Concentration-Dependent Effects of N-3 Long-Chain Fatty Acids on Na,K-ATPase Activity in Human Endothelial Cells
Abstract
1. Introduction
2. Results and Discussion
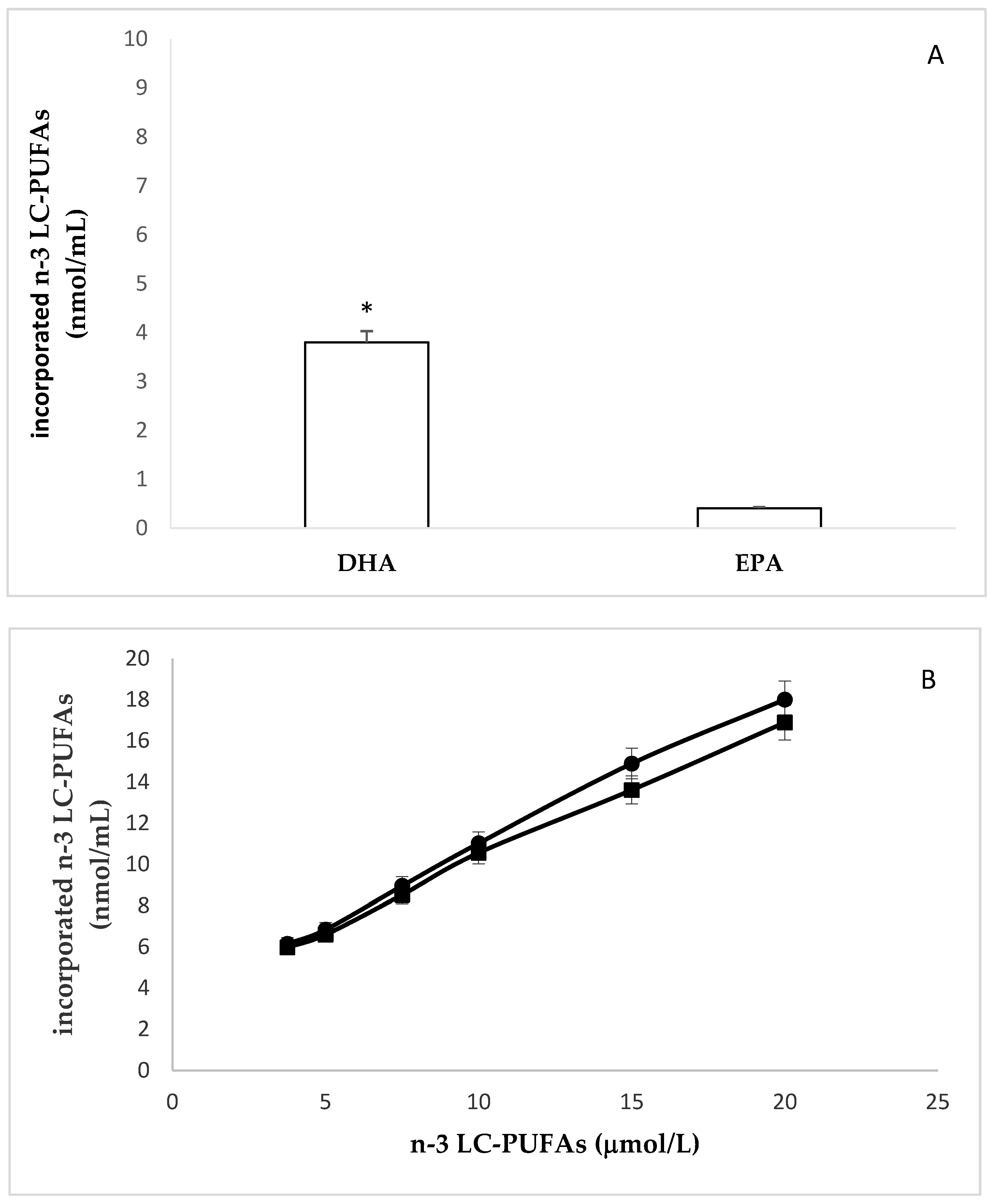
2.1. Incorporation of N-3 LC-PUFA in Confluent HMEC
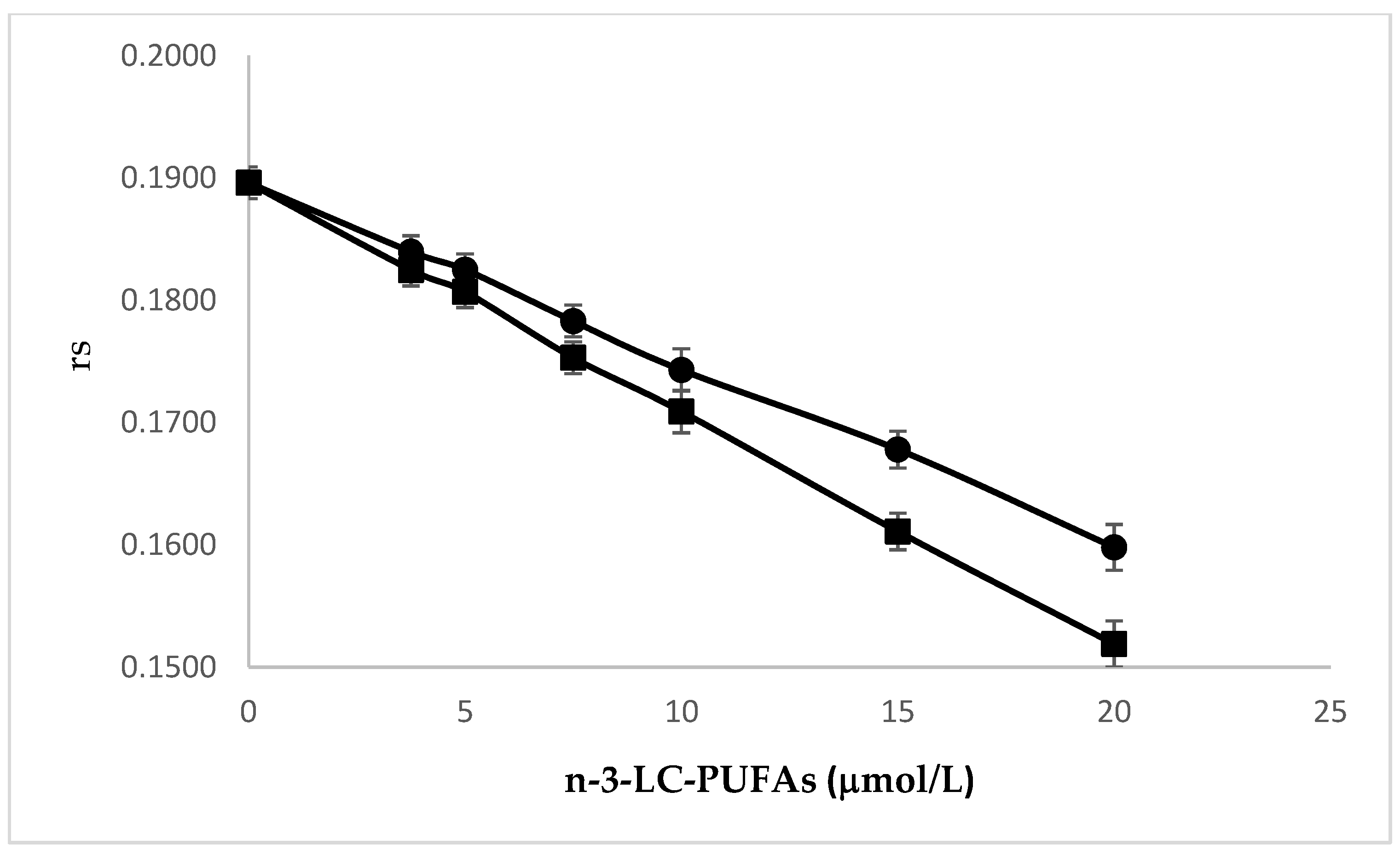
2.2. Effects of N-3 LC-PUFA Incorporation on Membrane Fluidity in HMEC
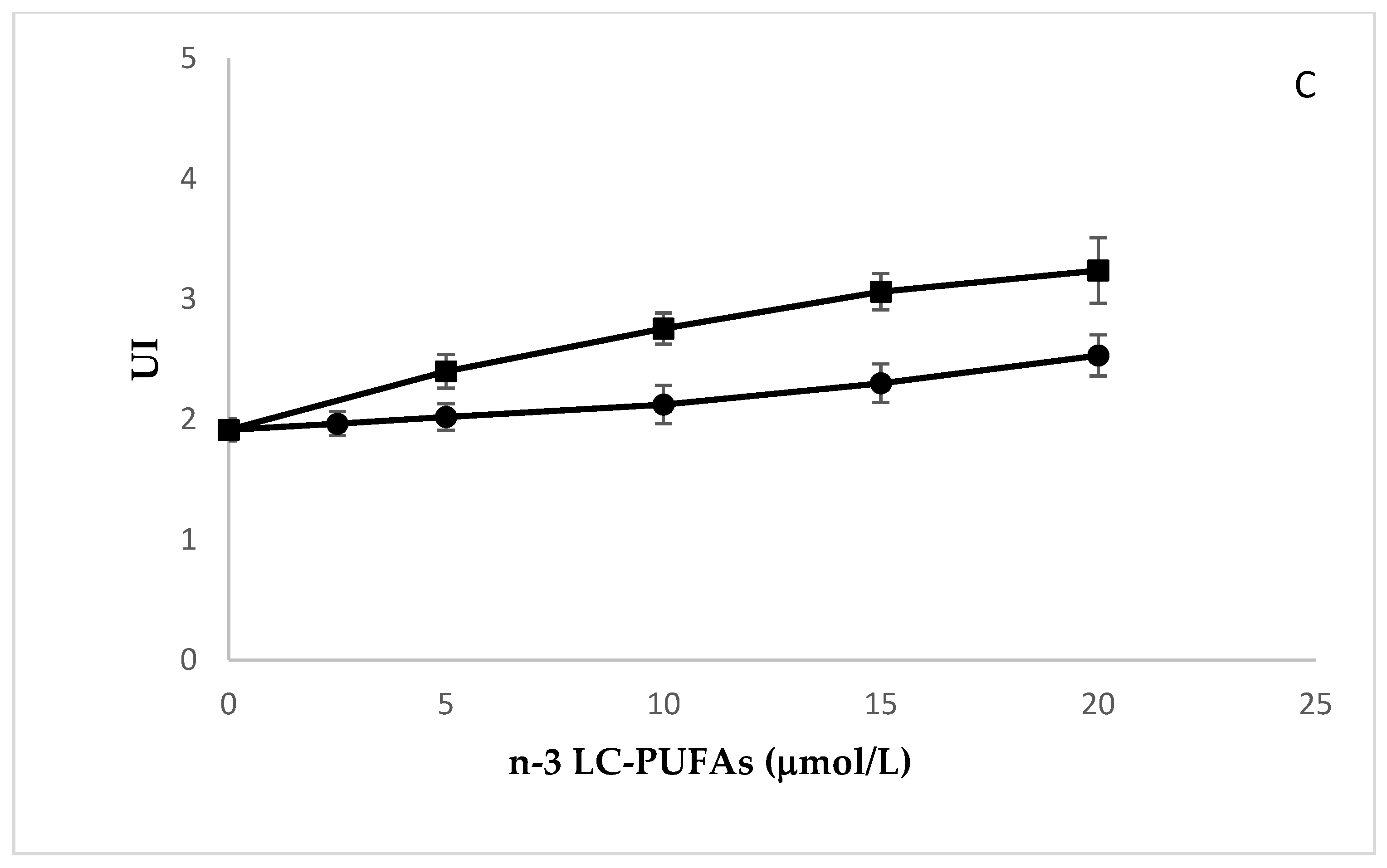
2.3. Effects of N-3 LC-PUFA Incorporation on Peroxidation Potential in HMEC
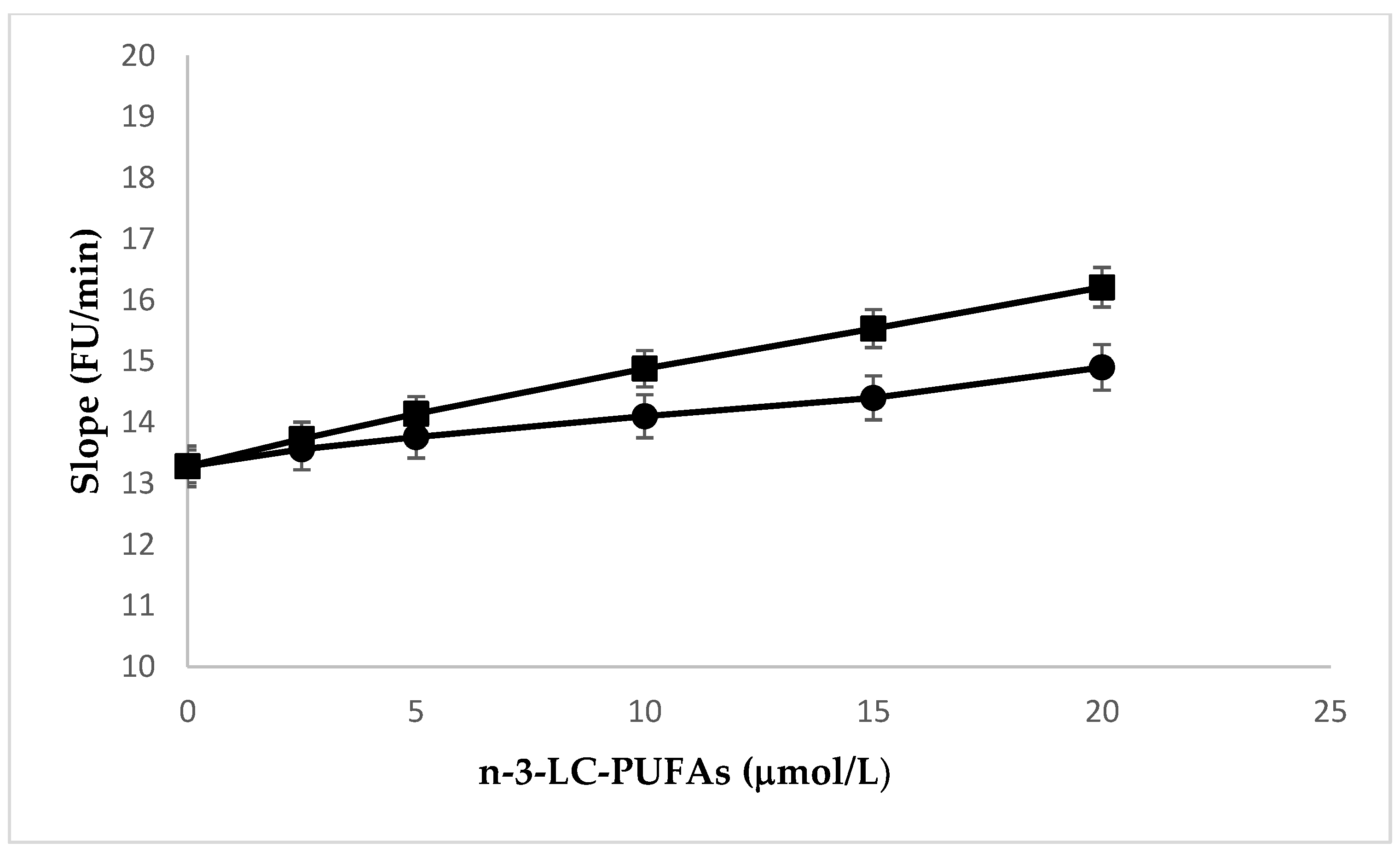
2.4. Effects of N-3 LC-PUFA Incorporation on Na,K-ATPase
3. Materials and Methods
3.1. Cell Culture
3.2. Fatty Acids Analysis
3.3. Membrane Fluidity and Peroxidation Potential
3.4. Na,K-ATPase Activity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marı́n, J.; Redondo, J. Vascular sodium pump: Endothelial modulation and alterations in some pathological processes and aging. Pharmacol. Ther. 1999, 84, 249–271. [Google Scholar] [CrossRef]
- Cornelius, F.; Habeck, M.; Kanai, R.; Toyoshima, C.; Karlish, S.J.D. General and specific lipid–protein interactions in Na,K-ATPase. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V.; Krivoi, I.I. Specialized Functional Diversity and Interactions of the Na,K-ATPase. Front. Physiol. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Maigaard, S.; Forman, A.; Andersson, K.-E. Digoxin inhibition of relaxation induced by prostacyclin and vasoactive intestinal polypeptide in small human placental arteries. Placenta 1985, 6, 435–443. [Google Scholar] [CrossRef]
