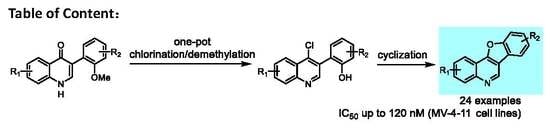
3.1.2. General Procedures for the Synthesis of 2-(4-Chloroquinolin-3-Yl)Phenol Substrates 4a–4x
A mixture of the substituted 3-phenylquinolin-4(1H)-one (1 equiv.), SOCl2 (4 equiv.), dichloromethane (5 mL) was refluxed until the reaction was completed (1 h), as evidenced using thin-layer chromatography (petroleum ether/ethyl acetate, 10:1). The cooled reaction mixture was concentrated in vacuo, diluted with water, then extracted with dichloromethane (3 × 20 mL). The combined organic layer were washed with water and brine, dried with anhydrous Na2SO4, and concentrated in vacuo. Then, the crude mixture was refluxed under 48% HBr without further purification until the reaction was completed. The mixture was poured into ice water and neutralized with saturated NaHCO3. The aqueous phase was extracted with ethyl acetate (3 × 20 mL), dried with anhydrous Na2SO4, and concentrated in vacuo. The crude mixture was purified using column chromatography over a silica gel using dichloromethane/methanol (80:1) as an eluent.
2-(4-Chloroquinolin-3-yl)phenol (4a): White solid (0.25 g, 96%), 1H-NMR (400 MHz, DMSO-d6) δ 9.76 (s, 1H), 8.78 (s, 1H), 8.29 (d, J = 8.3 Hz, 1H), 8.13 (d, J = 8.3 Hz, 1H), 7.89 (dd, J = 7.5, 4.1 Hz, 1H), 7.80 (dd, J = 7.8, 7.5 Hz, 1H), 7.34–7.28 (m, 2H), 7.02 (d, J = 8.1 Hz, 1H), 6.96 (dd, J = 7.4, 7.3 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 154.9, 152.2, 147.3, 139.6, 131.2, 130.9, 130.2, 130.0, 129.3, 128.2, 125.6, 124.0, 122.8, 119.0, 115.8.
2-(4-Chloroquinolin-3-yl)-6-methylphenol (4b): Orange solid (0.21 g, 78%), 1H-NMR (400 MHz, CDCl3) δ 8.94 (s, 1H), 8.47–8.44 (m, 1H), 8.36 (d, J = 8.2 Hz, 1H), 8.04–8.01 (m, 1H), 7.92–7.88 (m, 1H), 7.15 (d, J = 4.6 Hz, 1H), 7.01 (d, J = 5.7 Hz, 1H), 6.84–6.81 (m, 1H), 2.18 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 156.5, 156.3, 149.8, 140.7, 139.2, 137.2, 137.0, 135.3, 132.4, 131.8, 130.2, 130.0, 125.1, 124.3, 124.0, 20.2.
2-(4-Chloroquinolin-3-yl)-6-fluorophenol (4c): Yellow solid (0.24 g, 87%), 1H-NMR (400 MHz, DMSO-d6) δ 9.94 (br s, 1H), 8.91 (s, 1H), 8.34 (d, J = 8.1 Hz, 1H), 8.18 (d, J = 8.3 Hz, 1H), 7.97 (dd, J = 7.6, 7.1 Hz, 1H), 7.86 (dd, J = 7.5, 7.4 Hz, 1H), 7.35–7.30 (m, 1H), 7.14 (d, J = 7.6 Hz, 1H), 6.97 (ddd, J = 8.0, 7.9, 5.0 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 151.7 (d, JC-F = 238.1 Hz), 150.9, 145.7, 142.7 (d, JC-F = 14.6 Hz), 141.8, 131.3, 129.8 (d, JC-F = 3.0 Hz), 128.9, 128.0, 126.6 (d, JC-F = 2.8 Hz), 125.7, 125.5 (d, JC-F = 3.3 Hz), 124.3, 119.3 (d, JC-F = 7.4 Hz), 116.5 (d, JC-F = 18.2 Hz).
2-(4-Chloroquinolin-3-yl)-5-methylphenol (4d): Yellow-green solid (0.22 g, 81%), 1H-NMR (400 MHz, DMSO-d6) δ 9.64 (br s, 1H), 8.76 (s, 1H), 8.28 (d, J = 8.2 Hz, 1H), 8.12 (d, J = 7.9 Hz, 1H), 7.88 (dd, J = 7.4, 7.2 Hz, 1H), 7.79 (dd J = 7.1, 7.0 Hz, 1H), 7.16 (d, J = 7.6 Hz, 1H), 6.82 (s, 1H), 6.77 (d, J = 7.5 Hz, 1H), 2.31 (s, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 154.7, 152.3, 147.1, 139.6 (2C), 130.9, 130.2, 129.2, 128.2, 125.6, 124.0 (2C), 119.9, 119.8, 116.3, 21.0.
4-(4-Chloroquinolin-3-yl)benzene-1,3-diol (4e): Black oil (0.24 g, 90%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 2/3) δ 8.89–8.88 (m, 1H), 8.38–8.36 (m, 2H), 7.96–7.92 (m, 1H), 7.87–7.81 (m, 1H), 7.02 (dd, J = 8.3, 6.8 Hz, 1H), 6.47–6.46 (m, 1H), 6.35 (dd, J = 6.8, 6.4 Hz, 1H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 2/3) δ 157.4, 153.0, 148.7, 143.7, 134.1, 131.9, 129.9, 129.3, 128.4, 125.2, 123.2, 118.8, 108.2, 105.4, 100.4.
