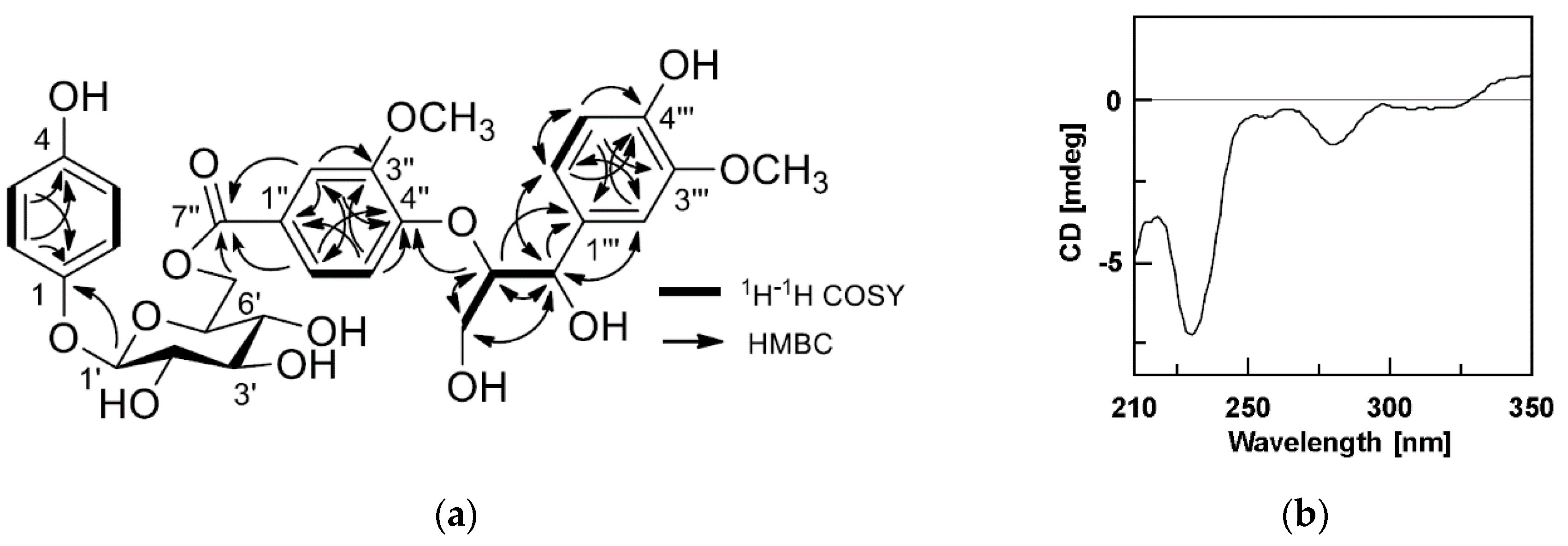
2.1. Elucidation of Chemical Structures of Compounds 1–24
2.2. Characterisation of Compounds 1–24
3. Materials and Methods
3.1. General Experiments
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of Compound 1
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lakornwong, W.; Kanokmedhakul, K.; Kanokmedhakul, S. Chemical constituents from the roots of Leea thorelii Gagnep. Nat. Prod. Res. 2014, 28, 1015–1057. [Google Scholar] [CrossRef]
- Leea. Available online: http://www.webcitation.org/76LSvAVrY (accessed on 21 January 2019).
