Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications
Abstract
:1. Introduction
2. Metal Organic Frameworks
2.1. Biological Metal Organic Frameworks (BioMOFs)
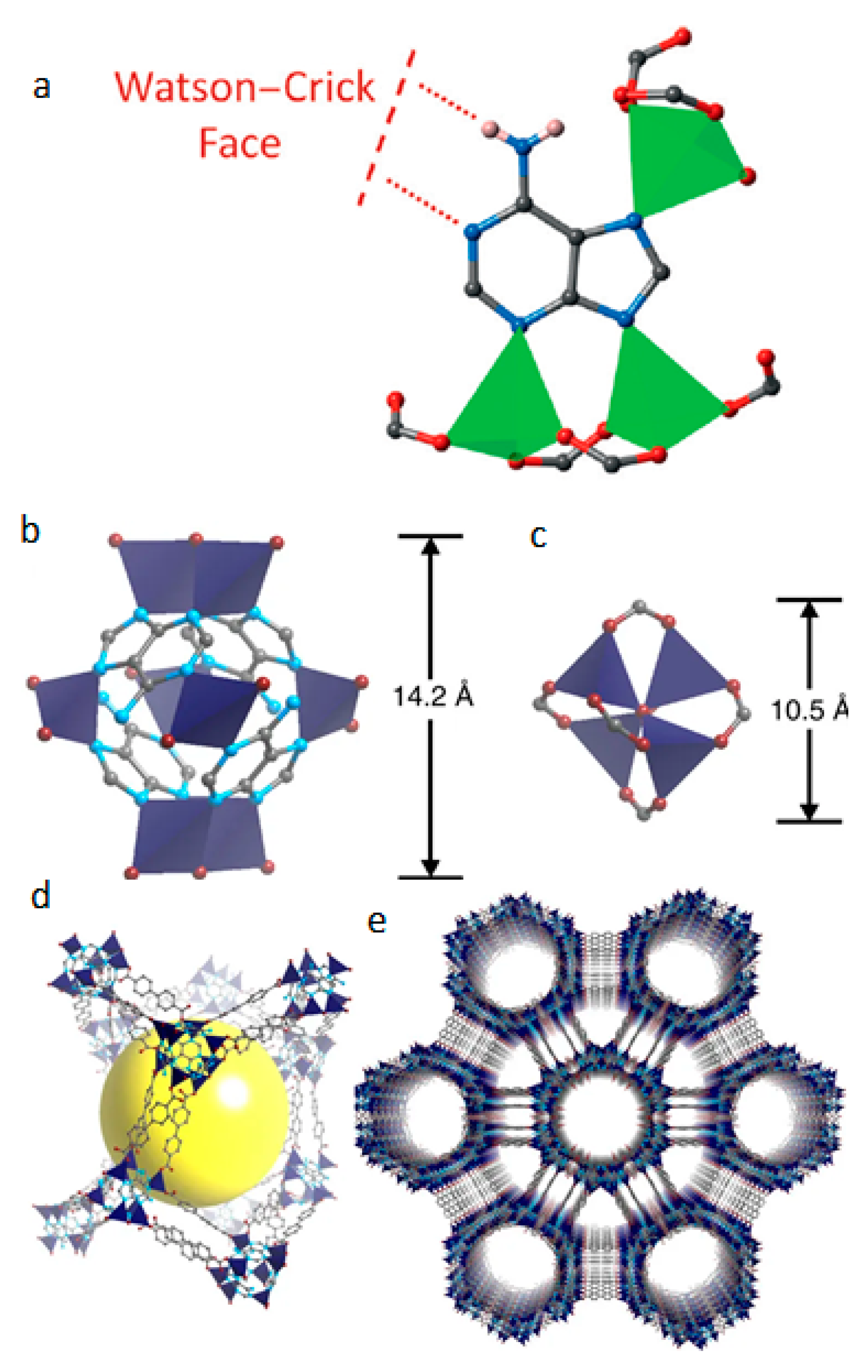
2.2. Metal Organic Frameworks (MOFs) for Biomedical Applications
3. Polymer/MOF Nanocomposites
3.1. Non-Covalent Attachment
3.2. Covalent Attachment
3.2.1. “Grafting to” Approaches
3.2.2. “Grafting from” Approaches
3.3. Polymer Coordination to Metal Ions
3.4. Encapsulation of MOF into Polymers
3.5. Other Strategies
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate-polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nanotechnol. 2008, 3, 327–331. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, characterization, propertles, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005, 5, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional nanomaterials for biomedical applications: Emerging trends and future prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- McKinlay, A.C.; Morris, R.E.; Horcajada, P.; Ferey, G.; Gref, R.; Couvreur, P.; Serre, C. BioMOFs: Metal–organic frameworks for biological and medical applications. Angew. Chem. Int. Ed. Engl. 2010, 49, 6260–6266. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of metal–organic frameworks: On the road to in vivo efficacy in biomedicine. Adv. Mater. 2018, 30, 1707365. [Google Scholar] [CrossRef]
- Wu, M.-X.; Yang, Y.-W. Metal–Organic Framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-Triggered drug release from a porous Zinc−adeninate metal−organic framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous metal organic frameworks as hybrid polymer–metal composites for drug delivery and biomedical applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A. Metal–organic-frameworks for biomedical applications in drug delivery, and as MRI contrast agents. J. Biomed. Mater. Res. Part A 2017, 105, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal–organic frameworks: A rapidly growing class of versatile nanoporous materials. Adv. Mater. 2011, 23, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Naka, K. Metal Organic Framework (MOF). In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1233–1238. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a Metal–organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, H.C. Recent progress in the synthesis of Metal–organic frameworks. Sci. Technol. Adv. Mater. 2015, 16, 054202. [Google Scholar] [CrossRef]
- Chui, S.S.; Lo, S.M.; Charmant, J.P.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous Metal–organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Alshammari, A.; Jiang, Z.; Cordova, K.E. Metal organic frameworks as emerging photocatalysts. In Semiconductor Photocatalysis: Materials, Mechanisms and Applications; Cao, W., Ed.; InTech: London, UK, 2016; pp. 302–341. [Google Scholar]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.O.; Hupp, J.T. Metal–organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrows, A.D. The Chemistry of Metal–Organic Frameworks. Synthesis, Characterization, and Applications, 2 Volumes. Edited by Stefan Kaskel. Angew. Chem. Int. Ed. 2017, 56, 1449. [Google Scholar] [CrossRef]