5-Bromo-2-(4-chloroquinolin-3-yl)phenol (4f): White solid (0.31g, 94%), 1H-NMR (400 MHz, CDCl3) δ 8.65 (s, 1H), 8.04 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 8.4 Hz, 1H), 7.69 (dd, J = 8.1, 7.2 Hz, 1H), 7.51 (dd, J = 8.0, 7.2 Hz, 1H), 7.30 (s, 1H), 7.19–7.16 (m, 1H), 7.08 (d, J = 8.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 154.2, 150.5, 145.9, 141.4, 131.4, 129.7, 128.2, 127.4, 127.2, 125.0, 123.5, 122.7, 122.5, 120.7, 119.5.
5-Chloro-2-(4-chloroquinolin-3-yl)phenol (4g): White solid (0.26 g, 88%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 2/3) δ 8.70 (s, 1H), 8.32 (d, J = 8.4 Hz, 1H), 8.05 (d, J = 8.3 Hz, 1H), 7.78 (dd, J = 8.0, 7.1 Hz, 1H), 7.67 (dd, J = 8.1, 7.2 Hz, 1H), 7.18 (d, J = 8.1 Hz, 1H), 6.98–6.93 (m, 2H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 2/3) δ 154.8, 150.8, 146.3, 141.0, 134.4, 131.4, 129.5, 129.4, 127.6, 127.1, 125.8, 123.8, 120.8, 118.8, 115.2.
2-(4-Chloroquinolin-3-yl)-4-fluorophenol (4h): Yellow solid (0.22 g, 80%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 2/3) δ 8.73 (s, 1H), 8.33 (d, J = 8.3 Hz, 1H), 8.06 (d, J = 8.4 Hz, 1H), 7.79 (dd, J = 7.9, 7.3 Hz, 1H), 7.68 (dd, J = 8.0, 7.2 Hz, 1H), 7.01–6.97 (m, 2H), 6.93–6.90 (m, 1H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 2/3) δ 155.2 (d, JC-F = 235.9 Hz), 150.7, 150.1 (d, JC-F = 1.8 Hz), 146.4, 141.0, 129.6, 129.3, 127.7, 127.2, 125.8, 123.8, 122.8 (d, JC-F = 8.0 Hz), 116.6 (d, JC-F = 23.4 Hz), 115.8 (d, JC-F = 8.0 Hz), 115.6 (d, JC-F = 22.7 Hz).
4-Chloro-2-(4-chloroquinolin-3-yl)phenol (4i): Yellow solid (0.28 g, 95%), 1H-NMR (400 MHz, CD3OD) δ 9.28 (s, 1H), 8.67 (d, J = 8.5 Hz, 1H), 8.30 (d, J = 8.5 Hz, 1H), 8.25 (dd, J = 8.5, 6.9 Hz, 1H), 8.10 (dd, J = 8.2, 7.2 Hz, 1H), 7.44 (d, J = 2.5 Hz, 1H), 7.38 (dd, J = 8.8, 2.6 Hz, 1H), 7.00 (d, J = 8.8 Hz, 1H). 13C-NMR (100 MHz, CD3OD) δ 155.3, 153.9, 147.7, 138.8, 136.8, 132.8, 132.7, 132.6, 131.8, 129.0, 127.2, 125.5, 122.8, 122.2, 118.5.
2-(4-Chloroquinolin-3-yl)-4-methylphenol (4j): Brown solid (0.25 g, 92%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 1/4) δ 8.92 (s, 1H), 8.47 (d, J = 7.9 Hz, 1H), 8.24 (s, 1H), 8.01–7.88 (m, 2H), 7.13–7.08 (m, 2H), 6.87 (d, J = 8.0 Hz, 1H), 2.28 (s, 3H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 1/4) δ 152.3, 148.5, 148.2, 140.6, 133.4, 132.3, 131.8, 131.2, 130.1, 129.0, 127.4, 125.5, 123.8, 120.3, 115.8, 20.0.
2-(4-Chloroquinolin-3-yl)-4-(trifluoromethoxy)phenol (4k): Yellow solid (0.29 g, 85%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 1/4) δ 8.81 (s, 1H), 8.31 (d, J = 8.2 Hz, 1H), 8.16 (d, J = 8.2 Hz, 1H), 7.80 (dd, J = 8.0, 6.7 Hz, 1H), 7.69 (dd, J = 7.8, 7.3 Hz, 1H), 7.14–7.12 (m, 2H), 6.96 (d, J = 7.8 Hz, 1H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 1/4) δ 150.9, 147.7, 142.3, 141.6, 138.9 (d, JC-F = 1.6 Hz), 129.1, 127.2, 126.2, 124.5, 124.3, 122.4, 121.8, 121.0, 120.2, 119.3, 116.7, 114.4.
2-(4-Chloroquinolin-3-yl)benzene-1,4-diol (4l): Brown solid (0.19 g, 68%), 1H-NMR (400 MHz, DMSO-d6) δ 9.02 (s, 1H), 8.38 (d, J = 8.2 Hz, 1H), 8.20 (d, J = 8.2 Hz, 1H), 8.01 (dd, J = 7.5, 7.1 Hz, 1H), 7.90 (dd, J = 7.5, 7.4 Hz, 1H), 6.84 (d, J = 8.6 Hz, 1H), 6.77–6.74 (m, 1H), 6.71 (d, J = 2.4 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 150.3, 149.7, 147.3, 143.5, 143.2, 131.9, 131.4, 129.3, 126.4, 126.0, 124.5, 122.0, 117.1, 117.0, 116.6.
