Abstract
Exploration of efficient catalysts is a priority for the electrochemical nitrogen reduction reaction (NRR) in order to receive a high product yield rate and faradaic efficiency of NH3, under ambient conditions. In the present contribution, the binding free energy of N2, NNH, and NH2 were used as descriptors to screen the potential NRR electrocatalyst among different single or binuclear transition metal atoms on N-doped nanoporous graphene. Results showed that the binuclear Mo catalyst might exhibit the highest catalytic activity. Further free energy profiles confirmed that binuclear Mo catalysts possess the lowest potential determining step (hydrogenation of NH2* to NH3). The improved activities could be ascribed to a down-shift of the density of states for Mo atoms. This investigation could contribute to the design of a highly active NRR electrocatalyst.
1. Introduction
Nitrogen fixation is one of the most important process from the perspectives of agriculture and human development [1,2,3,4,5,6]. Traditional Haber–Bosch process consumes plenty of energy, due to the difficulty of direct nitrogen-nitrogen triple bond cleavage reaction [7]. Electrocatalytic N2 reduction reaction (NRR) holds a great promise for realizing NH3 production, as it can decrease the barrier height of breakage for a nitrogen-nitrogen bond, to a certain degree [8,9,10,11,12,13]. Different kinds of electrocatalysts, such as single atom catalysts (SACs) [14,15,16,17,18,19,20], have been developed for boosting the nitrogen reduction performance. For the majority of catalysis, the intrinsic activities of SAC are very high, compared to the state-of-art catalysts. Zhao et al. have shown that, typically, a single Mo atom supported on a defective boron nitride monolayer is demonstrated to be an efficient electrocatalyst for nitrogen fixation, with a very low potential determining step (PDS) value, via the enzymatic pathway [21]. As the more widely used two-dimensional catalysts, single Mo and Fe atoms supported by N-doped nanoporous graphene (Mo- or Fe-N-C) have also been revealed to exhibit a good catalytic activity for NRR [22,23]. However, in general, the NRR strategy is still hindered by its high potential barrier, leading to an extremely low production rate and Faradaic efficiency of NH3.
Previous investigations have indicated that an excellent NRR electrocatalyst should have a moderate adsorption energy for N-related species [24,25]. In most cases, such as single Mo and Fe atoms supported by N-doped nanoporous graphene, SACs might show stronger or weaker interaction energy towards the reactant. Therefore, the electronic structure needs to be adjusted to get a proper binding energy. It has previously been reported that changing one atom in the ultrafine cluster might largely alter the electronic structure and drastically change its catalytic properties [26,27]. Such atom-dependent catalytic behaviors have been successfully demonstrated by the model catalysts of mass-selected metal clusters. Thus, atomically precise ultrafine metal clusters, such as dimers or trimer, on high-surface area supports, might improve the intrinsic activities of a catalyst, by adjusting the adsorption energy for N-related species.