- McGuire, C.V.; Forgan, R.S. The surface chemistry of metal–organic frameworks. Chem. Commun. 2015, 51, 5199–5217. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of Metal–organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Giannakoudakis, D.A.; Hu, Y.; Florent, M.; Bandosz, T.J. Smart textiles of MOF/g-3N4 nanospheres for the rapid detection/detoxification of chemical warfare agents. Nanoscale Horiz. 2017, 2, 356–364. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Travlou, N.A.; Secor, J.; Bandosz, T.J. Oxidized g-3N4 nanospheres as catalytically photoactive linkers in MOF/G-C3N4 composite of hierarchical pore structure. Small 2017, 13, 1601758. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Engineering the surface of a new class of adsorbents: Metal–organic framework/graphite oxide composites. J. Colloid Interface Sci. 2015, 447, 139–151. [Google Scholar] [CrossRef]
- Van Assche, T.R.C.; Duerinck, T.; Gutiérrez Sevillano, J.J.; Calero, S.; Baron, G.V.; Denayer, J.F.M. High adsorption capacities and two-step adsorption of polar adsorbates on copper–benzene-1,3,5-tricarboxylate metal–organic framework. J. Phys. Chem. C 2013, 117, 18100–18111. [Google Scholar] [CrossRef]
- Bandosz, T.J. (Ed.) Activated Carbon Surfaces in Environmental Remediation; Elsevier: Oxford, UK, 2006; Volume 7, pp. 1–571. [Google Scholar]
- Florent, M.; Giannakoudakis, D.A.; Bandosz, T.J. Mustard gas surrogate interactions with modified porous carbon fabrics: Effect of oxidative treatment. Langmuir 2017, 33, 11475–11483. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Fu, J.; Lazaridis, N.K.; Bikiaris, D.N.; Matis, K.A. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Stout, S.C.; Larsen, S.C.; Grassian, V.H. Adsorption, desorption and thermal oxidation of 2-CEES on nanocrystalline zeolites. Microporous Mesoporous Mater. 2007, 100, 77–86. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, P.; Zhou, H.-C.; Sharma, V.K. Water-stable Metal–organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere 2018, 209, 783–800. [Google Scholar] [CrossRef] [PubMed]
- Alfarra, A.; Frackowiak, E.; Béguin, F. The HSAB concept as a means to interpret the adsorption of metal ions onto activated carbons. Appl. Surf. Sci. 2004, 228, 84–92. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H. 2D metal–organic frameworks as multifunctional materials in heterogeneous catalysis and electro/photocatalysis. Adv. Mater. 2019, 31, e1900617. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.-L. Metal–organic frameworks: Structures and functional applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Freiberg, S.; Zhu, X.X. Polymer microspheres for controlled drug release. Int. J. Pharm. 2004, 282, 1–18. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Metal–Organic frameworks as efficient materials for drug delivery. Angew. Chem. Int. Ed. 2006, 45, 5974–5978. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Muñoz, B.; Rámila, A.; Pérez-Pariente, J.; Díaz, I.; Vallet-Regí, M. MCM-41 organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- An, J.; Farha, O.K.; Hupp, J.T.; Pohl, E.; Yeh, J.I.; Rosi, N.L. Metal-adeninate vertices for the construction of an exceptionally porous Metal–organic framework. Nat. Commun. 2012, 3, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurow, M.; Varotto, A.; Manichev, V.; Travlou, N.A.; Giannakoudakis, D.A.; Drain, C.M. Self-organized nanostructured materials of alkylated phthalocyanines and underivatized C60 on ITO. RSC Adv. 2013, 3, 21360–21364. [Google Scholar] [CrossRef]
- Cai, H.; Li, M.; Lin, X.R.; Chen, W.; Chen, G.H.; Huang, X.C.; Li, D. Spatial, hysteretic, and adaptive host-guest chemistry in a Metal–organic framework with open watson-crick sites. Angew. Chem. Int. Ed. 2015, 54, 10454–10459. [Google Scholar] [CrossRef]
- Agostoni, V.; Horcajada, P.; Noiray, M.; Malanga, M.; Aykaç, A.; Jicsinszky, L.; Vargas-Berenguel, A.; Semiramoth, N.; Daoud-Mahammed, S.; Nicolas, V.; et al. A “green” strategy to construct non-covalent, stable and bioactive coatings on porous MOF nanoparticles. Sci. Rep. 2015, 5, 7925. [Google Scholar] [CrossRef] [Green Version]
- Bellido, E.; Hidalgo, T.; Lozano, M.V.; Guillevic, M.; Simon-Vazquez, R.; Santander-Ortega, M.J.; Gonzalez-Fernandez, A.; Serre, C.; Alonso, M.J.; Horcajada, P. Heparin-engineered mesoporous iron Metal–organic framework nanoparticles: Toward stealth drug nanocarriers. Adv. Healthc. Mater. 2015, 4, 1246–1257. [Google Scholar] [CrossRef]
- Liu, S.; Zhai, L.; Li, C.; Li, Y.; Guo, X.; Zhao, Y.; Wu, C. Exploring and exploiting dynamic noncovalent chemistry for effective surface modification of nanoscale Metal–organic frameworks. ACS Appl. Mater. Interfaces 2014, 6, 5404–5412. [Google Scholar] [CrossRef]
- Azizi Vahed, T.; Naimi-Jamal, M.R.; Panahi, L. (Fe)MIL-100-Met@alginate: A hybrid polymer–MOF for enhancement of metformin’s bioavailability and pH-controlled release. New J. Chem. 2018, 42, 11137–11146. [Google Scholar] [CrossRef]
- Azizi Vahed, T.; Naimi-Jamal, M.R.; Panahi, L. Alginate-coated ZIF-8 Metal–organic framework as a green and bioactive platform for controlled drug release. J. Drug Deliv. Sci. Technol. 2019, 49, 570–576. [Google Scholar] [CrossRef]
- Wang, X.-G.; Dong, Z.-Y.; Cheng, H.; Wan, S.-S.; Chen, W.-H.; Zou, M.-Z.; Huo, J.-W.; Deng, H.-X.; Zhang, X.-Z. A multifunctional metal–organic framework based tumor targeting drug delivery system for cancer therapy. Nanoscale 2015, 7, 16061–16070. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Tan, S.; Yuan, D.; Lu, W.; Rezenom, Y.H.; Jiang, H.; Wang, L.Q.; Zhou, H.C. Surface functionalization of porous coordination nanocages via click chemistry and their application in drug delivery. Adv. Mater. 