3-Chloro-2-(4-chloroquinolin-3-yl)phenol (4m): Golden solid (0.27 g, 94%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 2/3) δ 8.62 (s, 1H), 8.32 (d, J = 8.0 Hz, 1H), 8.07 (d, J = 8.4 Hz, 1H), 7.80–7.76 (m, 1H), 7.68–7.64 (m, 1H), 7.22 (dd, J = 8.2, 8.1 Hz, 1H), 7.02 (d, J = 7.4 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 2/3) δ 162.3, 155.4, 150.7, 146.5, 142.2, 133.7, 129.6, 127.9, 127.7, 127.0, 125.7, 123.8, 121.5, 119.4, 113.2.
2-(4-Chloro-5-methylquinolin-3-yl)phenol (4n): Yellow solid (0.26 g, 95%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 3/2) δ 8.65 (s, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.63 (dd, J = 8.2, 7.4 Hz, 1H), 7.46 (d, J = 7.3 Hz, 1H), 7.31–7.27 (m, 1H), 7.21–7.19 (m, 1H), 6.98–6.94 (m, 2H), 3.06 (s, 3H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 3/2) δ 153.8, 148.9, 146.7, 143.3, 135.6, 132.1, 131.1, 130.2, 129.5, 129.4, 125.6 (2C), 122.5, 118.7, 115.0, 25.0.
2-(4,6-Dichloroquinolin-3-yl)phenol (4o): Orange solid (0.27 g, 94%), 1H-NMR (400 MHz, DMSO-d6) δ 9.83 (br s, 1H), 8.82 (s, 1H), 8.26 (d, J = 2.3 Hz, 1H), 8.15 (d, J = 8.9 Hz, 1H), 7.91 (dd, J = 9.0, 2.3 Hz, 1H), 7.34–7.27 (m, 2H), 7.01 (d, J = 7.6 Hz, 1H), 6.97–6.93 (m, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 154.8, 152.6, 145.5, 138.8, 133.0, 131.8, 131.5, 131.0, 130.9, 130.3, 126.5, 122.8, 122.3, 119.0, 115.8.
2-(4-Chloro-6-methylquinolin-3-yl)phenol (4p): Yellow solid (0.23 g, 86%), 1H-NMR (400 MHz, DMSO-d6) δ 9.65 (s, 1H), 8.61 (s, 1H), 7.97 (s, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.24–7.18 (m, 2H), 6.93 (d, J = 8.0 Hz, 1H), 6.8–6.84 (m, 1H), 2.42 (s, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 154.9, 151.2, 145.9, 138.8, 138.0, 132.2, 131.1, 130.8, 129.9, 129.1, 125.5, 122.9, 122.6, 119.0, 115.8, 21.4.
4-Chloro-3-(2-hydroxyphenyl)quinolin-6-ol (4q): Dark-red solid (0.25 g, 91%), 1H-NMR (400 MHz, DMSO-d6) δ 11.03 (br s, 1H), 9.93 (br s, 1H), 9.01 (s, 1H), 8.17–8.16 (m, 1H), 7.66–7.63 (m, 2H), 7.34 (s, 2H), 7.06–6.98 (m, 2H). 13C-NMR (100 MHz, DMSO-d6) δ 158.8, 154.8 (2C), 144.5, 135.2, 131.5, 131.1, 131.0, 130.7, 128.2, 121.2, 119.1, 115.9, 105.5, 99.5.
2-(4-Chloro-7-fluoroquinolin-3-yl)phenol (4r): Light yellow solid (0.25 g, 92%), 1H-NMR (400 MHz, DMSO-d6) δ 9.78 (s, 1H), 8.81 (s, 1H), 8.37 (dd, J = 9.3, 6.0 Hz, 1H), 7.91 (dd, J = 10.0, 2.5 Hz, 1H), 7.76–7.71 (m, 1H), 7.33–7.28 (m, 2H), 7.01 (d, J = 8.1 Hz, 1H), 6.97–6.93 (m, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 162.6 (d, JC-F = 247.7 Hz), 154.9, 153.5, 148.2 (d, JC-F = 12.8 Hz), 139.8 (d, JC-F = 0.8 Hz), 131.1, 130.5 (d, JC-F = 2.4 Hz), 130.1, 127.0 (d, JC-F = 10.1 Hz), 122.9, 122.4, 119.0, 118.3 (d, JC-F = 25.1 Hz), 115.8, 112.9 (d, JC-F = 20.4 Hz).
2-(4,7-Dichloroquinolin-3-yl)phenol (4s): Orange solid (0.26 g, 90%), 1H-NMR (400 MHz, CD3OD/CDCl3 = 3/2) δ 8.76 (s, 1H), 8.29 (d, J = 9.0 Hz, 1H), 8.05 (d, J = 2.0 Hz, 1H), 7.64 (dd, J = 9.0, 2.0 Hz, 1H), 7.32–7.28 (m, 1H), 7.24 (dd, J = 7.4, 1.0 Hz, 1H), 6.98–6.94 (m, 2H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 3/2) δ 156.1, 154.6, 148.8, 143.1, 137.6, 133.0, 132.6, 131.7, 130.1, 128.7, 127.6, 126.7, 123.9, 120.8, 117.1.
