Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery
Abstract
:1. Introduction
2. Metal Nanoparticles in Medicine
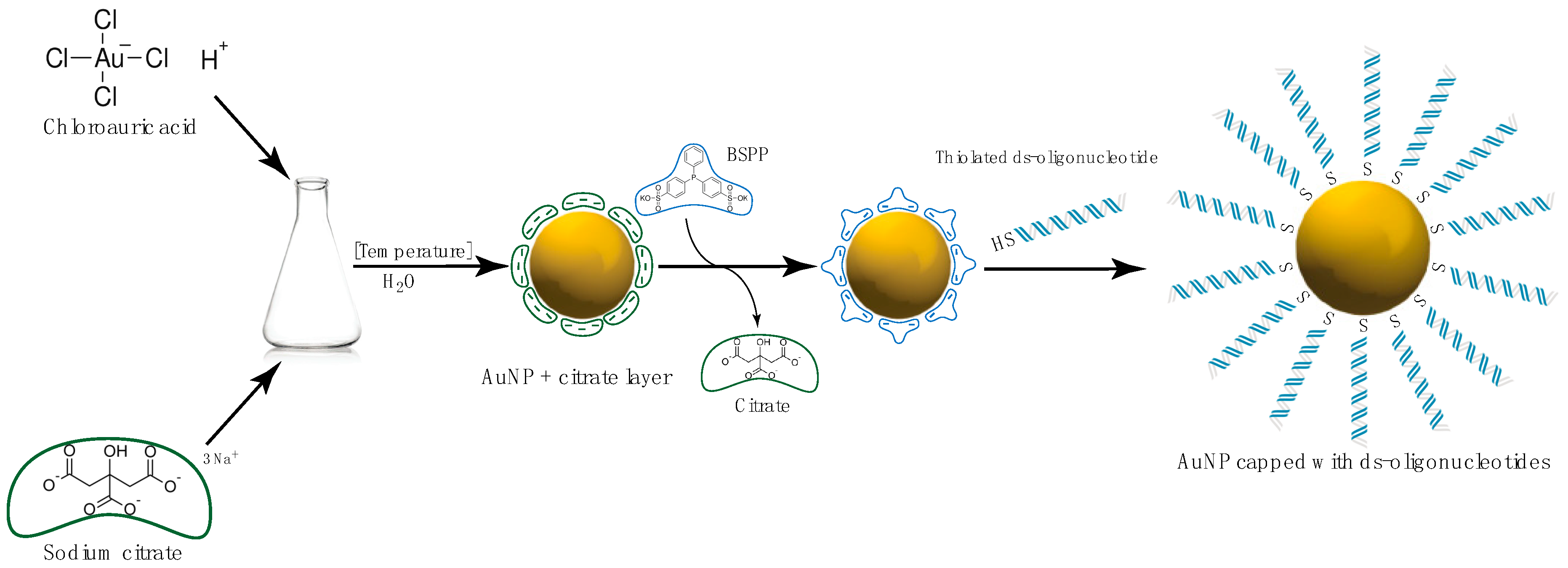
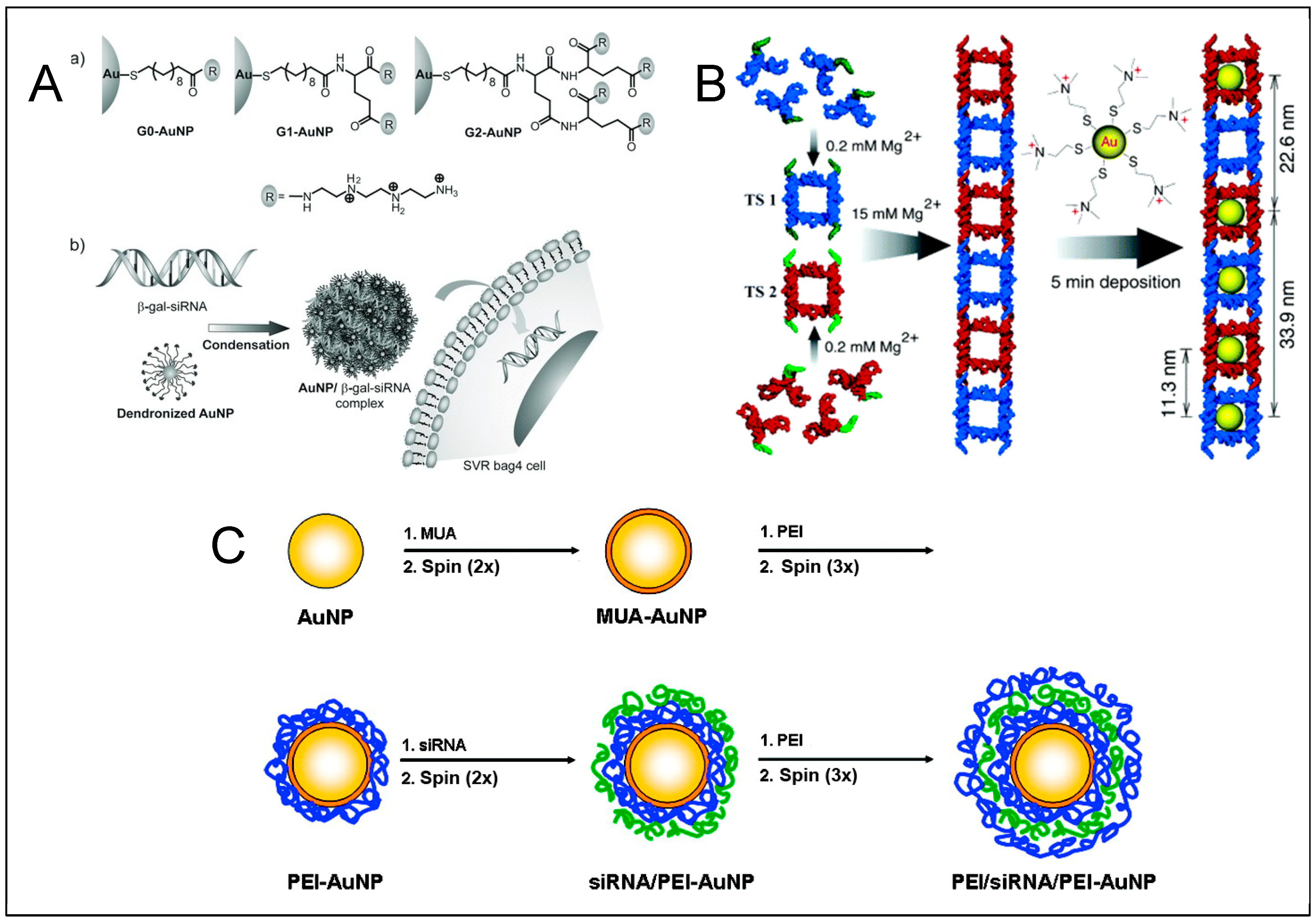
Gold Nanoparticles
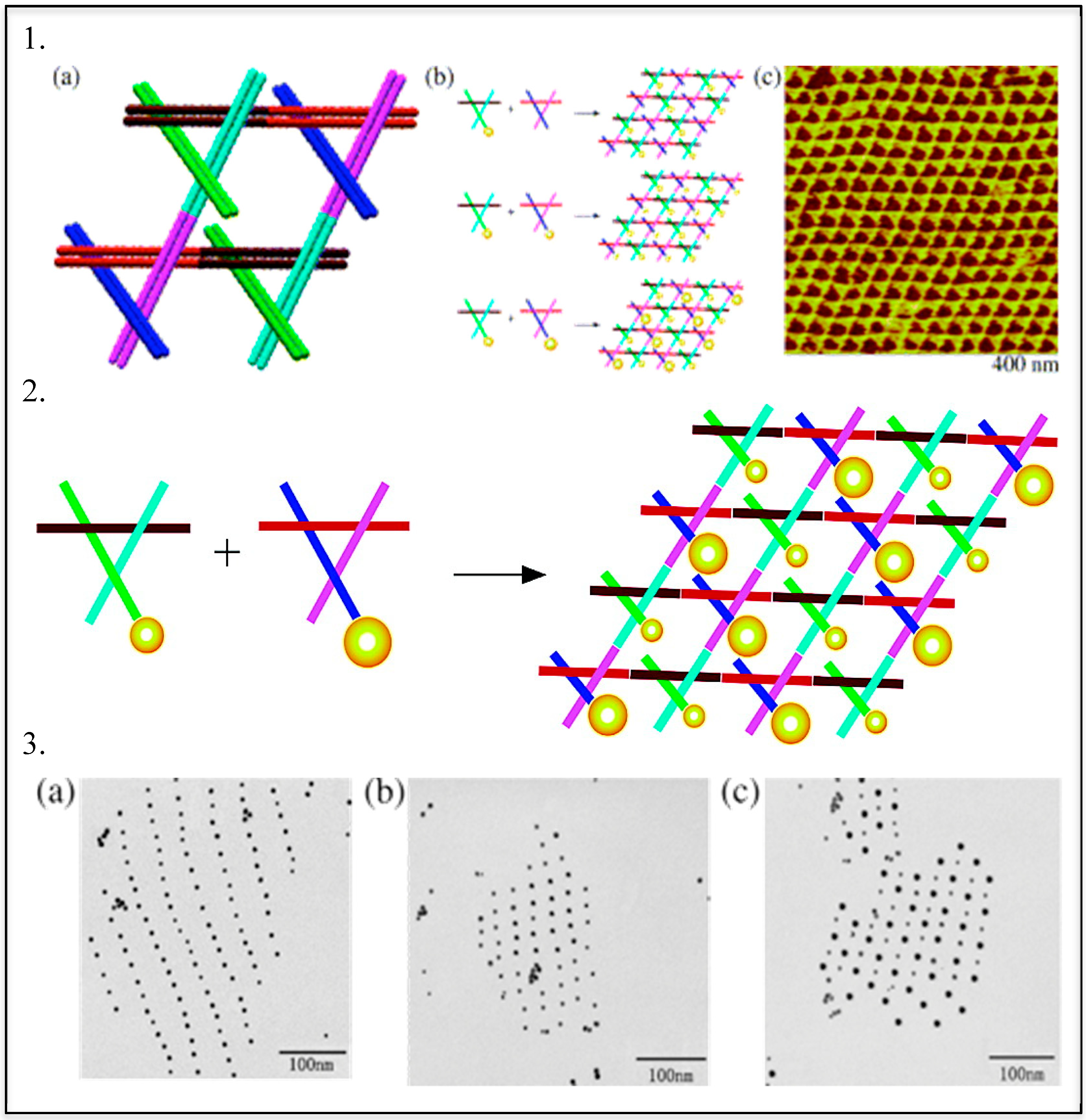
3. Templated DNA Structures—DNA Nanotechnology
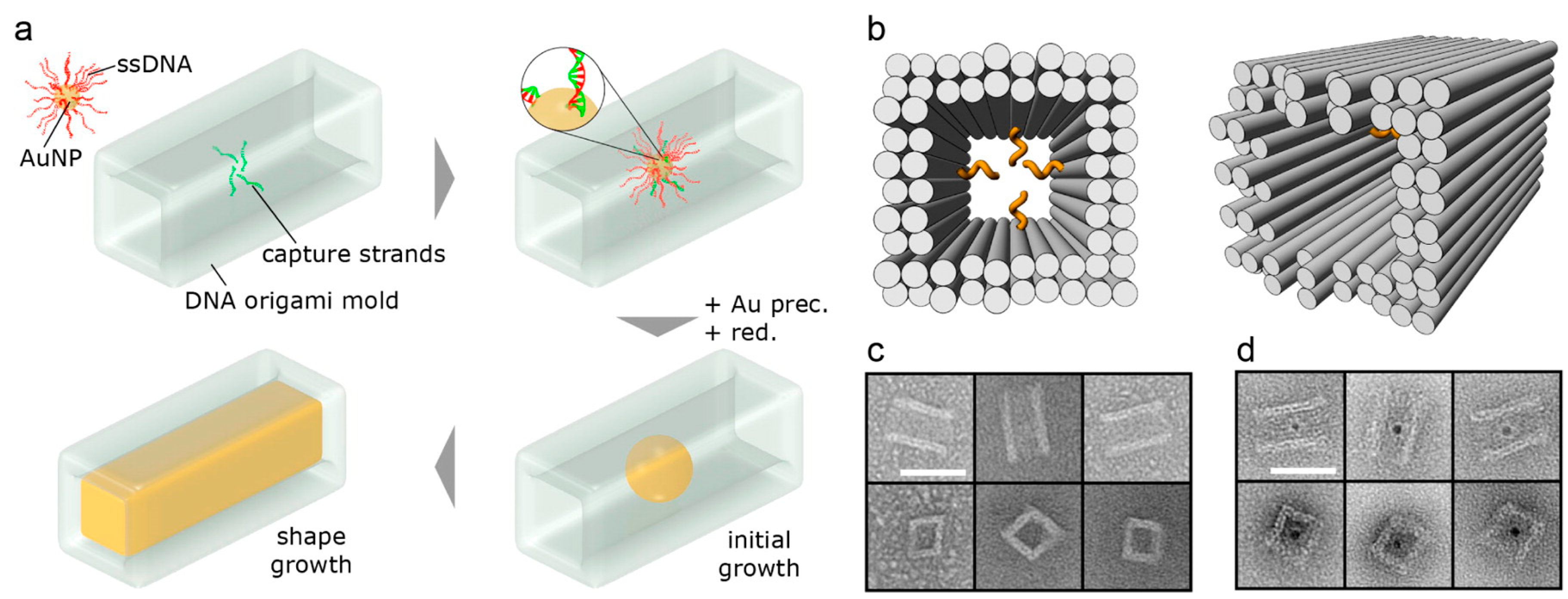
DNA—Metal Nanoparticles Conjugates
4. RNA—Gold Nanoparticles (AuNP) Conjugates
5. Transport of DNA and RNA Conjugated Nanoparticles inside a Cell
- Cell surface binding,
- Translocation across the plasma membrane and thus penetration inside the cell (include membrane invagination and sorting into early endosomes)
- Escape from endosomes or lysosomes
5.1. Intracellular Trafficking Organelle Distribution and Processing of Nanostructures