Recently, Lu et al. have showed that Pt2 dimers can be fabricated with a bottom-up approach on graphene, using the atomic layer deposition [28]. In the hydrolytic dehydrogenation of ammonia borane, Pt2 dimers were found to exhibit a higher specific rate of 2800 molH2 molPt−1 min−1, which was ~17-fold higher than the graphene-supported Pt single atoms. Li et al. developed a host–guest strategy to fabricate electrocatalysts with Fe-Co dual sites embedded on N-doped nanoporous graphene, and further showed an excellent oxygen reduction activity in the acidic electrolyte [29]. Li et al. proposed a new strategy that anchored an Fe3 or Co3Rh cluster on the oxide surfaces, as a heterogeneous catalyst for ammonia synthesis, from the first-principles study and microkinetic analysis [12,30]. The calculated turnover frequency is comparable to the Ru catalyst. However, to our knowledge, no binuclear atoms catalysts (BAC) on 2D materials has been reported for electrochemical NRR on experimental and theoretical fronts. Even to date, the intrinsic reaction mechanism still remains elusive.
Therefore, in this work, the adsorption energy of N2, NNH, and NH2 obtained by the density functional theory (DFT) were utilized to screen the electrochemical NRR catalyst for different SAC and BAC, indicating that an Mo BAC is potential excellent N2 electrocatalyst. Then, free energy differences along three possible pathways have confirmed that Mo BAC possess a low potential determining step than an SAC, due to the down-shift of states for the Mo atoms relative to the Fermi energy. Additionally, the stabilities of different single or binuclear transition metal atoms on N-doped graphene were investigated, demonstrating that most transition metal BAC were more stable than those of SAC. The conclusions in this work would provide an efficient method for exploring the NRR catalysts.
2. Results and Discussion
2.1. Stability for Various Mono- and Binuclear N-C Catalysts
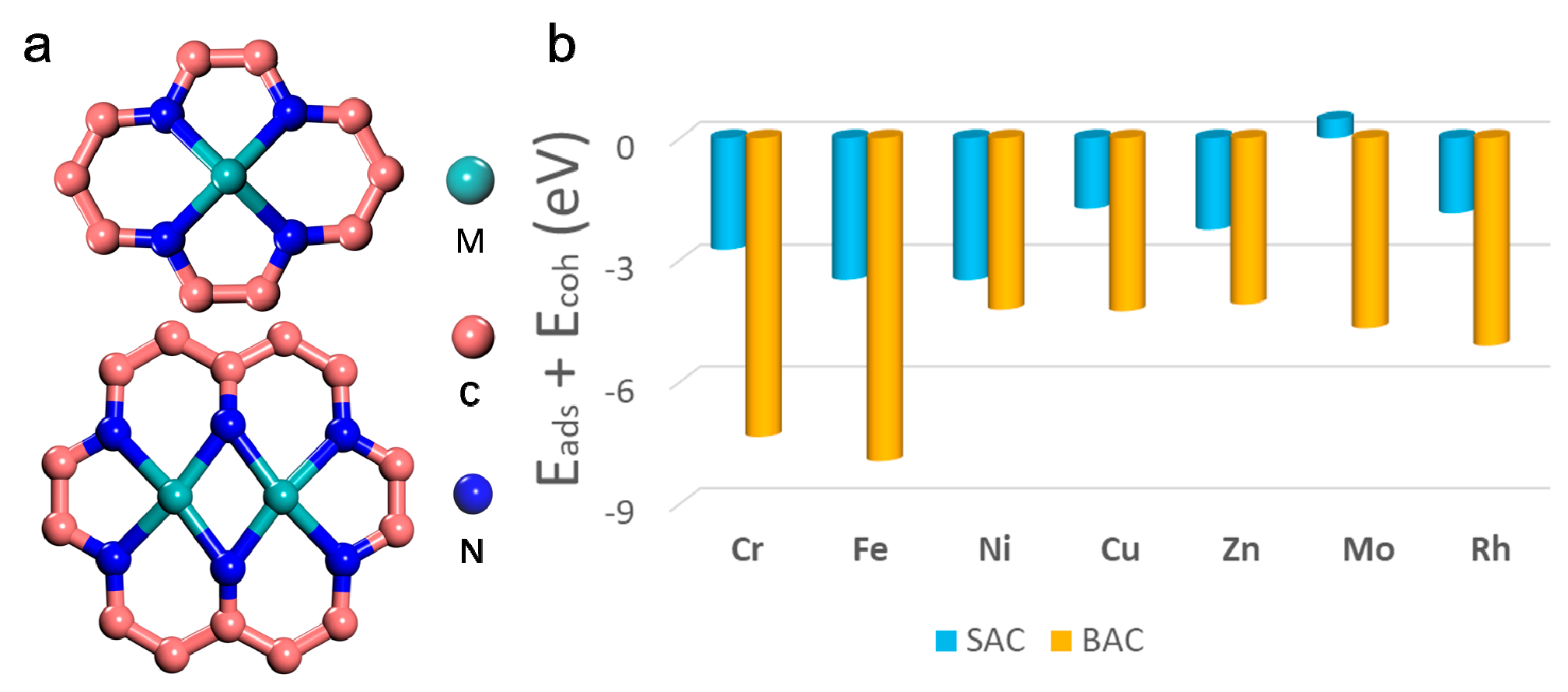
It is well-known that the duration of SAC or BAC is a key challenge in their applications that is yet to be solved [31]. As shown in Figure 1, the stability of different single or binuclear transition metal atoms on N-doped graphene was tested via their formation energies. Figure 1a shows the structural model of mono- and binuclear catalysts. Different possible structures of SAC and BAC, such as Mo-based catalysts, was explored, as depicted in Figure S1 in the Supplementary Materials (SM). Results showed that the configurations in Figure 1a were more favorable and, thus, stable (Table S1). Figure 1b and Table S2 shows the formation energies of various