Synthesis of Tris-pillar[5]arene and Its Association with Phenothiazine Dye: Colorimetric Recognition of Anions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Tris-pillar[5]arene
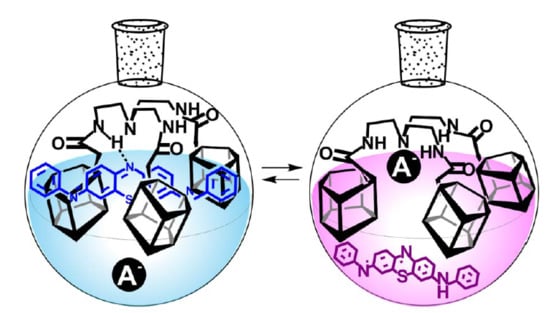
2.2. Study on the Interaction of Tris-pillar[5]arene 4 with PhTz
2.3. Investigation of Complex PhTz:4 Interaction with Anions
3. Materials and Methods
3.1. General Experimental Information
3.2. Synthesis of Tris-pillar[5]arene 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fréchet Hallberg, G.R. Agricultural chemicals in ground water: Extent and implications. Am. J. Alternative Agr. 1987, 2, 3–15. [Google Scholar] [CrossRef]
- Omar, S.A.; Webb, A.J.; Lundberg, J.O.; Weitzberg, E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J. Intern. Med. 2016, 279, 315–336. [Google Scholar] [CrossRef]
- Sorvin, M.; Belyakova, S.; Stoikov, I.; Shamagsumova, R.; Evtugyn, G. Solid-contact potentiometric sensors and multisensors based on polyaniline and thiacalixarene receptors for the analysis of some beverages and alcoholic drinks. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Sivaraman, G.; Iniya, M.; Siva, A.; Chellappa, D. Aminobenzohydrazide based colorimetric and ‘turn-on’ fluorescence chemosensor for selective recognition of fluoride. Anal. Chim. Acta 2015, 876, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kim, J.; Liu, Z.; Jo, S.; Pak, Y.L.; Swamy, K.M.K.; Yoon, J. Selective fluorescent and colorimetric recognition of cyanide via altering hydrogen bonding interaction in aqueous solution and its application in bioimaging. Dyes Pigm. 2016, 128, 256–262. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F. Synthesis of bisimidazole derivatives for selective sensing of fluoride ion. Molecules 2017, 22, 1519. [Google Scholar] [CrossRef] [PubMed]
- Langton, M.J.; Serpell, C.J.; Beer, P.D. Anion recognition in water: Recent advances from a supramolecular and macromolecular perspective. Angew. Chem. Int. Ed. 2016, 55, 1974–1987. [Google Scholar] [CrossRef] [PubMed]
- Vavilova, A.A.; Stoikov, I.I. p-tert-Butylthiacalix[4]arenes functionalized by N-(4’-nitrophenyl) acetamide and N, N-diethylacetamide fragments: Synthesis and binding of anionic guests. Beilstein J. Org. Chem. 2017, 13, 1940–1949. [Google Scholar] [CrossRef]
- Vavilova, A.A.; Nosov, R.V.; Stoikov, I.I. Selective fluoride ion recognition by a thiacalix[4]arene receptor containing N-(4-nitrophenyl) acetamide and 1-amidoanthraquinone fragments. Mendeleev Comm. 2016, 6, 508–510. [Google Scholar] [CrossRef]
- Saha, I.; Lee, J.T.; Lee, C.H. Recent Advancements in calix[4]pyrrole-based anion-receptor chemistry. Eur. J. Org. Chem. 2015, 2015, 3859–3885. [Google Scholar] [CrossRef]
- Zhu, H.; Shi, B.; Chen, K.; Wei, P.; Xia, D.; Mondal, J.H.; Huang, F. Cyclo[4]carbazole, an Iodide Anion Macrocyclic Receptor. Org. Lett. 2016, 18, 5054–5057. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Stoikov, I.I. Amide-functionalized pillar[5]arenes as a novel class of macrocyclic receptors for the sensing of H2PO4− anion. Chem. Commun. 2016, 52, 12462–12465. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Padnya, P.L.; Evtugyn, V.G.; Mukhametzyanov, T.A.; Khannanov, A.A.; Kutyreva, M.P.; Stoikov, I.I. Synthesis and properties of chiral nanoparticles based on (p S)-and (p R)-decasubstituted pillar[5]arenes containing secondary amide fragments. RSC Adv. 2016, 6, 9124–9131. [Google Scholar] [CrossRef]