- Kaewkrud, W.; Otsuka, H.; Ruchirawat, S.; Kanchanapoom, T. Leeaoside, a new megastigmane diglycoside from the leaves of Leea thorelii Gagnep. J. Nat. Med. 2007, 61, 449–451. [Google Scholar] [CrossRef]
- Wong, Y.H.; Kadir, H.A.; Ling, S.K. Bioassay-guided isolation of cytotoxic cycloartane triterpenoid glycosides from the traditionally used medicinal plant Leea indica. Evid. Based Complement. Alternat. Med. 2012. [Google Scholar] [CrossRef]
- Mishra, G.; Khosa, R.L.; Singh, P.; Tahseen, M.A. Ethnobotany and phytopharmacology of Leea indica: An overview. J. Coast. Life. Med. 2016, 4, 69–72. [Google Scholar] [CrossRef]
- Srinivasan, G.V.; Ranjith, C.; Vijayan, K.K. Identification of chemical compounds from the leaves of Leea indica. Acta Pharm. 2008, 58, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.S.; Navanesan, S.; Sinniah, S.K.; Wahab, N.A.; Sim, K.S. Phenolic content, antioxidant effect and cytotoxic activity of Leea indica leaves. BMC Complement. Altern. Med. 2012, 12, 128. [Google Scholar] [CrossRef]
- Patel, A.A.; Amin, A.A.; Patwari, A.H.; Shah, M.B. Validated high performance thin layer chromatography method for simultaneous determination of quercetin and gallic acid in Leea indica. Rev. Bras. Farmacogn. 2017, 27, 50–53. [Google Scholar] [CrossRef]
- Singh, D.; Siew, Y.Y.; Chong, T.I.; Yew, H.C.; Ho, S.S.; Lim, S.S.E.; Tan, W.X.; Neo, S.Y.; Koh, H.L. Identification of phytoconstituents in Leea indica (Burm. F.) Merr. leaves by high performance liquid chromatography micro time-of-flight mass spectrometry. Molecules 2019, 24, 714. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, Z.A.; Bachar, S.C.; Hasan, C.M.; Emran, T.B.; Qais, N.; Uddin, M.M.N. Phytochemical investigations and antioxidant potential of roots of Leea macrophylla (Roxb.). BMC Res. Notes 2017, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Prasad, S.K.; Joshi, V.K.; Hemalatha, S. Phytochemical standardization, antioxidant, and antibacterial evaluations of Leea macrophylla: A wild edible plant. J. Food Drug Anal. 2016, 24, 324–331. [Google Scholar]
- Op de Beck, P.; Cartier, G.; David, B.; Dijoux-Franca, M.G.; Mariotte, A.M. Antioxidant flavonoids and phenolic acids from leaves of Leea guineense G Don (Leeaceae). Phytother. Res. 2003, 17, 345–347. [Google Scholar] [CrossRef]
- Op de Beck, P.; Dijoux, M.G.; Cartier, G.; Mariotte, A.M. Quercitrin 3′-sulphate from leaves of Leea guineensis. Phytochemistry 1998, 47, 1171–1173. [Google Scholar] [CrossRef]
- Sen, S.; De, B.; Devanna, N.; Chakraborty, R. Anthelmintic and in vitro antioxidant evaluation of fractions of methanol extract of Leea asiatica leaves. Anc. Sci. Life 2012, 31, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; De, B.; Devanna, N.; Chakraborty, R. Cisplatin-induced nephrotoxicity in mice: Protective role of Leea asiatica leaves. Ren. Fail. 2013, 35, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; De, B.; Devanna, N.; Chakraborty, R. Hepatoprotective and antioxidant activity of Leea asiatica leaves against acetaminophen-induced hepatotoxicity in rats. Tang [Humanitas Medicine] 2014, 4, e18. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Y.P.; Wang, X.L.; Wang, S.J.; Bu, P.B.; Lin, S.; Zhu, C.G.; Shi, J.G. Aromatic glycosides from the flower buds of Lonicera japonica. J. Asian Nat. Prod. Res. 2013, 15, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhu, C.; Li, Y.; Tian, Y.; Lin, S.; Yuan, S.; Hu, J.; Hou, Q.; Chen, N.; Yang, Y.; et al. Lignans and neolignans from Sinocalamus affinis and their absolute configurations. J. Nat. Prod. 2011, 74, 1188–1200. [Google Scholar] [CrossRef]