2-(4-Chloro-7-methylquinolin-3-yl)phenol (4t): Earthy solid (0.23 g, 86%), 1H-NMR (400 MHz, DMSO-d6) δ 9.79 (br s, 1H), 8.80 (s, 1H), 8.20 (d, J = 8.0 Hz, 1H), 7.93 (s, 1H), 7.67 (d, J = 8.4 Hz, 1H), 7.33–7.27 (m, 2H), 7.01 (d, J = 8.0 Hz, 1H), 6.95 (dd, J = 7.4, 7.3 Hz, 1H), 2.58 (s, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 154.9, 151.5, 146.4, 140.9, 140.5, 131.2, 130.6, 130.2, 130.0, 127.3, 123.8 (2C), 122.5, 119.0, 115.8, 21.2.
4-Chloro-3-(2-hydroxyphenyl)quinolin-7-ol (4u): Brown solid (0.24 g, 89%), 1H-NMR (400 MHz, CD3OD) δ 8.96 (s, 1H), 8.51 (d, J = 9.3 Hz, 1H), 7.59 (dd, J = 9.4, 2.2 Hz, 1H), 7.46 (d, J = 2.2 Hz, 1H), 7.40–7.34 (m, 2H), 7.04–7.00 (m, 2H). 13C-NMR (100 MHz, CD3OD) δ 165.4, 156.4, 152.6, 146.7, 142.1, 132.4 (2C), 130.6, 129.2, 124.9, 123.5, 121.8, 120.8, 116.9, 103.5.
2-(4-Chloro-8-methylquinolin-3-yl)phenol (4v): Yellow solid (0.25 g, 91%), 1H-NMR (400 MHz, DMSO-d6) δ 9.74 (br s, 1H), 8.80 (s, 1H), 8.13 (d, J = 8.0 Hz, 1H), 7.73 (d, J = 6.8 Hz, 1H), 7.68–7.64 (m, 1H), 7.33–7.26 (m, 2H), 7.02 (d, J = 7.8 Hz, 1H), 6.97–6.93 (m, 1H), 2.77 (s, 3H). 13C-NMR (100 MHz, DMSO-d6) δ 154.9, 150.9, 146.1, 140.0, 137.0, 131.1, 130.7, 130.2, 130.0, 127.8, 125.6, 122.9, 121.9, 119.0, 115.8, 17.9.
2-(7-Bromo-4-chloroquinolin-3-yl)benzene-1,4-diol (4w): Yellow solid (0.34 g, 97%), 1H-NMR (400 MHz, CD3OD) δ 8.74 (s, 1H), 8.26–8.24 (m, 2H), 7.82 (dd, J = 9.0, 1.9 Hz, 1H), 6.83–6.76 (m, 2H), 6.72 (d, J = 2.7 Hz, 1H). 13C-NMR (100 MHz, CD3OD) δ 154.6, 151.3, 149.0 (2C), 142.7, 133.2, 132.5, 131.9, 127.4, 126.7, 125.5, 124.4, 118.3, 118.2, 117.9.
2-(4,7-Dichloroquinolin-3-yl)benzene-1,4-diol (4x): Brick-red solid (0.28 g, 91%), 1H-NMR (400 MHz, DMSO-d6) δ 8.83 (s, 1H), 8.31 (d, J = 9.0 Hz, 1H), 8.20 (d, J = 1.8 Hz, 1H), 7.84 (dd, J = 9.0, 1.8 Hz, 1H), 6.83 (d, J = 8.7 Hz, 1H), 6.74 (dd, J = 8.6, 2.7 Hz, 1H), 6.68 (d, J = 2.7 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 153.3, 149.7, 147.3, 147.0, 140.0, 135.0, 131.5, 128.9, 127.6, 126.3, 124.5, 122.5, 117.0, 116.9, 116.6.
3.1.3. General Procedures for the Synthesis of Benzofuro[3,2-c]Quionlines 2a–2x
To a solution of the 2-(4-chloroquinolin-3-yl)phenol (1 equiv.) in DMF (5 mL), KOt-Bu (2 equiv.) was added in one portion. The reaction mixture was first stirred at 25 °C for 0.5 h, then stirred at 60 °C for 2 h. The mixture was poured into ice water. The organic phase was separated and the water phase was extracted with ethyl acetate three times (3 × 20 mL). The combined organic phases were dried over anhydrous Na2SO4. Removal of the solvent was done in vacuo, and the crude mixture was further purified using column chromatography over silica gel using petroleum ether/ethyl acetate (15:1) as eluent.
Benzofuro[3,2-c]quinoline (2a): White solid (0.19 g, 88%), m.p. 130–132 °C. 1H-NMR (400 MHz, CDCl3) δ 9.48 (s, 1H), 8.40 (d, J = 8.1 Hz, 1H), 8.26 (d, J = 8.4 Hz, 1H), 8.08 (d, J = 7.6 Hz, 1H), 7.80–7.73 (m, 2H), 7.69 (dd, J = 7.6, 7.4 Hz, 1H), 7.54 (dd, J = 7.8, 7.4 Hz, 1H), 7.46 (dd, J = 7.4, 7.3 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 156.4, 154.9, 146.4, 143.4, 128.8, 128.2, 126.2, 126.0, 123.0, 121.6, 119.8, 119.6, 116.1, 115.3, 111.1; HRMS (ESI): calcd. for C15H10NO [M + H]+ 220.0757; found 220.0759.