- Marín, J.; Sánchez-Ferrer, C.F.; Salaices, M. Effects of ouabain on isolated cerebral and femoral arteries of the cat: a functional and biochemical study. Br. J. Pharmacol. 1988, 93, 43–52. [Google Scholar] [CrossRef]
- Vassalle, M. Contribution of the Na+/K+-pump to the membrane potential. Experientia 1987, 43, 1135–1140. [Google Scholar] [CrossRef]
- Fransen, P.; Hendrickx, J.; Brutsaert, D.L.; Sys, S.U. Distribution and role of Na+/K+-ATPase in endocardial endothelium. Cardiovasc. Res. 2001, 52, 487–499. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, M.Y.; Cavalli, M.; Chen, L.; Berra-Romani, R.; Balke, C.W.; Bianchi, G.; Ferrari, P.; Hamlyn, J.M.; Iwamoto, T.; et al. Sodium pump α2 subunits control myogenic tone and blood pressure in mice. J. Physiol. 2005, 569, 243–256. [Google Scholar] [CrossRef]
- Colussi, G.; Catena, C.; Novello, M.; Bertin, N.; Sechi, L.A. Impact of omega-3 polyunsaturated fatty acids on vascular function and blood pressure: Relevance for cardiovascular outcomes. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 191–200. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R. Omega-3 fatty acid supplementation and cardiovascular disease risk: Glass half full or time to nail the coffin shut? Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 Fatty Acids and Cardiovascular Disease: Are There Benefits? Curr. Treat. Options Cardiovasc. Med. 2016, 18. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.P.; Aggarwal, K.K.; Zhang, P.-Y. Omega-3 fatty acids and cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 441–445. [Google Scholar] [PubMed]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Balakumar, P.; Taneja, G. Fish oil and vascular endothelial protection: Bench to bedside. Free Radic. Biol. Med. 2012, 53, 271–279. [Google Scholar] [CrossRef]