mono- and binuclear catalysts for different transition metal anchored on the N-doped graphene (N-C). The stabilities at the given potentials for the different metal centers, were also collected, and is shown in Table S3. It can be seen that the majority of SAC and BAC had a good stability, even under harsh electrochemical conditions. Results indicate that the order of stability for mononuclear N-C was: Ni > Fe > Cr > Zn > Rh > Cu > Mo. The order of stability for the binuclear N-C was: Fe > Cr > Rh > Mo > Cu > Ni > Zn. An interesting result was that, for certain metal-doped monolayer, formation of a second metal center was energetically favorable. Typically, the stability of the BAC for Mo and Ru is much higher than that of SAC, which further confirmed the rationality of our catalyst design.
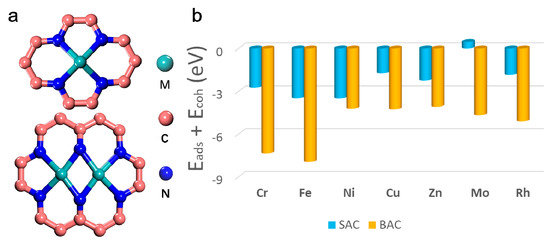
Figure 1.
(a) Structures of single and binuclear atoms catalysts, and (b) calculated formation energy of different mono- and binuclear N-C catalysts.
2.2. Screen of Potential NRR Electrocatalyst Combining Different Descriptors
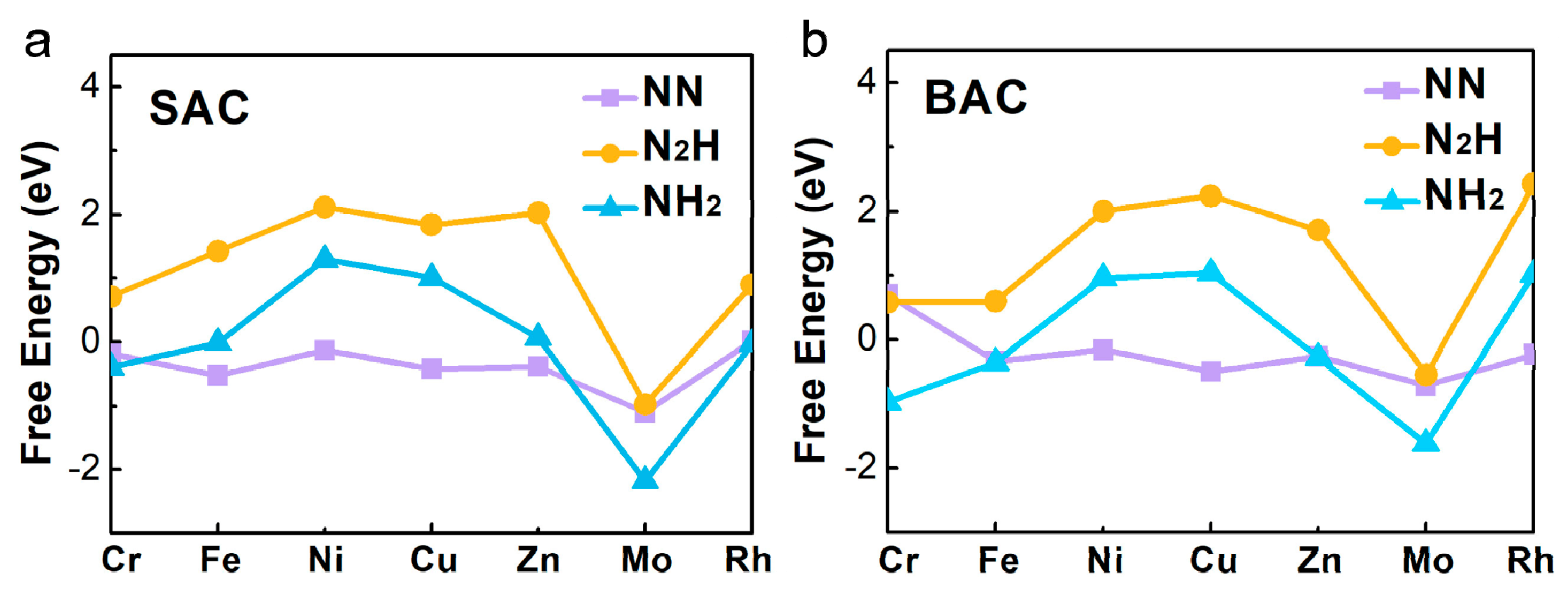
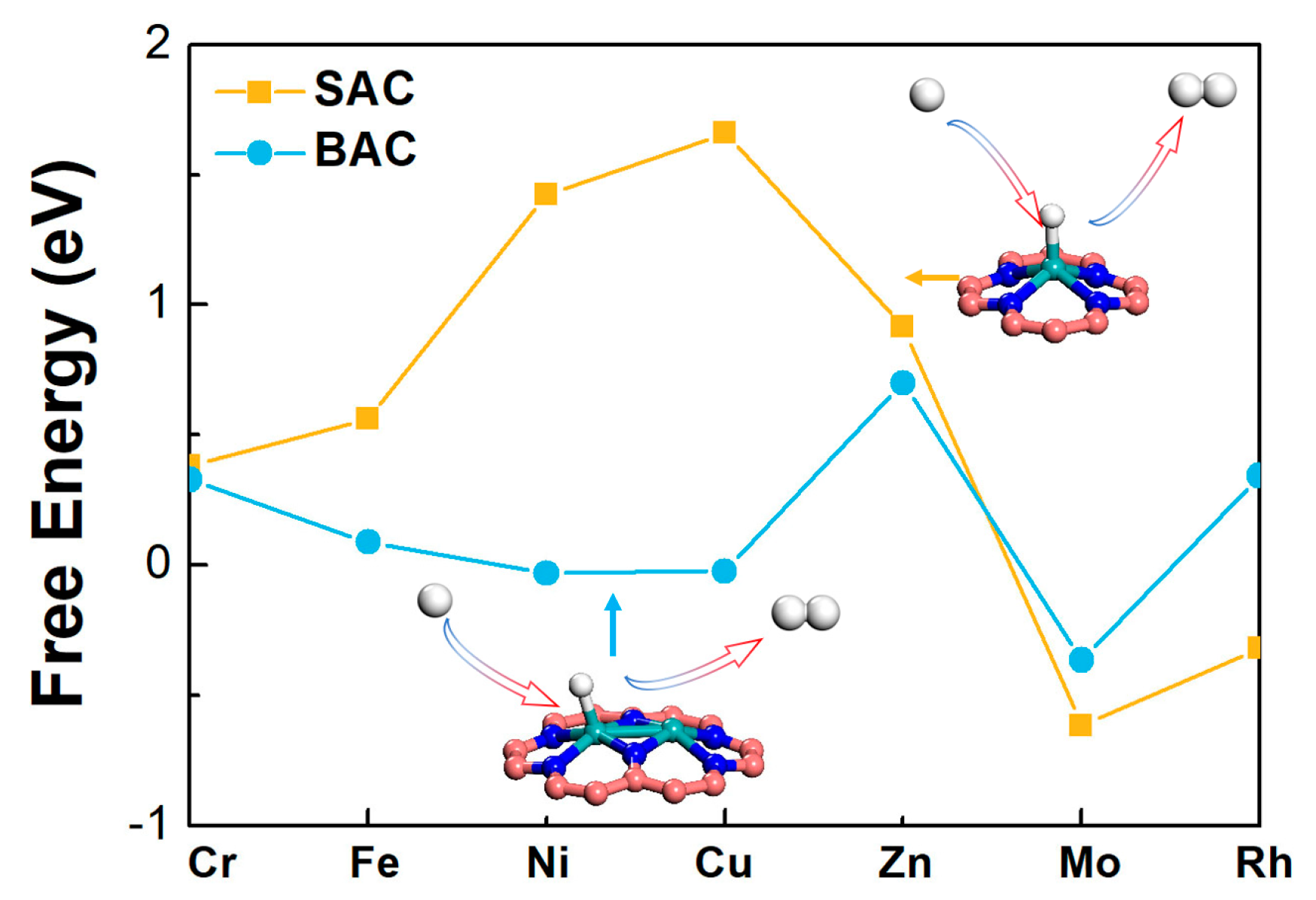
Based on the three screeners of eligible electrocatalyst for the NRR proposed by the previous studies [21,24,25], we explored a series of metal atoms, supported by defective N-C nanosheets, by calculating the adsorption free energy (∆G) of N2, NNH, and NH2 (Figure 2 and Table S4). As shown in Figure 2a, it indicated that Fe- and Mo- mononuclear catalyst had a lower ∆G for N2 adsorption (∆GFe = −0.52 eV, ∆GMo = −1.10 eV). For the binuclear catalysts, Fe and Mo had a lower ∆G (∆GFe = −0.33 eV, ∆GMo = −0.71 eV) than the other metals, for the adsorption of N2. It could be inferred that these two metal-doped nanosheets were more favorable NRR electrocatalysts because of the strong N2 activation. Figure 2 also shows the adsorption free energies of NNH and NH2 on the mononuclear and binuclear catalysts. We found that the properties of mononuclear and binuclear catalysts for Fe and Mo were interesting. As shown in Figure 2a, ∆GNNH on Fe-N-C was calculated to be as high as 1.43 eV, and ∆GNH2 on Mo-N-C was as low as −2.18 eV, showing that for Fe and Mo, anchored SAC was not suitable for NRR. However, in the BAC catalysts, ∆GNNH on Fe2-N-C was 0.60 eV, which was much smaller than that on the Fe-N-C. Binding free energy of NH2 on Mo2-N-C was −1.62 eV, which was also smaller than that on Mo-N-C. More importantly, Mo2-N-C possessed the lowest difference value between NNH and NH2, than the others. Therefore, it could be inferred that Mo2-N-C might have a better performance for NRR than Mo-N-C and the other SAC. Combined with the above analyses results, our interest on further exploration of Mo-anchored catalysts, was aroused.
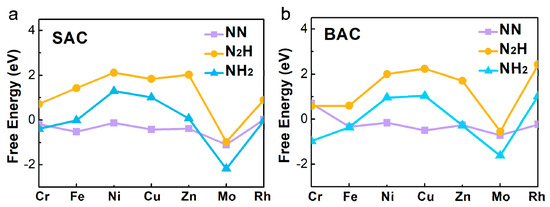
Figure 2.
The calculated Gibbs free energies of N2, NNH, and NH2 species on various (a) mono- and (b) binuclear N-C catalysts.