6-Methylbenzofuro[3,2-c]quinoline (2b): White solid (0.15 g, 85%), m.p. 139–141 °C. 1H-NMR (400 MHz, CDCl3) δ 9.48–9.46 (m, 1H), 8.45 (dd, J = 7.8, 6.7 Hz, 1H), 8.26 (d, J = 8.3 Hz, 1H), 7.91 (dd, J = 8.6, 6.8 Hz, 1H), 7.80–7.76 (m, 1H), 7.69 (dd, J = 8.0, 6.9 Hz, 1H), 7.38–7.34 (m, 2H), 2.70 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 156.2, 153.9, 146.3, 143.6, 128.8, 128.1, 127.2, 125.8, 123.0, 121.4, 121.1, 119.8, 116.9, 116.2, 115.7, 14.2; HRMS (ESI): calcd. for C16H12NO [M + H]+ 234.0913; found 234.0906.
6-Fluorobenzofuro[3,2-c]quinoline (2c): White solid (0.17 g, 82%), m.p. 154–157 °C. 1H-NMR (400 MHz, CDCl3) δ 9.47 (s, 1H), 8.46 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.5 Hz, 1H), 7.85 (d, J = 7.7 Hz, 1H), 7.84–7.79 (m, 1H), 7.72 (dd, J = 7.4, 7.2 Hz, 1H), 7.40 (ddd, J = 8.0, 7.9, 4.3 Hz, 1H), 7.31–7.27 (m, 1H). 13C-NMR (100 MHz, CDCl3) δ 156.8, 147.3 (d, JC-F = 249.5 Hz), 146.7, 143.3, 141.7 (d, JC-F = 11.3 Hz), 128.8, 128.7, 126.3, 125.2 (d, JC-F = 2.6 Hz), 123.8 (d, JC-F = 5.8 Hz), 119.8, 116.0, 115.1 (d, JC-F = 4.0 Hz), 115.0 (d, JC-F = 2.2 Hz), 112.7 (d, JC-F = 16.1 Hz); HRMS (ESI): calcd. for C15H9FNO [M + H]+ 238.0663; found 238.0667.
5-Methylbenzofuro[3,2-c]quinoline (2d): White solid (0.17 g, 92%), m.p. 146–149 °C. 1H-NMR (400 MHz,CDCl3) δ 9.40 (s, 1H), 8.34 (d, J = 8.0 Hz, 1H), 8.23 (d, J = 8.4 Hz, 1H), 7.88 (d, J = 7.8 Hz, 1H), 7.74 (dd, J = 7.3, 7.0 Hz, 1H), 7.64 (dd, J = 7.8, 7.1 Hz, 1H), 7.48 (s, 1H), 7.23 (d, J = 7.8 Hz, 1H), 2.53 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 156.2, 155.3, 146.0, 143.1, 136.8, 128.7, 127.9, 125.8, 124.2, 119.6, 119.0, 116.1, 115.3, 111.2, 20.9; HRMS (ESI): calcd. for C16H12NO [M + H]+ 234.0913; found 234.0907.
Benzofuro[3,2-c]quinolin-5-ol (2e): Earthy solid (0.03 g, 12%), m.p. >250 °C. 1H-NMR (400 MHz, CD3OD/CDCl3 = 3/2) δ 9.30 (s, 1H), 8.33 (d, J = 8.1 Hz, 1H), 8.13 (d, J = 8.4 Hz, 1H), 7.88 (d, J = 8.4 Hz, 1H), 7.74 (dd, J = 8.3, 7.0 Hz, 1H), 7.67 (dd, J = 7.4, 7.3 Hz, H), 7.17 (s, 1H), 6.97 (d, J = 8.4 Hz, 1H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 3/2) δ 158.0, 157.6, 157.2, 145.7, 143.3, 128.9, 128.3, 127.0, 120.8, 120.4, 117.0, 116.9, 114.2, 113.1, 98.6; HRMS (ESI): calcd. for C15H10NO2 [M + H]+ 236.0706; found 236.0707.
5-Bromobenzofuro[3,2-c]quinoline (2f): White solid (0.24 g, 85%), m.p. 195–196 °C. 1H-NMR (400 MHz, CDCl3) δ 9.46 (s, 1H), 8.40 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.4 Hz, 1H), 7.96–7.93 (m, 2H), 7.81 (ddd, J = 8.3, 7.0, 1.2 Hz, 1H), 7.71 (ddd, J = 8.0, 7.1, 1.1 Hz, 1H), 7.61 (d, J = 8.2 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 156.7, 155.1, 146.5, 143.1, 128.9, 128.6, 126.5, 126.3, 120.8, 120.5, 119.8, 119.4, 116.0, 114.7 (2C); HRMS (ESI): calcd. for C15H9BrNO [M + H]+ 297.9862; found 297.9869.