- Mayol, V.; Duran, M.J.; Gerbi, A.; Dignat-George, F.; Lévy, S.; Sampol, J.; Maixent, J.M. Cholesterol and omega-3 fatty acids inhibit Na, K-ATPase activity in human endothelial cells. Atherosclerosis 1999, 142, 327–333. [Google Scholar] [CrossRef]
- Singh, T.; Garg, S.; Mishra, S. Evaluation of effects of eicosapentaenoic acid on Na + -K + -ATPase in sheep pulmonary artery. Hum. Exp. Toxicol. 2012, 31, 579–587. [Google Scholar] [CrossRef]
- Soltoff, S.P.; Mandel, L.J. Active ion transport in the renal proximal tubule. II. Ionic dependence of the Na pump. J. Gen. Physiol. 1984, 84, 623–642. [Google Scholar] [CrossRef]
- Ewart, H.S.; Klip, A. Hormonal regulation of the Na + -K +-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am. J. Physiol. Physiol. 1995, 269, C295–C311. [Google Scholar] [CrossRef]
- Therien, A.G.; Blostein, R. Mechanisms of sodium pump regulation. Am. J. Physiol. Physiol. 2000, 279, C541–C566. [Google Scholar] [CrossRef]
- Cazzola, R.; Rondanelli, M.; Russo-Volpe, S.; Ferrari, E.; Cestaro, B. Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J. Lipid Res. 2004, 45, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Miranda-Merchak, A.; Valenzuela Grau, R.; Bachler, J.P.; Vergara, L. Modulation of (Na,K)-ATPase activity by membrane fatty acid composition: therapeutic implications in human hypertension. Clin. Exp. Hypertens. 