2.3. Mo- and Mo2-N-C Monolayer for NRR
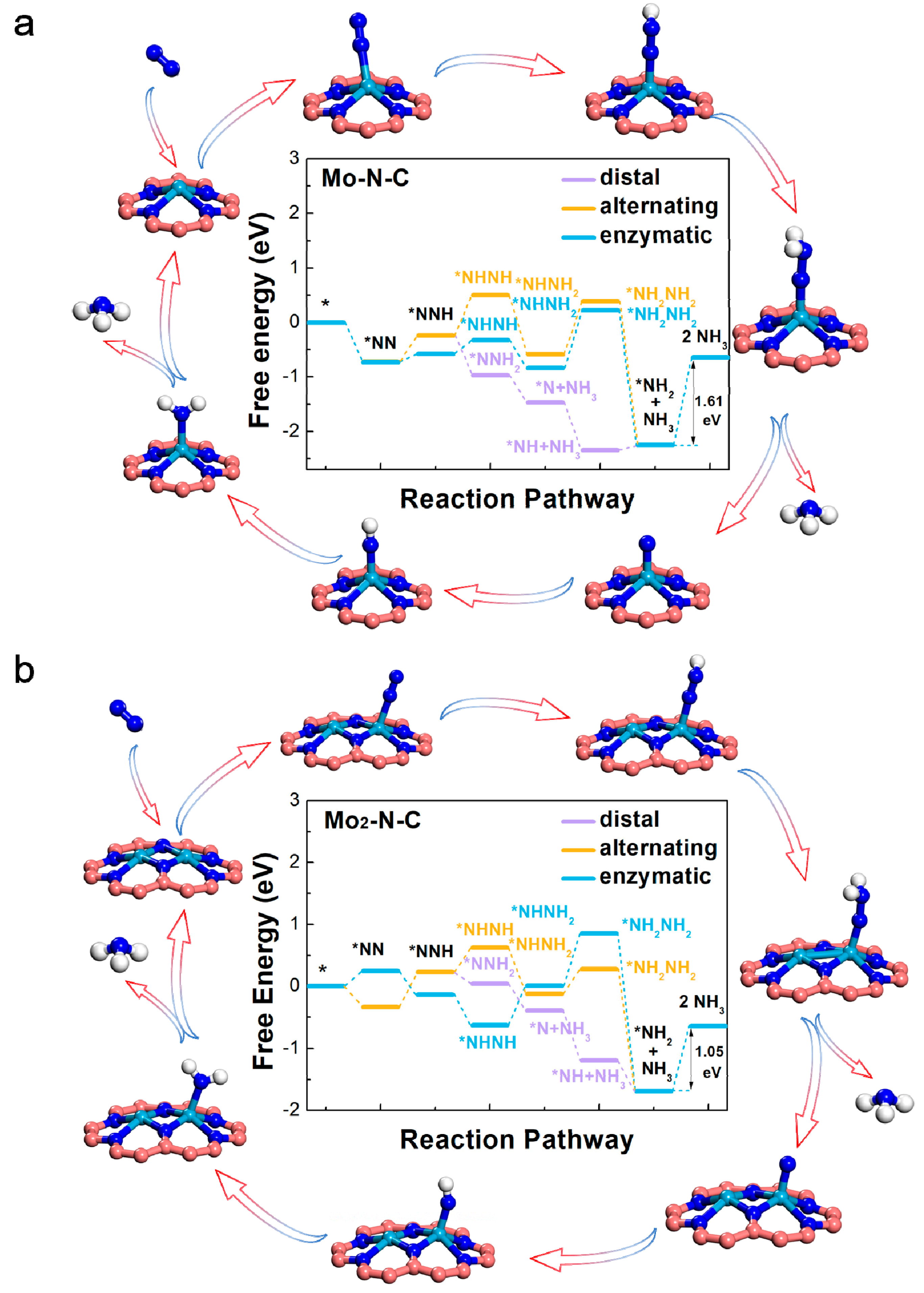
To assess the ability of Mo-N-C and Mo2-N-C monolayer as an electrocatalyst to reduce activated N2 to NH3, we explored four possible NRR pathways, including distal, alternating, enzymatic, and dissociative mechanisms. Figure 3, and Tables S5 and S6 summarize the atomic configurations and corresponding free energy changes of each elementary steps, corresponding to the different paths. Energy zero was defined as the energy of pure SAC or BAC, and the free N2 molecule. For Mo-N-C, the ΔG values of PDS, through distal, alternating, and enzymatic pathways were calculated to be 1.61 eV, corresponding to the hydrogenation of NH2* to NH3. For Mo2-N-C, hydrogenation of NH2* to NH3 was also calculated to be PDS (1.05 eV), through the distal, alternating, and enzymatic pathways. By further comparing the ΔG values of PDS, it could be inferred that the catalytic performance of Mo2-N-C was better than that of Mo-N-C. Single and binuclear Mo atoms on N-doped graphene also exhibited an excellent NRR activity, in comparison to other electrocatalysts, especially for the carbon-based materials with the same PDS (Table S7).
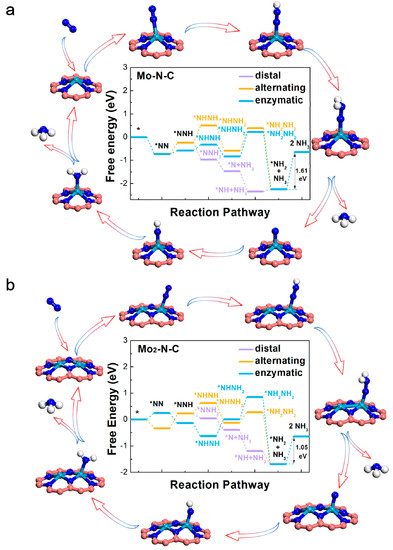
Figure 3.
Free-energy diagrams for the nitrogen reduction reaction (NRR) on the Mo-N-C (a) and Mo2-N-C (b) catalysts.