5-Chlorobenzofuro[3,2-c]quinoline (2g): White solid (0.17 g, 75%), m.p. 194–195 °C. 1H-NMR (400 MHz, CDCl3) δ 9.45 (s, 1H), 8.39 (d, J = 8.0 Hz, 1H), 8.27 (d, J = 8.4 Hz, 1H), 8.00 (d, J = 8.3 Hz, 1H), 7.81 (ddd, J = 8.3, 7.0, 0.4 Hz, 1H), 7.76 (s, 1H), 7.71 (ddd, J = 8.0, 7.1, 0.7 Hz, 1H), 7.46 (d, J = 8.3 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 156.8, 155.0, 146.4, 143.1, 131.9, 128.9, 128.6, 126.2, 123.8, 120.4, 120.1, 119.7, 116.0, 114.6, 111.8; HRMS (ESI): calcd. for C15H9ClNO [M + H]+ 254.0367; found 254.0362.
4-Fluorobenzofuro[3,2-c]quinoline (2h): White solid (0.17 g, 89 %), m.p. 187–188 °C. 1H-NMR (400 MHz, CDCl3) δ 9.42 (s, 1H), 8.38 (d, J = 8.2 Hz, 1H), 8.26 (d, J = 8.4 Hz, 1H), 7.80 (dd, J = 8.2, 7.1 Hz,1H), 7.74–7.66 (m, 3H), 7.26–7.22 (m, 1H). 13C-NMR (100 MHz, CDCl3) δ 158.7 (d, JC-F = 239.8 Hz), 157.6, 150.9, 146.5, 143.3, 128.9, 128.6, 126.2, 122.6 (d, JC-F = 10.6 Hz), 119.8, 116.0, 115.1 (d, JC-F = 3.7 Hz), 113.7 (d, JC-F = 25.8 Hz), 111.9 (d, JC-F = 9.4 Hz), 105.7 (d, JC-F = 25.5 Hz); HRMS (ESI): calcd. for C15H9FNO [M + H]+ 238.0663; found 238.0664.
4-Chlorobenzofuro[3,2-c]quinoline (2i): White solid (0.21 g, 86%), m.p. 181–182 °C. 1H-NMR (400 MHz, CDCl3) δ 9.43 (s, 1H), 8.38 (d, J = 8.1 Hz, 1H), 8.26 (d, J = 8.4 Hz, 1H), 8.05 (d, J = 1.7 Hz, 1H), 7.80 (dd, J = 8.2, 7.1 Hz, 1H), 7.70 (dd, J = 7.7, 7.4 Hz, 1H), 7.66 (d, J = 8.8 Hz, 1H), 7.49 (dd, J = 8.8, 1.9 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 157.2, 153.2, 146.6, 143.2, 128.9, 128.7 (2C), 126.3, 126.2, 123.1, 119.8, 119.4, 116.0, 114.5, 112.1; HRMS (ESI): calcd. for C15H9ClNO [M + H]+ 254.0367; found 254.0361.
4-Methylbenzofuro[3,2-c]quinoline (2j): Yellow solid (0.20 g, 94%), m.p. 149–150 °C. 1H-NMR (400 MHz, CDCl3) δ 9.45 (s, 1H), 8.40–8.38 (m, 1H), 8.25 (d, J = 8.4 Hz, 1H), 7.87 (s, 1H), 7.79–7.75 (m, 1H), 7.70–7.66 (m, 1H), 7.61 (d, J = 8.4 Hz, 1H), 7.34–7.32 (m, 1H), 2.55 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 157.7, 154.4, 147.3, 144.4, 133.8, 129.8, 129.2, 128.3, 126.9, 122.7, 120.8, 120.5, 117.3, 116.3, 111.6, 21.4; HRMS (ESI): calcd. for C16H12NO [M + H]+ 234.0913; found 234.0916.
4-(Trifluoromethoxy)benzofuro[3,2-c]quinoline (2k): White solid (0.16 g, 62%), m.p. 143–144 °C. 1H-NMR (400 MHz, CDCl3) δ 9.45 (s, 1H), 8.40–8.38 (m, 1H), 8.27 (d, J = 8.4 Hz, 1H), 7.94 (d, J = 1.0 Hz, 1H), 7.84–7.79 (m, 1H), 7.75–7.69 (m, 2H), 7.40 (dd, J = 8.9,1.6 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 157.6, 152.8, 146.6, 144.7 (d, JC-F = 2.1 Hz), 143.2, 129.0, 128.8, 126.3, 122.7, 119.8, 119.7, 119.6 (q, JC-F = 255.5 Hz), 115.9, 114.8, 112.5, 112.0. 19F NMR (376 MHz, CDCl3): δ −58.1. HRMS (ESI): calcd. for C16H9F3NO2 [M + H]+ 304.0580; found 304.0584.
Benzofuro[3,2-c]quinolin-4-ol (2l): Light yellow solid (0.04 g, 23%), m.p. >250 °C. 1H-NMR (400 MHz, CD3OD/CDCl3 = 3/2) δ 9.30 (s, 1H), 8.36 (d, J = 7.9 Hz, 1H), 8.14 (d, J = 8.4 Hz, 1H), 7.76 (dd, J = 8.4, 8.2 Hz, 1H), 7.68 (dd, J = 7.7, 7.3 Hz, 1H), 7.55 (d, J = 8.8 Hz, 1H), 7.46 (s, 1H), 7.03 (dd, J = 8.9, 2.5 Hz, 1H). 13C-NMR (100 MHz, CD3OD/CDCl3 = 3/2) δ 157.4, 153.3, 149.5, 145.5, 143.2, 128.8, 127.6, 126.4, 122.2, 120.0, 116.4, 115.9, 115.1, 111.6, 104.7; HRMS (ESI): calcd. for C15H10NO2 [M + H]+ 236.0706; found 236.0710.