2011, 23, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Abánades Lázaro, I.; Haddad, S.; Sacca, S.; Orellana-Tavra, C.; Fairen-Jimenez, D.; Forgan, R.S. Selective surface PEGylation of UiO-66 nanoparticles for enhanced stability, cell uptake, and pH-responsive drug delivery. Chem 2017, 2, 561–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J.M.; Orellana-Tavra, C.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. mechanistic investigation into the selective anticancer cytotoxicity and immune system response of surface-functionalized, dichloroacetate-loaded, UiO-66 nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 5255–5268. [Google Scholar] [CrossRef] [Green Version]
- Abánades Lázaro, I.; Haddad, S.; Rodrigo-Muñoz, J.M.; Marshall, R.J.; Sastre, B.; del Pozo, V.; Fairen-Jimenez, D.; Forgan, R.S. Surface-Functionalization of Zr-fumarate MOF for selective cytotoxicity and immune system compatibility in nanoscale drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 31146–31157. [Google Scholar] [CrossRef]
- Rijnaarts, T.; Mejia-Ariza, R.; Egberink, R.J.M.; van Roosmalen, W.; Huskens, J. Metal–Organic Frameworks (MOFs) as multivalent materials: Size control and surface functionalization by monovalent capping ligands. Chem. Eur. J. 2015, 21, 10296–10301. [Google Scholar] [CrossRef]
- Mejia-Ariza, R.; Huskens, J. The effect of PEG length on the size and guest uptake of PEG-capped MIL-88A particles. J. Mater. Chem. B 2016, 4, 1108–1115. [Google Scholar] [CrossRef]
- He, Z.; Dai, Y.; Li, X.; Guo, D.; Liu, Y.; Huang, X.; Jiang, J.; Wang, S.; Zhu, G.; Zhang, F.; et al. Hybrid nanomedicine fabricated from photosensitizer-terminated Metal–organic framework nanoparticles for photodynamic therapy and hypoxia-activated cascade chemotherapy. Small 2019, 15, e1804131. [Google Scholar] [CrossRef]
- Zimpel, A.; Preiß, T.; Röder, R.; Engelke, H.; Ingrisch, M.; Peller, M.; Rädler, J.O.; Wagner, E.; Bein, T.; Lächelt, U.; et al. Imparting functionality to MOF nanoparticles by external surface selective covalent attachment of polymers. Chem. Mater. 2016, 28, 3318–3326. [Google Scholar] [CrossRef]
- Giménez-Marqués, M.; Bellido, E.; Berthelot, T.; Simón-Yarza, T.; Hidalgo, T.; Simón-Vázquez, R.; González-Fernández, Á.; Avila, J.; Asensio, M.C.; Gref, R.; et al. GraftFast surface engineering to improve MOF nanoparticles furtiveness. Small 2018, 14, 1801900. [Google Scholar] [CrossRef]
- Benzaqui, M.; Semino, R.; Carn, F.; Tavares, S.R.; Menguy, N.; Giménez-Marqués, M.; Bellido, E.; Horcajada, P.; Berthelot, T.; Kuzminova, A.I.; et al. Covalent and selective grafting of polyethylene glycol brushes at the surface of ZIF-8 for the processing of membranes for pervaporation. ACS Sustain. Chem. Eng. 2019, 7, 6629–6639. [Google Scholar] [CrossRef]
- Cai, X.; Deng, X.; Xie, Z.; Shi, Y.; Pang, M.; Lin, J. Controllable synthesis of highly monodispersed nanoscale Fe-soc-MOF and the construction of Fe-soc-MOF@polypyrrole core-shell nanohybrids for cancer therapy. Chem. Eng. J. 2019, 358, 369–378. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, K.; Lei, L.; Lei, Z. UiO-66-NH2@PMAA: A hybrid Polymer–MOFs architecture for pectinase immobilization. Ind. Eng. Chem. Res. 2018, 57, 559–567. [Google Scholar] [CrossRef]