2014, 36, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Yamasaki, H.; Yazawa, K.; Masumura, S. Effects of eicosapentaenoic acid and docosahexaenoic acid on plasma membrane fluidity of aortic endothelial cells. Lipids 1999, 34, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef]
- Simonetto, M.; Infante, M.; Sacco, R.L.; Rundek, T.; Della-Morte, D. A Novel Anti-Inflammatory Role of Omega-3 PUFAs in Prevention and Treatment of Atherosclerosis and Vascular Cognitive Impairment and Dementia. Nutrients 2019, 11, 2279. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Kinnun, J.J.; Leng, X.; Williams, J.A.; Wassall, S.R. How polyunsaturated fatty acids modify molecular organization in membranes: Insight from NMR studies of model systems. Biochim. Biophys. Acta Biomembr. 2015, 1848, 211–219. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n−3 and n−6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Bonafini, S.; Fava, C. Omega-3 fatty acids and cytochrome P450-derived eicosanoids in cardiovascular diseases: Which actions and interactions modulate hemodynamics? Prostaglandins Other Lipid Mediat. 2017, 128–129, 34–42. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Catalá, A. Five decades with polyunsaturated Fatty acids: chemical synthesis, enzymatic formation, lipid peroxidation and its biological effects. J. Lipids 2013, 2013, 710290. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, R.; Rondanelli, M.; Trotti, R.; Cestaro, B. Effects of weight loss on erythrocyte membrane composition and fluidity in overweight and moderately obese women. J. Nutr. Biochem. 