3-Chlorobenzofuro[3,2-c]quinoline (2m): White solid (0.10 g, 42%), m.p. 160–162 °C. 1H-NMR (400 MHz, CDCl3) δ 9.79 (s, 1H), 8.40–8.38 (m, 1H), 8.28 (d, J = 8.4 Hz, 1H), 7.83–7.79 (m, 1H), 7.72–7.68 (m, 1H), 7.65 (dd, J = 6.7, 2.3 Hz, 1H), 7.48–7.43 (m, 2H). 13C-NMR (100 MHz, CDCl3) δ 156.5, 155.2, 146.4, 144.5, 128.9, 128.7, 127.0, 126.6, 126.1, 123.5, 120.7, 119.8, 115.7, 114.5, 109.4; HRMS (ESI): calcd. for C15H9ClNO [M + H]+ 254.0367; found 254.0348.
7-Methylbenzofuro[3,2-c]quinoline (2n): Light yellow solid (0.19 g, 85%), m.p. 146–147 °C. 1H-NMR (400 MHz, CDCl3) δ 9.46 (s, 1H), 8.11–8.09 (m, 2H), 7.75 (d, J = 8.1 Hz, 1H), 7.64 (dd, J = 8.0, 7.6 Hz, 1H), 7.54 (dd, J = 8.2, 7.1 Hz, 1H), 7.49–7.44 (m, 2H), 3.12 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 158.4, 155.8, 148.4, 144.1, 133.6, 128.9, 128.3, 127.6, 127.0, 124.0, 122.1, 120.4, 117.3, 116.6, 112.1, 22.4; HRMS (ESI): calcd. for C16H12NO [M + H]+ 234.0913; found 234.0914.
8-Chlorobenzofuro[3,2-c]quinoline (2o): White solid (0.22 g, 91%), m.p. 168–169 °C. 1H-NMR (400 MHz, CDCl3) δ 9.42 (s, 1H), 8.32 (d, J = 2.3 Hz, 1H), 8.16 (d, J = 9.0 Hz, 1H), 8.07–8.05 (m, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.67 (dd, J = 9.0, 2.4 Hz, 1H), 7.57–7.53 (m, 1H), 7.47 (ddd, J = 7.6, 7.5, 1.0 Hz, 1H). 13C-NMR (100 MHz, CDCl3) δ 155.3, 155.0, 144.5, 143.5, 131.9, 130.4, 129.0, 126.6, 123.2, 121.3, 119.7, 118.8, 116.7, 115.9, 111.2; HRMS (ESI): calcd. for C15H9ClNO [M + H]+ 254.0367; found 254.0363.
8-Methylbenzofuro[3,2-c]quinoline (2p): White solid (0.17 g, 83%), m.p. 153–156 °C. 1H-NMR (400 MHz, CDCl3) δ 9.41 (s, 1H), 8.17 (br s, 1H), 8.15 (d, J = 8.6 Hz, 1H), 8.09–8.07 (m, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.61 (dd, J = 8.6, 1.9 Hz, 1H), 7.56–7.51 (m, 1H), 7.46 (ddd, J = 7.6, 7.4, 0.9 Hz, 1H), 2.63 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 156.1, 154.9, 145.0, 142.4, 136.1, 130.5, 128.5, 126.1, 122.9, 121.8, 119.6, 118.6, 116.0, 115.2, 111.0, 20.8. HRMS (ESI): calcd. for C16H12NO [M + H]+ 234.0913; found 234.0919.
Benzofuro[3,2-c]quinolin-8-ol (2q): Yellow solid (0.03 g, 13%), m.p. >250 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.41 (s, 1H), 9.44 (s, 1H), 8.30 (d, J = 7.6 Hz, 1H), 8.08 (d, J = 9.1 Hz, 1H), 7.92 (d, J = 8.2 Hz, 1H), 7.61 (dd, J = 7.9, 7.5 Hz, 1H), 7.55–7.50 (m, 2H), 7.39 (dd, J = 9.1, 2.3 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 156.4, 155.5, 155.2, 142.0, 141.3, 131.3, 127.5, 124.2, 122.3, 121.6, 121.3, 117.5, 115.7, 112.1, 101.3; HRMS (ESI): calcd. for C15H10NO2 [M + H]+ 236.0706; found 236.0715.
9-Fluorobenzofuro[3,2-c]quinoline (2r): White solid (0.17 g, 77%), m.p. 150–151 °C. 1H-NMR (400 MHz, CDCl3) δ 9.47 (s, 1H), 8.39 (dd, J = 9.0, 6.0 Hz, 1H), 8.07 (d, J = 7.6 Hz, 1H), 7.89 (dd, J = 10.3, 2.4 Hz, 1H), 7.73 (d, J = 8.2 Hz, 1H), 7.54 (dd, J = 7.7, 7.5 Hz, 1H), 7.50–7.46 (m, 2H). 13C-NMR (100 MHz, CDCl3) δ 161.9 (d, JC-F = 248.2 Hz), 156.5 (d, JC-F = 1.0 Hz), 154.8, 147.4 (d, JC-F = 12.4 Hz), 144.5, 126.3, 123.2, 121.9 (d, JC-F = 9.8 Hz), 121.4, 119.6, 116.3 (d, JC-F = 25.3 Hz), 115.0 (d, JC-F = 1.9 Hz), 113.1 (d, JC-F = 1.1 Hz), 112.9 (d, JC-F = 20.7 Hz), 111.1; HRMS (ESI): calcd. for C15H9FNO [M + H]+ 238.0663; found 238.0650.