- Nagata, S.; Kokado, K.; Sada, K. Metal–Organic framework tethering PNIPAM for ON–OFF controlled release in solution. Chem. Commun. 2015, 51, 8614–8617. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Liao, W.-C.; Sohn, Y.S.; Fadeev, M.; Cecconello, A.; Nechushtai, R.; Willner, I. Stimuli-Responsive nucleic acid-based polyacrylamide hydrogel-coated metal–organic framework nanoparticles for controlled drug release. Adv. Funct. Mater. 2018, 28, 1705137. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; He, Y.; Kim, J.; Goh, S.J.; Nam, E.; Qiao, G.G.; Webley, P.A. Synthesis of well dispersed polymer grafted metal–organic framework nanoparticles. Chem. Commun. 2015, 51, 15566–15569. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, H.; Zhu, S. Reversibly dispersible/collectable Metal–organic frameworks prepared by grafting thermally responsive and switchable polymers. Macromol. Mater. Eng. 2015, 300, 191–197. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.; Wang, J.; Gao, H.; Yang, X.; Wu, S.; Liu, D.; Chen, Q. Transcellular delivery of messenger RNA payloads by a cationic supramolecular MOF platform. Chem. Commun. 2018, 54, 11304–11307. [Google Scholar] [CrossRef]
- Dong, S.; Chen, Q.; Li, W.; Jiang, Z.; Ma, J.; Gao, H. A dendritic catiomer with an MOF motif for the construction of safe and efficient gene delivery systems. J. Mater. Chem. B 2017, 5, 8322–8329. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Q.; Dong, S.; Ma, J.; Yang, Y.-W.; Chen, L.; Gao, H. Polymer brush decorated MOF nanoparticles loaded with AIEgen, anticancer drug, and supramolecular glue for regulating and in situ observing DOX release. Macromol. Biosci. 2018, 18, 1800317. [Google Scholar] [CrossRef] [PubMed]
- McDonald, K.A.; Feldblyum, J.I.; Koh, K.; Wong-Foy, A.G.; Matzger, A.J. Polymer@MOF@MOF: “grafting from” atom transfer radical polymerization for the synthesis of hybrid porous solids. Chem. Commun. 2015, 51, 11994–11996. [Google Scholar] [CrossRef] [PubMed]
- Molavi, H.; Shojaei, A.; Mousavi, S.A. Improving mixed-matrix membrane performance via PMMA grafting from functionalized NH2–UiO-66. J. Mater. Chem. A 2018, 6, 2775–2791. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Zhang, N.; Xie, Z.; Dong, D. Polymer brushes on metal–organic frameworks by UV-induced photopolymerization. Polym. Chem. 2016, 7, 5828–5834. [Google Scholar] [CrossRef]
- Rowe, M.D.; Chang, C.-C.; Thamm, D.H.; Kraft, S.L.; Harmon, J.F.; Vogt, A.P.; Sumerlin, B.S.; Boyes, S.G. Tuning the magnetic resonance imaging properties of positive contrast agent nanoparticles by surface modification with RAFT polymers. Langmuir 2009, 25, 9487–9499. [Google Scholar] [CrossRef]
- Rowe, M.D.; Thamm, D.H.; Kraft, S.L.; Boyes, S.G. Polymer-Modified gadolinium Metal–organic framework nanoparticles used as multifunctional nanomedicines for the targeted imaging and treatment of cancer. Biomacromolecules 2009, 10, 983–993. [Google Scholar] [CrossRef]