- Seebacher, W.; Simic, N.; Weis, R.; Saf, R.; Kunert, O. Complete assignments of 1H and 13C-NMR resonances of oleanolic acid, 18a-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Taniguchi, S.; Imayoshi, Y.; Kobayashi, E.; Takamatsu, Y.; Ito, H.; Hatano, T.; Sakagami, H.; Tokuda, H.; Nishino, H.; Sugita, D.; et al. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 2002, 59, 315–323. [Google Scholar] [CrossRef]
- Wen, X.; Sun, H.; Liu, J.; Wu, G.; Zhang, L.; Wu, X.; Ni, P. Pentacyclic triterpenes. Part 1: The first examples of naturally occurring pentacyclic triterpenes as a new class of inhibitors of glycogen phosphorylases. Bioorg. Med. Chem. Lett. 2005, 15, 4944–4948. [Google Scholar] [CrossRef]
- Kundu, A.P.; Mahato, S.B. Triterpenoids and their glycosides from Terminalia chebula. Phytochemistry 1993, 32, 999–1002. [Google Scholar] [CrossRef]
- Bisoli, E.; Garcez, W.S.; Hamerski, L.; Tieppo, C.; Garcez, F.R. Bioactive pentacyclic triterpenes from the stems of Combretum laxum. Molecules 2008, 13, 2717–2728. [Google Scholar] [CrossRef]
- Kohda, H.; Tanaka, S.; Yamaoka, Y. Saponins from leaves of Acanthopanax hypoleucus Makino. Chem. Pharm. Bull. 1990, 38, 3380–3383. [Google Scholar] [CrossRef]
- Kawai, H.; Kuroyanagi, M.; Umehara, K.; Ueno, A.; Satake, M. Studies on the saponins of Lonicera japonica THUNB. Chem. Pharm. Bull. 1988, 36, 4769–4775. [Google Scholar] [CrossRef]
- Choi, J.; Cho, J.Y.; Kim, Y.D.; Htwe, K.M.; Lee, W.S.; Lee, J.C.; Kim, J.; Yoon, K.D. Phenolic compounds and triterpenes from the barks of Diospyros burmanica. Nat. Prod. Sci. 2015, 21, 76–81. [Google Scholar]
- Kpegba, K.; Agbonon, A.; Petrovic, A.G.; Amouzou, E.; Gbeassor, M.; Proni, G.; Nesnas, N. Epiafzelechin from the root bark of Cassia sieberiana: Detection by DART mass spectrometry, spectroscopic characterization, and antioxidant properties. J. Nat. Prod. 2011, 74, 455–459. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Wu, H.K.; Aisa, H.A. Kaempferol and quercetin flavonoids from Rosa rugosa. Chem. Nat. Comp. 2006, 42, 736–737. [Google Scholar] [CrossRef]
- Mahmoud, I.I.; Marzouk, M.S.; Moharram, F.A.; El-Gindi, M.R.; Hassan, A.M. Acylated flavonol glycosides from Eugenia jambolana leaves. Phytochemistry 2001, 58, 1239–1244. [Google Scholar] [CrossRef]
- Mok, S.Y.; Lee, S. Identification of flavonoids and flavonoid rhamnosides from Rhododendron mucronulatum for albiflorum and their inhibitory activities against aldose reductase. Food Chem. 2013, 136, 969–974. [Google Scholar]
- Bai, J.; Fang, Z.; Chen, H.; Yu, S.; Zhang, D.; Wei, H.; Ma, S.; Li, Y.; Qu, J.; Xu, S.; et al. Antioxidant phenolic glycosides from the roots of Illicium dunnianum. Carbohydr. Res. 2012, 361, 206–211. [Google Scholar] [CrossRef]