9-Chlorobenzofuro[3,2-c]quinoline (2s): Light yellow solid (0.16 g, 69%), m.p. 155–157 °C. 1H-NMR (400 MHz, CDCl3) δ 9.46 (s, 1H), 8.32 (d, J = 8.8 Hz, 1H), 8.24 (d, J = 1.9 Hz, 1H), 8.07 (d, J = 7.6 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.63 (dd, J = 8.8, 2.0 Hz, 1H), 7.58–7.53 (m, 1H), 7.50–7.46 (m, 1H). 13C-NMR (100 MHz, CDCl3) δ 156.2, 154.9, 146.7, 144.4, 134.2, 128.0, 126.9, 126.5, 123.2, 121.4, 121.1, 119.7, 115.6, 114.5, 111.1; HRMS (ESI): calcd. for C15H9ClNO [M + H]+ 254.0367; found 254.0367.
9-Methylbenzofuro[3,2-c]quinoline (2t): Yellow-green solid (0.18 g, 92%), m.p. 159–161 °C. 1H-NMR (400 MHz, CDCl3) δ 9.44 (s, 1H), 8.30 (d, J = 8.4 Hz, 1H), 8.07 (d, J = 7.1 Hz, 1H), 8.04 (br s, 1H), 7.73 (d, J = 8.1 Hz, 1H), 7.54–7.50 (m, 2H), 7.48–7.44 (m, 1H), 2.62 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 156.6, 154.8, 146.7, 143.3, 138.7, 128.1, 128.0, 126.0, 122.9, 121.8, 119.5, 114.7, 114.0, 111.0, 21.0; HRMS (ESI): calcd. for C16H12NO [M + H]+ 234.0913; found 234.0909.
Benzofuro[3,2-c]quinolin-9-ol (2u): White solid (0.03 g, 15%), m.p. >250 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.39 (s, 1H), 9.53 (s, 1H), 8.28–8.25 (m, 2H), 7.88 (d, J = 8.1 Hz, 1H), 7.58–7.54 (m, 1H), 7.50 (dd, J = 7.5, 6.8 Hz, 1H), 7.47 (d, J = 2.1 Hz, 1H), 7.34 (dd, J = 8.9, 2.3 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 158.8, 157.0, 154.9, 149.0, 145.1, 126.9, 124.2, 122.4, 121.9, 120.8, 119.6, 113.7, 111.9, 111.3, 109.9; HRMS (ESI): calcd. for C15H10NO2 [M + H]+ 236.0706; found 236.0709.
10-Methylbenzofuro[3,2-c]quinoline (2v): Off-white solid (0.17 g, 79%), m.p. 203–204 °C. 1H-NMR (400 MHz, CDCl3) δ 9.49 (s, 1H), 8.27 (d, J = 8.0 Hz, 1H), 8.10 (d, J = 7.6 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.64 (d, J = 6.6 Hz, 1H), 7.60–7.52 (m, 2H), 7.48–7.45 (m, 1H), 2.91 (s, 3H). 13C-NMR (100 MHz, CDCl3) δ 156.8, 154.9, 145.4, 142.0, 136.7, 128.7, 126.1, 125.6, 122.9, 121.8, 119.6, 117.7, 116.0, 115.0, 111.0, 17.9; HRMS (ESI): calcd. for C16H12NO [M + H]+ 234.0913; found 234.0906.
9-Bromobenzofuro[3,2-c]quinolin-4-ol (2w): Light pink solid (0.05 g, 17%), m.p. >250 °C. 1H-NMR (400 MHz, DMSO-d6) δ 9.71–9.63 (m, 2H), 8.41 (s, 1H), 8.32 (d, J = 8.6 Hz, 1H), 7.90 (d, J = 8.3 Hz, 1H), 7.72 (d, J = 8.8 Hz, 1H), 7.62 (s, 1H), 7.06 (d, J = 8.4 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 156.8, 154.6, 149.3, 147.2, 146.5, 131.5, 130.3, 122.6, 122.5 (2C), 116.8, 116.2, 115.3, 112.6, 106.1; HRMS (ESI): calcd. for C15H9BrNO2 [M + H]+ 314.9844; found 314.0560.
9-Chlorobenzofuro[3,2-c]quinolin-4-ol (2x): Pink solid (0.03 g, 12%), m.p. >250 °C. 1H-NMR (400 MHz, DMSO-d6) δ 9.86 (br s, 1H), 9.62 (s, 1H), 8.38 (d, J = 8.8 Hz, 1H), 8.23 (d, J = 1.9 Hz, 1H), 7.77 (dd, J = 8.8, 2.0 Hz, 1H), 7.71 (d, J = 8.9 Hz, 1H), 7.63 (d, J = 2.4 Hz, 1H), 7.09 (dd, J = 8.9, 2.4 Hz, 1H). 13C-NMR (100 MHz, DMSO-d6) δ 156.7, 154.7, 149.2, 147.0, 146.5, 133.8, 128.3, 127.7, 122.5, 116.7, 116.2, 115.1, 112.5, 106.1; HRMS (ESI): calcd. for C15H9ClNO2 [M + H]+ 270.0316; found 270.0318.