- Aykaç, A.; Noiray, M.; Malanga, M.; Agostoni, V.; Casas-Solvas, J.M.; Fenyvesi, É.; Gref, R.; Vargas-Berenguel, A. A non-covalent “click chemistry” strategy to efficiently coat highly porous MOF nanoparticles with a stable polymeric shell. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1606–1616. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Lai, X.; Chu, Y.; Chen, Y. Biocompatible surface modification of nano-scale zeolitic imidazolate frameworks for enhanced drug delivery. RSC Adv. 2018, 8, 23623–23628. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, T.; Giménez-Marqués, M.; Bellido, E.; Avila, J.; Asensio, M.C.; Salles, F.; Lozano, M.V.; Guillevic, M.; Simón-Vázquez, R.; González-Fernández, A.; et al. Chitosan-coated mesoporous MIL-100(Fe) nanoparticles as improved bio-compatible oral nanocarriers. Sci. Rep. 2017, 7, 43099. [Google Scholar] [CrossRef]
- Filippousi, M.; Turner, S.; Leus, K.; Siafaka, P.I.; Tseligka, E.D.; Vandichel, M.; Nanaki, S.G.; Vizirianakis, I.S.; Bikiaris, D.N.; van Der Voort, P.; et al. Biocompatible Zr-based nanoscale MOFs coated with modified poly(epsilon-caprolactone) as anticancer drug carriers. Int. J. Pharm. 2016, 509, 208–218. [Google Scholar] [CrossRef]
- He, S.; Wang, H.; Zhang, C.; Zhang, S.; Yu, Y.; Lee, Y.; Li, T. A generalizable method for the construction of MOF@polymer functional composites through surface-initiated atom transfer radical polymerization. Chem. Sci. 2019, 10, 1816–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Márquez, A.G.; Hidalgo, T.; Lana, H.; Cunha, D.; Blanco-Prieto, M.J.; Álvarez-Lorenzo, C.; Boissière, C.; Sánchez, C.; Serre, C.; Horcajada, P. Biocompatible polymer–metal–organic framework composite patches for cutaneous administration of cosmetic molecules. J. Mater. Chem. B 2016, 4, 7031–7040. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Zhu, W.; Yi, X.; Dong, Z.; Xu, X.; Chen, M.; Yang, K.; Lu, G.; Jiang, L.; et al. Nanoscale metal−organic frameworks for combined photodynamic & radiation therapy in cancer treatment. Biomaterials 2016, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering phototheranostic nanoscale metal–organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Li, Y.; Liu, S.; Xie, Z.; Jing, X. Nanoscale polymer metal–organic framework hybrids for effective photothermal therapy of colon cancers. Adv. Mater. 2016, 28, 9320–9325. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, C.; Ge, J.; Liu, Z. Polydopamine tethered enzyme/metal–organic framework composites with high stability and reusability. Nanoscale 2015, 7, 18883–18886. [Google Scholar] [CrossRef]
- Feng, J.; Xu, Z.; Dong, P.; Yu, W.; Liu, F.; Jiang, Q.; Wang, F.; Liu, X. Stimuli-responsive multifunctional metal–organic framework nanoparticles for enhanced chemo-photothermal therapy. J. Mater. Chem. B 2019, 7, 994–1004. [Google Scholar] [CrossRef]