- Low-motility particles (adhered to the membrane or bound to receptors)
- High-motility particles (particles wrapped in early endosomal vesicles)
- Low-motility particles (wrapped in late endosomes or lysosomes in the perinuclear region of the cell)
- The fast-moving single particle chases a slow-moving one to merge (this type of motion is associated with vesicular fusion of early endosomes)
- The single nanoparticle connects to small cluster on a different track, then rapidly separates and both molecules continue the moving along original tracks.
- The small cluster moves rapidly towards another one, which is static and suddenly reverses its moving direction. This motion is similar to a dynein- and kinesin-based bidirectional cargo transport along the microtubule.
- The two small clusters move along two tracks in the cross-section of the microtubules and one of them looses its mobility and becomes static while the second one departs with high speed.
5.2. Physico-Chemical Properties of Nanoparticles and Their Cellular Uptake and Transport
6. Summary
Funding
Acknowledgments
Conflicts of Interest
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Khan, Z.U.; Khan, A.; Chen, Y.M.; Shah, N.S.; Muhammad, N.; Khan, A.U.; Tahir, K.; Khan, F.U.; Murtaza, B.; Ul Hassan, S.; et al. Biomedical applications of green synthesized Nobel metal nanoparticles. J. Photochem. Photobiol. B 2017, 173, 150–164. [Google Scholar] [CrossRef]
- Meziani, M.J.; Sun, Y.P. Protein-conjugated nanoparticles from rapid expansion of supercritical fluid solution into aqueous solution. J. Am. Chem. Soc. 2003, 125, 8015–8018. [Google Scholar] [CrossRef]
- Piella, J.; Bastus, N.G.; Puntes, V. Size-Dependent Protein-Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjug. Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef]
- Draz, M.S.; Fang, B.A.; Zhang, P.F.; Hu, Z.; Gu, S.D.; Weng, K.C.; Gray, J.W.; Chen, F.F. Nanoparticle-Mediated Systemic Delivery of siRNA for Treatment of Cancers and Viral Infections. Theranostics 2014, 4, 872–892. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Mirkin, C.A.; Letsinger, R.L.; Mucic, R.C.; Storhoff, J.J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. [Google Scholar] [CrossRef]
- Exicure. Available online: http://www.exicuretx.com/index.php (accessed on 27 November 2019).