- Morikawa, H.; Kasai, R.; Otsuka, H.; Hirata, E.; Shinzato, T.; Aramoto, M.; Takeda, Y. Terpenic and phenolic glycosides from leaves of Breynia officinalis HEMSL. Chem. Pharm. Bull. 2004, 52, 1086–1090. [Google Scholar] [CrossRef]
- Hase, T.; Kawamoto, Y.; Ohtani, K.; Kasai, R.; Yamasaki, K.; Picheansoonthon, C. Cyclohexylethanoids and related glucosides from Millingtonia hortensis. Phytochemistry 1995, 39, 235–241. [Google Scholar] [CrossRef]
- Ono, M.; Yoshida, A.; Ito, Y.; Nohara, T. Phenethyl alcohol glycosides and isopentenol glycoside from fruit of Bupleurum falcatum. Phytochemistry 1999, 51, 819–823. [Google Scholar] [CrossRef]
- Song, S.J.; Li, L.Z.; Gao, P.Y.; Peng, Y.; Yang, J.Y.; Wu, C.F. Terpenoids and hexenes from the leaves of Crataegus pinnatifida. Food Chem. 2011, 129, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Morikawa, T.; Nakamura, S.; Ninomiya, K.; Matsuda, H.; Muraoka, O.; Wu, L.-J.; Yoshikawa, M. Medicinal flowers. XXVIII. Structures of five new glycosides, everlastosides, A, B, C, D, and E, from the flowers of Helichrysum arenarium. Heterocycles 2009, 78, 1235–1242. [Google Scholar]
- Ito, T.; Hara, Y.; Oyama, M.; Tanaka, T.; Murata, J.; Darnaedi, D.; Iinuma, M. Occurrence of bergenin phenylpropanoates in Vatica bantamensis. Phytochem. Lett. 2012, 5, 743–746. [Google Scholar] [CrossRef]
- Umehara, K.; Hattori, I.; Miyase, T.; Ueno, A.; Hara, S.; Kageyama, C. Studies on the constituents of leaves of Citrus unshiu MARCOV. Chem. Pharm. Bull. 1988, 36, 5004–5008. [Google Scholar] [CrossRef]
- Njoku, C.J.; Zeng, L.; Asuzu, I.U.; Oberlies, N.H.; Mclaughlin, J.L. Oleanolic acid, a bioactive Component of the leaves of Ocimum gratissimum (Lamiaceae). Int. J. Pharmacogn. 1997, 35, 134–137. [Google Scholar] [CrossRef]
- Vijaya; Yadav, A.K..; Gogoi, S. In vitro and in vivo anthelmintic efficacy of two pentacyclic triterpenoids, ursolic acid and betulinic acid against mice pinworm, Syphacia obvelata. J. Parasit. Dis. 2018, 42, 144–149. [Google Scholar] [CrossRef]
- Borges, D.G.L.; Echeverria, J.T.; de Oliveira, T.L.; Heckler, R.P.; de Freitas, M.G.; Damasceno-Junior, G.A.; Carollo, C.A.; Borges, F.A. Discovery of potential ovicidal natural products using metabolomics. PLoS ONE 2019, 14, e0211237. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids and antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A dietary molecule with diverse biological activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Choi, J.; Htwe, K.M.; Chin, Y.W.; Kim, J.; Yoon, K.D. Flavonoid glycosides from the aerial parts of Acacia pennata in Myanmar. Phytochemistry 2015, 118, 17–22. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1, 2, 4, 6, 9, 10, 12 and 17 are stored in DMSO and available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kil, H.W.; Rho, T.; Yoon, K.D. Phytochemical Study of Aerial Parts of Leea asiatica. Molecules 2019, 24, 1733. https://doi.org/10.3390/molecules24091733
Kil HW, Rho T, Yoon KD. Phytochemical Study of Aerial Parts of Leea asiatica. Molecules. 2019; 24(9):1733. https://doi.org/10.3390/molecules24091733
Chicago/Turabian StyleKil, Hyun Woo, Taewoong Rho, and Kee Dong Yoon. 2019. "Phytochemical Study of Aerial Parts of Leea asiatica" Molecules 24, no. 9: 1733. https://doi.org/10.3390/molecules24091733