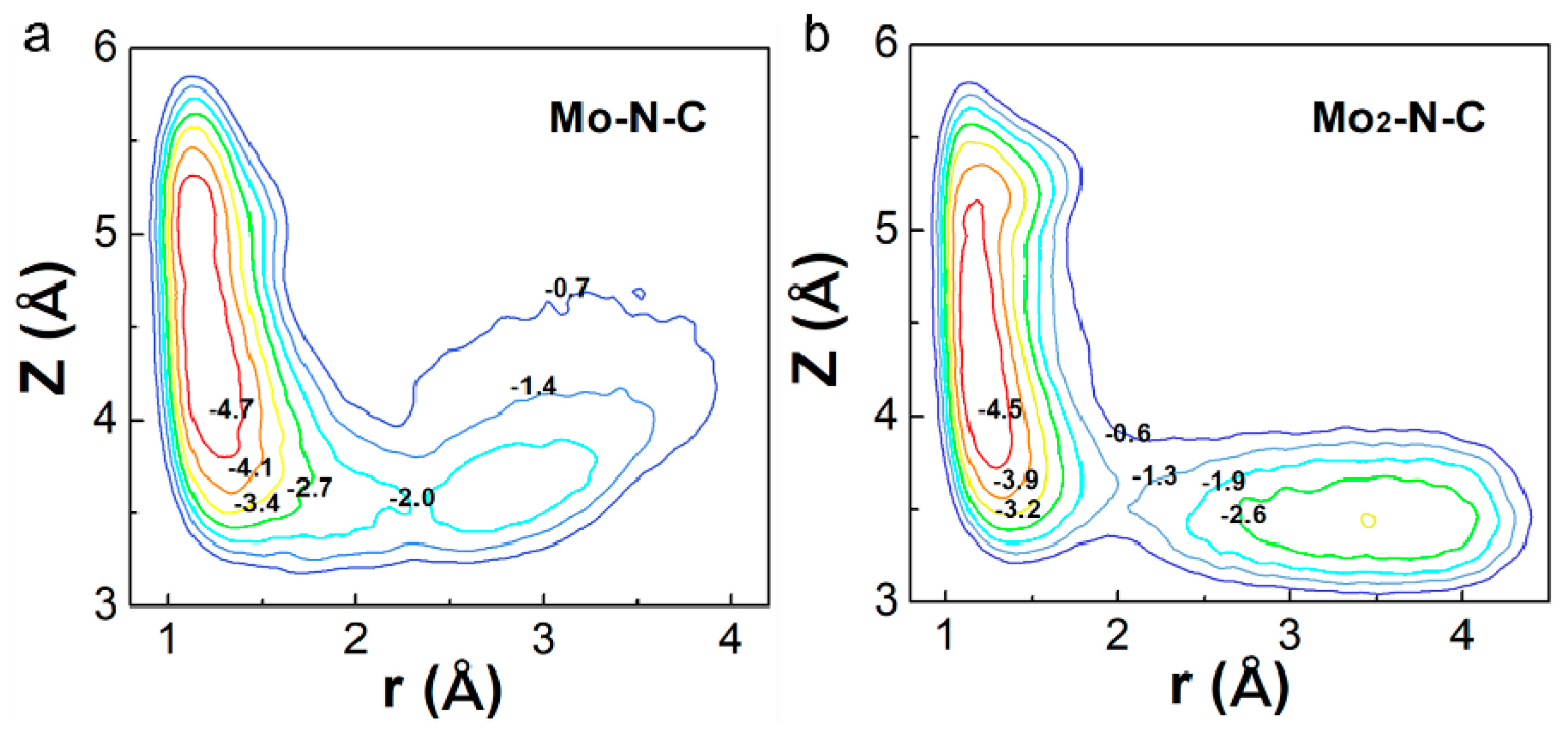
Generally, a dissociative pathway is difficult to achieve because of the extremely stable nitrogen–nitrogen triple bond. However, Mo2-N-C might facilitate a direct dissociation of N2, due to its unique electronic structure and catalytic properties. Thus, the potential energy surface (PES) as a function of the molecular coordinates r and z (see Figure 4) were calculated for Mo-N-C and Mo2-N-C. PES were obtained using DFT–molecular dynamics (MD) simulations. To estimate the free energy barriers for the dissociative reaction, we employed metadynamics, which allowed to sample the free energy landscape spanned by the two collective variables whose combination was able to describe the mechanism under study. The method has been successfully used to investigate the dissociative pathway of different small molecules on the catalyst surface [32]. For Mo-N-C, it could be seen that only one remarkable minimum was obtained, demonstrating that N2 was hardly directly decomposed. As for Mo2-N-C, another minimum was observed, in which the N2 had been broken into two N atoms absorbed on two Mo atoms. Results showed that binuclear Mo N-C nanosheet were the more favorable NRR electrocatalyst due to the strong N2 activation.
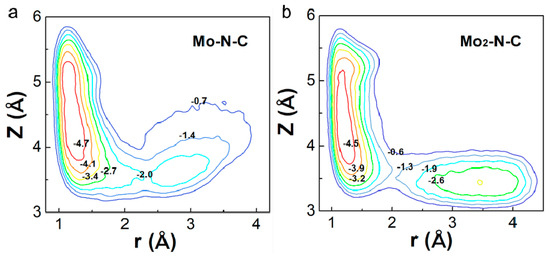
Figure 4.
The potential energy surfaces of N2 dissociation on Mo (a) mono- and (b) binuclear N-C catalysts.
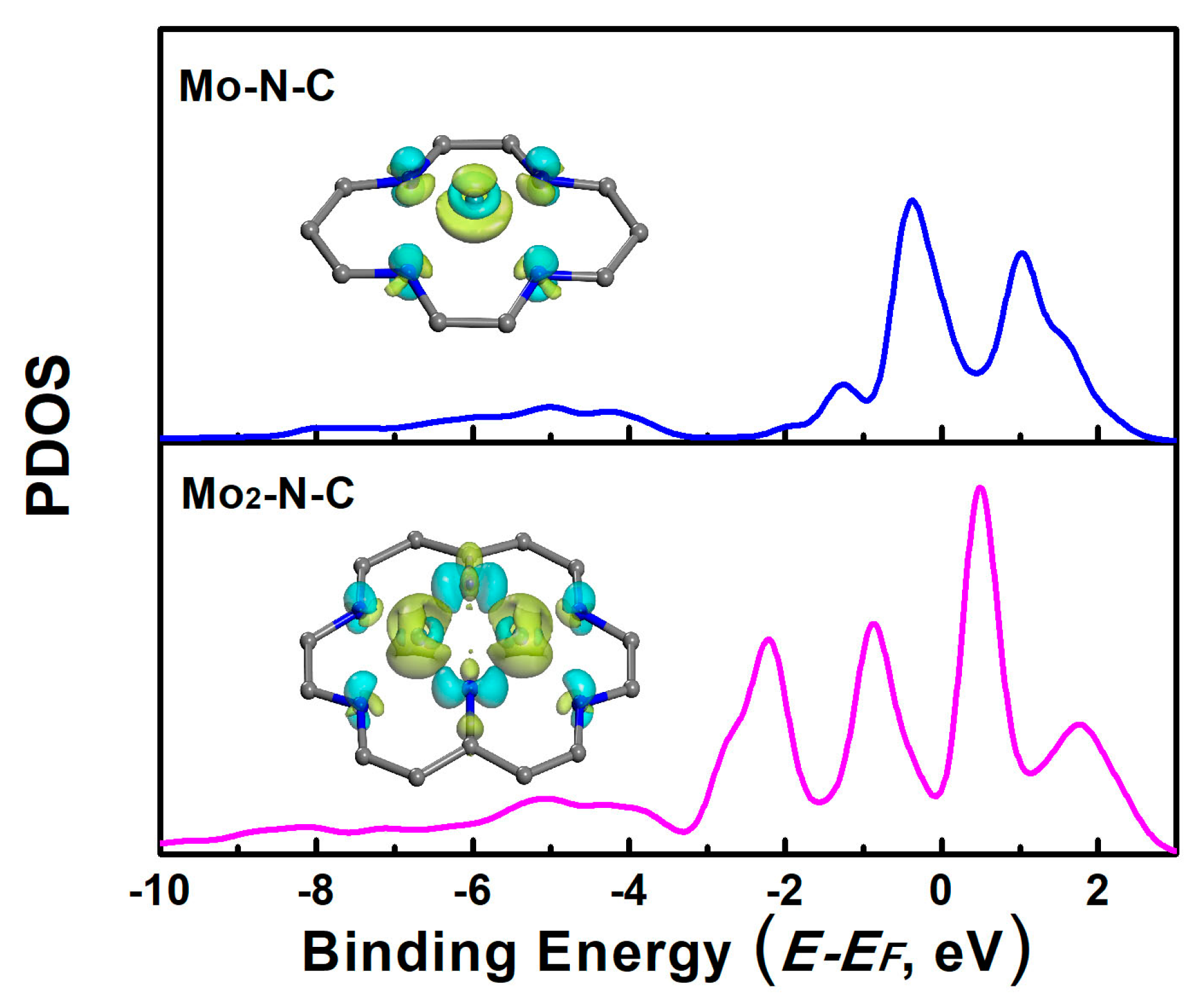
In order to further investigate the factors influencing the performance of the catalyst, we further calculated the projected density of state (PDOS) of Mo-N-C and Mo2-N-C. As shown in Figure 5, the PDOS of Mo on the Mo-N-C had a peak on the left sides of the Fermi level, and the peak was very close to the Fermi level. It could be inferred that the Mo-N-C would have an excessive interaction with the reactants. The excessive adsorption caused it to be difficult to get detached from the catalyst surface. As for the Mo2-N-C, the peak width of Mo was not sharp, and the highest peak was far away from the Fermi level. This indicated that the 4d orbital of Mo was hybridized, due to the interactions with the carrier. The shift down of the peak of the PDOS resulted in the moderate binding strength of the reactants. It could also be deduced from the charge density difference of the Mo-embedded configuration on the monolayer (insets of Figure 5). For the Mo-N-C, electrons, both assumption and depletion towards the adsorbate appeared, while for the Mo2-N-C, electrons depletion was dominant, leading to a decrease of the binding ability.
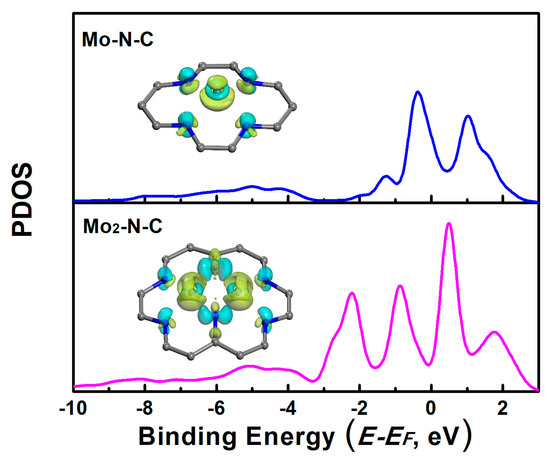
Figure 5.
Projected density of the states (PDOS) of Mo atoms on mono- and binuclear N-C catalysts. Inset: the charge density difference of the Mo adsorption configuration on Mo-N-C and Mo2-N-C catalysts (the cyan and yellow colors represent charge accumulation and depletion in the space, respectively).