2011, 22, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.E.; Reed, D.J. Radical-induced inactivation of kidney Na+,K+-ATPase: Sensitivity to membrane lipid peroxidation and the protective effect of vitamin E. Arch. Biochem. Biophys. 1990, 281, 96–105. [Google Scholar] [CrossRef]
- Héliès-Toussaint, C.; Gambert, S.; Roller, P.; Tricot, S.; Lacour, B.; Grynberg, A. Lipid metabolism in human endothelial cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.R.; Andresen, T.L.; Feldborg, L.N.; Duelund, L.; Ipsen, J.H. Understanding detergent effects on lipid membranes: a model study of lysolipids. Biophys. J. 2010, 98, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.T. Cholesterol enrichment inhibits Na+/K+ pump in endothelial cells. Atherosclerosis 1994, 110, 251–257. [Google Scholar] [CrossRef]
- Niki, E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990, 186, 100–108. [Google Scholar]
- Pinchuk, I.; Lichtenberg, D. Analysis of the kinetics of lipid peroxidation in terms of characteristic time-points. Chem. Phys. Lipids 2014, 178, 63–76. [Google Scholar] [CrossRef]
- Cazzola, R.; Cassani, E.; Barichella, M.; Cestaro, B. Impaired fluidity and oxidizability of HDL hydrophobic core and amphipathic surface in dyslipidemic men. Metabolism. 2013, 62. [Google Scholar] [CrossRef]
- Kurauchi, Y.; Hisatsune, A.; Seki, T.; Katsuki, H. Na+, K+-ATPase dysfunction causes cerebrovascular endothelial cell degeneration in rat prefrontal cortex slice cultures. Brain Res. 2016, 1644, 249–257. [Google Scholar] [CrossRef]