- Yang, S.; Peng, L.; Sun, D.T.; Asgari, M.; Oveisi, E.; Trukhina, O.; Bulut, S.; Jamali, A.; Queen, W.L. A new post-synthetic polymerization strategy makes metal–organic frameworks more stable. Chem. Sci. 2019, 10, 4542–4549. [Google Scholar] [CrossRef] [Green Version]
- Castells-Gil, J.; Novio, F.; Padial, N.M.; Tatay, S.; Ruíz-Molina, D.; Martí-Gastaldo, C. Surface functionalization of metal–organic framework crystals with catechol coatings for enhanced moisture tolerance. ACS Appl. Mater. Interfaces 2017, 9, 44641–44648. [Google Scholar] [CrossRef]
- Chun, J.; Kang, S.; Park, N.; Park, E.J.; Jin, X.; Kim, K.-D.; Seo, H.O.; Lee, S.M.; Kim, H.J.; Kwon, W.H.; et al. Metal–Organic framework@microporous organic network: Hydrophobic adsorbents with a crystalline inner porosity. J. Am. Chem. Soc. 2014, 136, 6786–6789. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Zheng, X.; Li, Z.; Xie, Z. Nanoscale fluorescent metal–organic framework@microporous organic polymer composites for enhanced intracellular uptake and bioimaging. Chem. Eur. J. 2017, 23, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Liu, S.; Xie, Z. Metal–organic frameworks@polymer composites containing cyanines for near-infrared fluorescence imaging and photothermal tumor therapy. Bioconj. Chem. 2017, 28, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, R.; Cravillon, J.; Weidmann, C.; Wiebcke, M.; Smarsly, B.M. Metal–organic framework nanofibers via electrospinning. Chem. Commun. 2011, 47, 442–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamage, N.-D.H.; McDonald, K.A.; Matzger, A.J. MOF-5-Polystyrene: Direct production from monomer, improved hydrolytic stability, and unique guest adsorption. Angew. Chem. 2016, 128, 12278–12282. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ge, J.; Jiang, H.L.; Yu, S.H. A facile and general coating approach to moisture/water-resistant Metal–organic frameworks with intact porosity. J. Am. Chem. Soc. 2014, 136, 16978–16981. [Google Scholar] [CrossRef]
- Pastore, V.J.; Cook, T.R.; Rzayev, J. Polymer–MOF hybrid composites with high porosity and stability through surface-selective ligand exchange. Chem. Mater. 2018, 30, 8639–8649. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules 2020, 25, 185. https://doi.org/10.3390/molecules25010185
Giliopoulos D, Zamboulis A, Giannakoudakis D, Bikiaris D, Triantafyllidis K. Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules. 2020; 25(1):185. https://doi.org/10.3390/molecules25010185
Chicago/Turabian StyleGiliopoulos, Dimitrios, Alexandra Zamboulis, Dimitrios Giannakoudakis, Dimitrios Bikiaris, and Konstantinos Triantafyllidis. 2020. "Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications" Molecules 25, no. 1: 185. https://doi.org/10.3390/molecules25010185
APA StyleGiliopoulos, D., Zamboulis, A., Giannakoudakis, D., Bikiaris, D., & Triantafyllidis, K. (2020). Polymer/Metal Organic Framework (MOF) Nanocomposites for Biomedical Applications. Molecules, 25(1), 185. https://doi.org/10.3390/molecules25010185