Owing to the oxophilicity of the metal atom, *OH could be formed spontaneously in an aqueous environment, at applied potentials. Previous investigation has demonstrated that the pre-adsorbed OH on SAC or BAC acted as a modifying ligand to promote electrochemical activity [27]. In order to clarify this effect, PDOS of metal atoms on Mo-N-C and Mo2-N-C with pre-adsorbed OH were, thus, analyzed preliminarily (Figures S2 and S3). It could be inferred that the pre-adsorbed OH induced the shift down of peak of PDOS, under the Fermi Energy for Mo-N-C (from −0.38 eV to −0.92 eV) and Mo2-N-C (from −0.88 eV to −2.36 eV), leading to a weaker binding strength of the reactants, such as NH2*. Free energy diagrams of NRR on Mo-N-C and Mo2-N-C have shown that hydrogenation of NH2* to NH3 was a PDS, along different pathways. Therefore, Mo-N-C and Mo2-N-C with pre-adsorbed *OH might also exhibit excellent NRR activity. Moreover, in order to preliminarily explore the activity on a clean Mo surface, PDOS of the metal atoms on Mo (001) were calculated (Figure S4). It can be seen that the peak of PDOS under Fermi Energy was located at −0.37 eV, which was closer to the Fermi Energy, compared to those of Mo-N-C (−0.38 eV) and Mo2-N-C (−0.88 eV). Thus, the binding ability of NH2* might be stronger on the Mo surface and the NRR activity of Mo surface should be less favorable than those of Mo-N-C and Mo2-N-C.
2.4. Hydrogen Evolution Reaction
It is important to consider the unwanted hydrogen evolution reaction, since most current densities observed in the experiments for the NRR were hydrogen evolution reaction (HER). Therefore, the selectivity of the catalyst for NRR was evaluated by calculating the reaction free energy of HER (Figure 6) [33,34]. Results indicated that the order of HER activities for mononuclear N-C was: Rh > Cr > Fe > Mo > Zn > Ni > Cu. The order of HER activities for the binuclear N-C was: Cu > Ni > Fe > Cr > Rh > Mo > Zn. It could be seen that, for most BAC, the HER activities were also improved, accompanied by the enhancement of NRR. However, the free energy change was much smaller, especially for the Mo-based catalyst. The improved activities could also be ascribed to the down-shift of the density of states for the Mo atom. Furthermore, electrode potentials of different reaction were estimated, based on the potential determining step. Under this applied potential, all steps involving the proton-electron pairs would go downhill, on the free energy diagram, using a computational hydrogen electrode scheme. It could be seen that the applied potentials of HER for Mo-N-C (U = −0.62 V) and Mo2-N-C (U = −0.37 V) was smaller than those of NRR for Mo-N-C (U = −1.61 V) and Mo2-N-C (U = −1.05 V) (Figure S5), demonstrating that HER would go in parallel with NRR. Thus, most current densities observed in the experiments for the NRR were HER. However, for the Mo-based SAC and BAC, the free energy change in HER was smaller than that in NRR, therefore, the overall activity of NRR for Mo2-N-C should be improved.