- Liu, W.; Xie, X.; Liu, M.; Zhang, J.; Liang, W.; Chen, X. Serum ω-3 Polyunsaturated Fatty Acids and Potential Influence Factors in Elderly Patients with Multiple Cardiovascular Risk Factors. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Casati, S.; Ottria, R.; Ciuffreda, P.; Jeanette, A.M.M. N6-Isopentenyladenosine and its Analogue N6-Benzyladenosine Induce Cell Cycle Arrest and Apoptosis in Bladder Carcinoma T24 Cells. Anticancer. Agents Med. Chem. 2013, 13, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Cazzola, R.; Cestaro, B. Red wine polyphenols protect n-3 more than n-6 polyunsaturated fatty acid from lipid peroxidation. Food Res. Int. 2011, 44, 3065–3071. [Google Scholar] [CrossRef]
- Cazzola, R.; Viani, P.; Allevi, P.; Cighetti, G.; Cestaro, B. pH sensitivity and plasma stability of liposomes containing N-stearoylcysteamine. Biochim. Biophys. Acta Biomembr. 1997, 1329. [Google Scholar] [CrossRef]
- Royall, J.A.; Ischiropoulos, H. Evaluation of 2’,7’-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem. Biophys. 1993, 302, 348–355. [Google Scholar] [CrossRef]
- Raccah, D.; Dadoun, F.; Coste, T.; Vague, P. Decreased Na/K ATPase ouabain binding sites in red blood cells of patients with insulin-dependent diabetes and healthy north African control subjects: relationship with diabetic neuropathy. Horm. Metab. Res. 1996, 28, 128–132. [Google Scholar] [CrossRef]
- LOWRY, O.H.; ROSEBROUGH, N.J.; FARR, A.L.; RANDALL, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar]