- Smolko, V.; Shurpik, D.; Evtugyn, V.; Stoikov, I.; Evtugyn, G. Organic acid and DNA sensing with electrochemical sensor based on carbon black and pillar[5]arene. Electroanalysis 2016, 28, 1391–1400. [Google Scholar] [CrossRef]
- Stoikova, E.E.; Sorvin, M.I.; Shurpik, D.N.; Budnikov, H.C.; Stoikov, I.I.; Evtugyn, G.A. Solid-contact potentiometric sensor based on polyaniline and unsubstituted pillar[5]arene. Electroanalysis 2015, 27, 440–449. [Google Scholar] [CrossRef]
- Yakimova, L.S.; Shurpik, D.N.; Gilmanova, L.H.; Makhmutova, A.R.; Rakhimbekova, A.; Stoikov, I.I. Highly selective binding of methyl orange dye by cationic water-soluble pillar[5]arenes. Org. Biomol. Chem. 2016, 14, 4233–4238. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Zheng, F.; Liu, L.; Mao, P.P.; Zhang, Y.M.; Yao, H.; Wei, T.B. Efficient sensing of fluoride ions in water using a novel water soluble self-assembled supramolecular sensor based on pillar[5]arene. RSC Adv. 2016, 6, 111928–111933. [Google Scholar] [CrossRef]
- Bojtár, M.; Kozma, J.; Szakács, Z.; Hessz, D.; Kubinyi, M.; Bitter, I. Pillararene-based fluorescent indicator displacement assay for the selective recognition of ATP. Sens. Actuators B Chem. 2017, 248, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Pei, Y.; Wen, J.; Pei, Z. Recent advances in pillar [n] arenes: Synthesis and applications based on host–guest interactions. Chem. Commun. 2016, 52, 9316–9326. [Google Scholar] [CrossRef]
- Nosov, R.V.; Stoikov, I.I. Pentakis-amidothiacalix [4] arene stereoisomers: Synthesis and effect of central core conformation on their aggregation properties. Macroheterocycles 2015, 8, 120–127. [Google Scholar] [CrossRef]
- Nosov, R.; Padnya, P.; Shurpik, D.; Stoikov, I. Synthesis of water-soluble amino functionalized multithiacalix[4]arene via quaternization of tertiary amino groups. Molecules 2018, 23, 1117. [Google Scholar] [CrossRef] [PubMed]
- Shurpik, D.N.; Stoikov, I.I. Covalent assembly of tris-pillar[5]arene. Russ. J. Gen. Chem. 2016, 86, 752–755. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Yakimova, L.S.; Gorbachuk, V.V.; Sevastyanov, D.A.; Padnya, P.L.; Bazanova, O.B.; Stoikov, I.I. Hybrid multicyclophanes based on thiacalix[4]arene and pillar[5]arene: Synthesis and influence on the formation of polyaniline. Org. Chem. Front. 2018, 5, 2780–2786. [Google Scholar] [CrossRef]
- Jowett, L.A.; Ricci, A.; Wu, X.; Howe, E.N.W.; Gale, P.A. Investigating the influence of steric hindrance on selective anion transport. Molecules 2019, 24, 1278. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Das, G. A selective fluoride encapsulated neutral tripodal receptor capsule: Solvatochromism and solvatomorphism. Chem. Commun. 2011, 47, 4983–4985. [Google Scholar] [CrossRef]
- Yang, K.; Wen, J.; Chao, S.; Liu, J.; Yang, K.; Pei, Y.; Pei, Z. A supramolecular photosensitizer system based on the host–guest complexation between water-soluble pillar[6]arene and methylene blue for durable photodynamic therapy. Chem. Commun. 2018, 54, 5911–5914. [Google Scholar] [CrossRef]
- Liu, X.; Weinert, Z.J.; Sharafi, M.; Liao, C.; Li, J.; Schneebeli, S.T. Regulating molecular recognition with C-Shaped strips attained by chirality-assisted synthesis. Angew. Chem. Int. Ed. 2015, 54, 12772–12776. [Google Scholar] [CrossRef]