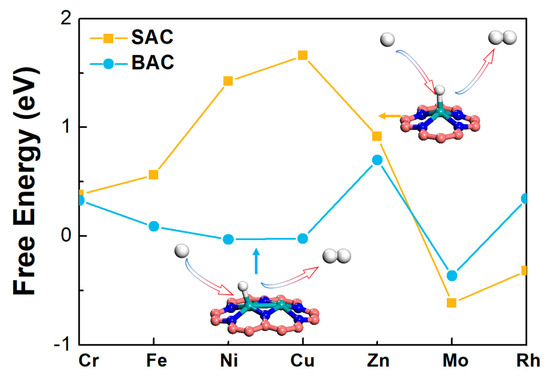
Figure 6.
The calculated Gibbs free energy changes of HER on mono- and binuclear N-C catalysts.
3. Models and Methods
All computations were implemented by means of spin-polarized density functional theory (DFT) methods, using the DMol3 code [35]. The Perdew, Burke, and Ernzerhof (PBE) exchange-correlation functional was employed [36] within a generalized gradient approximation (GGA). The double numerical plus polarization (DNP) was chosen as the base set [37]. Core treatment was adopted as the Effective Core Potentials, to conduct metal relativistic effect. Self-consistent field (SCF) calculations were performed with a convergence criterion of 2*10−5 Ha on the total energy and electronic computations. The maximum force and displacement for the geometric optimization were 0.004 Ha/Å and 0.005 Å. We chose a real-space global orbital cutoff radius as high as 4.5 Å. A smearing of 0.005 Ha to the orbital occupation was applied. Using a 5 × 5 × 1 Monkhorst-Pack, grid k-points were employed for the geometric optimization. Structural optimization was carried out without any constraints.
To model the defective N-C monolayer, we first built a supercell containing 60 carbon atoms with a vacuum of at least 15 Å in the z-direction, and then removed six carbon atoms to provide an anchoring site for the single transition metal atom, or removed ten carbon atoms for the binuclear transition metal atoms. Based on the approach described in [38], formation energy was calculated, first, by the adsorption energy of metal atoms over N-doped graphene, and then further correlated with the experimentally obtained cohesive energy. Adsorption energy was calculated according to the equation: ∆E = EM-N-C − (mEM + ENC), where EM-N-C was the total energy of M-N-C sheet; ENC was the energy of the N-doped graphene without the metal atoms; EM was the energy of the isolated metal atom and m was the number of metal atoms in the system. The Gibbs free energy change (∆G) of every basic step involving the electron/proton transferred, was calculated by using the computational hydrogen electrode (CHE) model presented by Nørskov [39]. Based on this method, the ∆G value could be determined as follows: ∆G = ∆E + ∆ZPE − T∆S + ∫CpdT, where ∆E was the electronic energy difference calculated from DFT, ∆ZPE was the change in zero-point energies, here T was the ambient temperature, Cp was the heat capacity, and ∆S was the entropy change. The thermodynamic properties of molecules in the gas- and adsorbed-phase were obtained through the vibrational frequencies.
Potential energy surfaces (PES) were obtained using semi-empirical PM6 DFT [40] molecular dynamics (MD) simulations, implemented with the open-source CP2K/QUICKSTEP code [41,42]. The choice of PM6, due to its less computational cost, allowed us to explore the long dynamics trajectories, and to recognize the intermediate states of the reaction. To sample the catalyst surface in MD calculations, NVT simulations were performed over 0.5 ns at 500 K, using time-steps of 1 fs.
4. Conclusions
In summary, through the spin-polarized DFT calculation and molecular dynamics simulation, we systematically studied the potential of doping the excess metal atoms (Cr, Ni, Fe, Cu, Zn, Mo, and Rh) on the defective N-C catalyst, for NRR. Our calculations showed that the catalytic activity of Mo-doped dual-core catalysts was much higher than that of the mononuclear and other catalysts. Hydrogenation of NH2* to NH3 was calculated to be a PDS, along the different pathways. The distal pathway was preferred for the Mo2-N-C in the NRR reaction. The boosted NRR activity was mainly due to the fact that the DOS of the Mo atom moved far away from the Fermi Energy. The electronic structure was supposed to be adjusted to get the proper binding energy to reactants. This study provided new insights into the development of robust electrocatalysts for the high-efficiency conversion of N2 into valuable chemicals.
Supplementary Materials
The following are available online, Figure S1: Different possible structures of SAC and BAC for the Mo atoms; Figure S2: PDOS of Mo atoms on OH pre-adsorbed Mo-N-C catalysts; Figure S3: PDOS of Mo atoms on OH pre-adsorbed Mo2-N-C catalysts; Figure S4: PDOS of Mo atoms on Mo (001) catalysts; Figure S5: Free-energy diagrams for the NRR on Mo-N-C (a) and Mo2-N-C (b) catalysts under different potentials via distal pathway; Table S1: The adsorption energy (Eads, eV) and the cohesive energy (Ecoh, eV) of the corresponding SAC and BAC for the Mo atoms in Figure S1; Table S2: The adsorption energy (Eads, eV) and the cohesive energy (Ecoh, eV) of the different transition metals doped on N-C nanosheets; Table S3: Calculated values of (V); Table S4: Adsorption energy (eV) of N2, NNH, and NH2 intermediates on the different N-C monolayers; Table S5: Atomic configurations and corresponding adsorption energy and free energy correction of each elementary steps along the different pathways for Mo-N-C; Table S6: Atomic configurations and corresponding adsorption energy and free energy correction of each elementary steps, along the different pathways for Mo2-N-C; Table S7: Different two-dimensional NRR electrocatalysts reported in the literature.
Author Contributions
Conceptualization and supervision, J.H.; formal analysis and investigation, R.G.; writing—original draft preparation, R.G. and M.H.; writing—review and editing, J.H. and W.Z.