- Rimm, E.B.; Appel, L.J.; Chiuve, S.E.; Djoussé, L.; Engler, M.B.; Kris-Etherton, P.M.; Mozaffarian, D.; Siscovick, D.S.; Lichtenstein, A.H. Seafood Long-Chain n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: A Science Advisory From the American Heart Association. Circulation 2018, 138. [Google Scholar] [CrossRef] [PubMed]
- Preston Mason, R. New Insights into Mechanisms of Action for Omega-3 Fatty Acids in Atherothrombotic Cardiovascular Disease. Curr. Atheroscler. Rep. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazzola, R.; Della Porta, M.; Castiglioni, S.; Pinotti, L.; Maier, J.A.M.; Cestaro, B. Concentration-Dependent Effects of N-3 Long-Chain Fatty Acids on Na,K-ATPase Activity in Human Endothelial Cells. Molecules 2020, 25, 128. https://doi.org/10.3390/molecules25010128
Cazzola R, Della Porta M, Castiglioni S, Pinotti L, Maier JAM, Cestaro B. Concentration-Dependent Effects of N-3 Long-Chain Fatty Acids on Na,K-ATPase Activity in Human Endothelial Cells. Molecules. 2020; 25(1):128. https://doi.org/10.3390/molecules25010128
Chicago/Turabian StyleCazzola, Roberta, Matteo Della Porta, Sara Castiglioni, Luciano Pinotti, Jeanette A.M. Maier, and Benvenuto Cestaro. 2020. "Concentration-Dependent Effects of N-3 Long-Chain Fatty Acids on Na,K-ATPase Activity in Human Endothelial Cells" Molecules 25, no. 1: 128. https://doi.org/10.3390/molecules25010128
APA StyleCazzola, R., Della Porta, M., Castiglioni, S., Pinotti, L., Maier, J. A. M., & Cestaro, B. (2020). Concentration-Dependent Effects of N-3 Long-Chain Fatty Acids on Na,K-ATPase Activity in Human Endothelial Cells. Molecules, 25(1), 128. https://doi.org/10.3390/molecules25010128