- Wainwright, M.; Grice, N.J.; Pye, L.E. Phenothiazine photosensitizers: Part 2. 3, 7-Bis (arylamino) phenothiazines. Dyes Pigm. 1999, 42, 45–51. [Google Scholar] [CrossRef]
- Pauliukaite, R.; Ghica, M.E.; Barsan, M.M.; Brett, C.M. Phenazines and polyphenazines in electrochemical sensors and biosensors. Anal. Lett. 2010, 43, 1588–1608. [Google Scholar] [CrossRef]
- Khadieva, A.I.; Gorbachuk, V.V.; Evtugyn, G.A.; Belyakova, S.V.; Latypov, R.R.; Drobyshev, S.V.; Stoikov, I.I. Phenyliminophenothiazine based self-organization of polyaniline nanowires and application as redox probe in electrochemical sensors. Sci. Rep. 2019, 9, 417. [Google Scholar] [CrossRef]
- Wainwright, M.; McLean, A. Rational design of phenothiazinium derivatives and photoantimicrobial drug discovery. Dyes Pigm. 2017, 136, 590–600. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, F.; Zhao, Y.; Liu, Y.; Zhu, L. Phenothiazine-cyanine-functionalized upconversion nanoparticles for LRET and colorimetric sensing of cyanide ions in water samples. J. Photochem. Photobiol. A Chem. 2016, 319, 53–61. [Google Scholar] [CrossRef]
- Kuzin, Y.; Ivanov, A.; Evtugyn, G.; Hianik, T. Voltammetric detection of oxidative DNA damage based on interactions between polymeric dyes and DNA. Electroanalysis 2016, 28, 2956–2964. [Google Scholar] [CrossRef]
- Othman, A.B.; Lee, Y.H.; Ohto, K.; Abidi, R.; Kim, Y.; Vicens, J. Multi-calixarenes with multidentate coordination sites. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 187–191. [Google Scholar] [CrossRef]
- Kaur, M.; Cho, M.J.; Choi, D.H. A phenothiazine-based “naked-eye” fluorescent probe for the dual detection of Hg2+ and Cu2+: Application as a solid state sensor. Dyes Pigm. 2016, 125, 1–7. [Google Scholar] [CrossRef]
- Weng, J.; Mei, Q.; Zhang, B.; Jiang, Y.; Tong, B.; Fan, Q.; Ling, Q.; Huang, W. Multi-functional fluorescent probe for Hg2+, Cu2+ and ClO− based on a pyrimidin-4-yl phenothiazine derivative. Analyst 2013, 138, 6607–6616. [Google Scholar] [CrossRef]
- Ghanadzadeh Gilani, A.; Dezhampanah, H.; Poormohammadi-Ahandani, Z. A comparative spectroscopic study of thiourea effect on the photophysical and molecular association behavior of various phenothiazine dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 179, 132–143. [Google Scholar] [CrossRef]
- Bindfit v0.5, Open Data Fit. Available online: http://supramolecular.org/bindfit/ (accessed on 8 April 2019).
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadieva, A.; Gorbachuk, V.; Shurpik, D.; Stoikov, I. Synthesis of Tris-pillar[5]arene and Its Association with Phenothiazine Dye: Colorimetric Recognition of Anions. Molecules 2019, 24, 1807. https://doi.org/10.3390/molecules24091807
Khadieva A, Gorbachuk V, Shurpik D, Stoikov I. Synthesis of Tris-pillar[5]arene and Its Association with Phenothiazine Dye: Colorimetric Recognition of Anions. Molecules. 2019; 24(9):1807. https://doi.org/10.3390/molecules24091807
Chicago/Turabian StyleKhadieva, Alena, Vladimir Gorbachuk, Dmitriy Shurpik, and Ivan Stoikov. 2019. "Synthesis of Tris-pillar[5]arene and Its Association with Phenothiazine Dye: Colorimetric Recognition of Anions" Molecules 24, no. 9: 1807. https://doi.org/10.3390/molecules24091807
APA StyleKhadieva, A., Gorbachuk, V., Shurpik, D., & Stoikov, I. (2019). Synthesis of Tris-pillar[5]arene and Its Association with Phenothiazine Dye: Colorimetric Recognition of Anions. Molecules, 24(9), 1807. https://doi.org/10.3390/molecules24091807