Funding
This study was supported by the National Natural Science Foundation of China (21603161). We also acknowledge the National Supercomputing Center in Shenzhen for providing the computational resources and materials studio (DMol3).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Amar, I.A.; Lan, R.; Petit, C.T.G.; Tao, S. Solid-state electrochemical synthesis of ammonia: A review. J. Solid State Electrochem. 2011, 15, 1845–1860. [Google Scholar] [CrossRef]
- Honkala, K. Ammonia synthesis from first-principles calculations. Science 2005, 307, 555–558. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Licht, S.; Cui, B.; Wang, B.; Li, F.F.; Lau, J.; Liu, S. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science 2014, 345, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. New recipe produces ammonia emissions from the air, water, and sunlight. Science 2014, 345, 610. [Google Scholar] [CrossRef]
- Schlögl, R. Catalytic synthesis of ammonia—A “never-ending story”? Angew. Chem. Int. Ed. 2003, 42, 2004–2008. [Google Scholar] [CrossRef]
- Lv, C.; Yan, C.S.; Chen, G.; Ding, Y.; Sun, J.X.; Zhou, Y.S.; Yu, G. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions. Angew. Chem. Int. Ed. 2018, 57, 6073–6076. [Google Scholar] [CrossRef]
- Kuriyama, S.; Arashiba, K.; Nakajima, K.; Matsuo, Y.; Tanaka, H.; Ishii, K.; Yoshizawa, K.; Nishibayashi, Y. Catalytic transformation of dinitrogen into ammonia and hydrazine by iron-dinitrogen complexes bearing pincer ligand. Nat. Commun. 2016, 7, 12181. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient visible light nitrogen Fixation with BiOBr nanosheets of oxygen vacancies on the exposed {001} facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar] [CrossRef]
- Li, X.; Wang, W.; Jiang, D.; Sun, S.; Zhang, L.; Sun, X. Efficient solar-driven nitrogen fixation over carbon-tungstic-acid hybrids. Chem. Eur. J. 2016, 22, 13819–13822. [Google Scholar] [CrossRef]
- Liu, J.C.; Ma, X.L.; Li, Y.; Wang, Y.G.; Xiao, H.; Li, J. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 2018, 9, 1610. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, L.; Hu, L.; Chen, G.; Xin, H.; Feng, X. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential. Nat. Commun. 2018, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.F.; Delariva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.S.; Oh, S.; Wiebenga, M.H.; Hernandez, X.I.P.; et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Lin, J.; Wang, A.; Qiao, B.; Liu, X.; Yang, X.; Wang, X.; Liang, J.; Li, J.; Liu, J.; Zhang, T. Remarkable performance of Ir1/FeO(x) single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 2013, 135, 15314–15317. [Google Scholar] [CrossRef] [PubMed]
- Moses-DeBusk, M.; Yoon, M.; Allard, L.F.; Mullins, D.R.; Wu, Z.; Yang, X.; Veith, G.; Stocks, G.M.; Narula, C.K. CO oxidation on supported single Pt atoms: Experimental and ab initio density functional studies of CO interaction with Pt atom on Theta-Al2O3(010) surface. J. Am. Chem. Soc. 2013, 135, 12634–12645. [Google Scholar] [CrossRef]
- Narula, C.K.; Allard, L.F.; Stocks, G.M.; Moses-DeBusk, M. Remarkable NO oxidation on single supported platinum atoms. Sci. Rep. 2014, 4, 7238. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Alam, M.Z.; De Leon, I.; Boyd, R.W. Large optical nonlinearity of indium tin oxide in its epsilon-near-zero region. Science 2016, 352, 795–797. [Google Scholar] [CrossRef]
- Zhao, J.X.; Chen, Z.F. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: A computational study. J. Am. Chem. Soc. 2017, 139, 12480–12487. [Google Scholar] [CrossRef]
- Han, L.L.; Liu, X.J.; Chen, J.P.; Lin, R.Q.; Liu, H.X.; Lu, F.; Bak, S.; Liang, Z.X.; Zhao, S.Z.; Stavitski, E.; et al. Atomically dispersed molybdenum catalysts for high-efficiency ambient N2 fixation. Angew. Chem. Int. Ed. 2018, 58, 2321–2325. [Google Scholar] [CrossRef]
- Li, X.F.; Li, Q.K.; Cheng, J.; Liu, L.; Yan, Q.; Wu, Y.; Zhang, X.H.; Wang, Z.Y.; Qiu, Q.; Luo, Y. Conversion of dinitrogen to ammonia by FeN3-embedded graphene. J. Am. Chem. Soc. 2016, 138, 8706–8709. [Google Scholar] [CrossRef]
- Skulason, E.; Bligaard, T.; Gudmundsdottir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jonsson, H.; Norskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef]
- Montoya, J.H.; Tsai, C.; Vojvodic, A.; Norskov, J.K. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem 2015, 8, 2180–2186. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, K.R.; Wang, Z.; Yan, X.; Cao, S.; Ye, Y.; Dong, Q.; Zhang, X.; Thorne, J.E.; Jin, L.; et al. Stable iridium dinuclear heterogeneous catalysts supported on metal-oxide substrate for solar water oxidation. Proc. Natl. Acad. Sci. USA 2018, 115, 2902–2907. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, H.; Chen, Y.; Zhu, J.; Gao, L.; Jin, Z.; Ge, J.; Jiang, Z.; Chen, S.; Liu, C.; et al. Identification of binuclear Co2N5 active sites for oxygen reduction reaction with more than one magnitude higher activity than single atom CoN4 Site. Nano Energy 2018, 46, 396–403. [Google Scholar] [CrossRef]
- Yan, H.; Lin, Y.; Wu, H.; Zhang, W.; Sun, Z.; Cheng, H.; Liu, W.; Wang, C.; Li, J.; Huang, X.; et al. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 2017, 8, 1070. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Liu, W.; Chang, C.; Tang, H.; Li, Z.; Chen, W.; Jia, C.; Yao, T.; Wei, S.; et al. Design of N-coordinated dual-metal sites: A stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef]
- Ma, X.L.; Liu, J.C.; Xiao, H.; Li, J. Surface single-cluster catalyst for N2-to-NH3 thermal conversion. J. Am. Chem. Soc. 2018, 140, 46–49. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.; Zhao, J. Computational screening of a single transition metal atom supported on the C2N monolayer for electrochemical ammonia synthesis. Phys. Chem. Chem. Phys. 2018, 20, 12835–12844. [Google Scholar] [CrossRef]
- Pignedoli, C.A.; Laino, T.; Treier, M.; Fasel, R.; Passerone, D. A simple approach for describing metal-supported cyclohexaphenylene dehydrogenation. Eur. Phys. J. B 2010, 75, 65–70. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, 23–26. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P (001) surface: The importance of ensemble effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar] [CrossRef]
- Pasti, I.A.; Jovanovic, A.; Dobrota, A.S.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Atomic adsorption on graphene with a single vacancy: Systematic DFT study through the periodic table of elements. Phys. Chem. Chem. Phys. 2018, 20, 858–865. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Logadottir, A.; Nørskov, J.K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 2005, 319, 178–184. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Hutter, J.; Iannuzzi, M.; Schiffmann, F.; VandeVondele, J. CP2K: Atomistic simulations of condensed matter systems. WIREs Comput. Mol. Sci. 2014, 4, 15–25. [Google Scholar] [CrossRef]
- VandeVondele, J.; Krack, M.; Mohamed, F.; Parrinello, M.; Chassaing, T.; Hutter, J. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 2005, 167, 103–128. [Google Scholar] [CrossRef]
Sample Availability: not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).