- Cutler, J.I.; Auyeung, E.; Mirkin, C.A. Spherical Nucleic Acids. J. Am. Chem. Soc. 2012, 134, 1376–1391. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, B.; Lu, X.; Tan, X.; Jia, F.; Xiao, Y.; Cheng, Z.; Li, Y.; Silva, D.O.; Schrekker, H.S.; et al. Molecular spherical nucleic acids. Proc. Natl. Acad. Sci. USA 2018, 115, 4340–4344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutler, J.I.; Zhang, K.; Zheng, D.; Auyeung, E.; Prigodich, A.E.; Mirkin, C.A. Polyvalent Nucleic Acid Nanostructures. J. Am. Chem. Soc. 2011, 133, 9254–9257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; MeneghettI, M.; Stener, M.; Guo, Y.; Chen, S.; Crespo, P.; Garcia, M.A.; Hernando, A.; Pengo, P.; Pasquato, L. Physico-Chemical Characteristics of Gold Nanoparticles. In Gold Nanoparticles in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 81–152. [Google Scholar]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Han, G.; You, C.C.; Kim, B.J.; Turingan, R.S.; Forbes, N.S.; Martin, C.T.; Rotello, V.M. Light-regulated release of DNA and its delivery to nuclei by means of photolabile gold nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 3165–3169. [Google Scholar] [CrossRef]
- Weintraub, K. The new gold standard. Nature 2013, 495, S14–S16. [Google Scholar] [CrossRef]
- Hong, R.; Han, G.; Fernandez, J.M.; Kim, B.J.; Forbes, N.S.; Rotello, V.M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Sotnikov, D.V.; Berlina, A.N.; Ivanov, V.S.; Zherdev, A.V.; Dzantiev, B.B. Adsorption of proteins on gold nanoparticles: One or more layers? Colloids Surf. B Biointerfaces 2019, 173, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Khashayar, P.; Amoabediny, G.; Larijani, B.; Hosseini, M.; Vanfleteren, J. Fabrication and Verification of Conjugated AuNP-Antibody Nanoprobe for Sensitivity Improvement in Electrochemical Biosensors. Sci. Rep. 2017, 7, 16070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafaoglu, N.; Kiziltepe, T.; Bilgicer, B. Site-specific conjugation of an antibody on a gold nanoparticle surface for one-step diagnosis of prostate specific antigen with dynamic light scattering. Nanoscale 2017, 9, 8684–8694. [Google Scholar] [CrossRef] [PubMed]
- Pissuwan, D.; Niidome, T.; Cortie, M.B. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J. Control. Release 2011, 149, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Camerin, M.; Magaraggia, M.; Soncin, M.; Jori, G.; Moreno, M.; Chambrier, I.; Cook, M.J.; Russell, D.A. The in vivo efficacy of phthalocyanine-nanoparticle conjugates for the photodynamic therapy of amelanotic melanoma. Eur. J. Cancer 2010, 46, 1910–1918. [Google Scholar] [CrossRef]
- Labala, S.; Jose, A.; Venuganti, V.V. Transcutaneous iontophoretic delivery of STAT3 siRNA using layer-by-layer chitosan coated gold nanoparticles to treat melanoma. Colloids Surf. B Biointerfaces 2016, 146, 188–197. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Sazgarnia, A.; Rajabi, O.; Seilanian Toosi, M. Comparative study of X-ray treatment and photodynamic therapy by using 5-aminolevulinic acid conjugated gold nanoparticles in a melanoma cell line. Artif. Cells Nanomed. Biotechnol. 2017, 45, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S.; West, J.L.; Drezek, R.A. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef]
- Day, E.S.; Zhang, L.; Thompson, P.A.; Zawaski, J.A.; Kaffes, C.C.; Gaber, M.W.; Blaney, S.M.; West, J.L. Vascular-targeted photothermal therapy of an orthotopic murine glioma model. Nanomedicine 2012, 7, 1133–1148. [Google Scholar] [CrossRef] [Green Version]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Acunto, M. Detection of Intracellular Gold Nanoparticles: An Overview. Materials 2018, 11, 882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huefner, A.; Septiadi, D.; Wilts, B.D.; Patel, I.I.; Kuan, W.L.; Fragniere, A.; Barker, R.A.; Mahajan, S. Gold nanoparticles explore cells: Cellular uptake and their use as intracellular probes. Methods 2014, 68, 354–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.H.; Kim, C.; Maslov, K.; Wang, L.V. Noninvasive in vivo spectroscopic nanorod-contrast photoacoustic mapping of sentinel lymph nodes. Eur. J. Radiol. 2009, 70, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, N.J.; Jeffery, H.C.; Claire, S.; Lewis, D.J.; Zikeli, G.; Hodges, N.J.; Egginton, S.; Nash, G.B.; Pikramenou, Z. Tailoring iridium luminescence and gold nanoparticle size for imaging of microvascular blood flow. Nanomedicine 2017, 12, 2725–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meola, A.; Rao, J.; Chaudhary, N.; Sharma, M.; Chang, S.D. Gold Nanoparticles for Brain Tumor Imaging: A Systematic Review. Front. Neurol. 2018, 9, 328. [Google Scholar] [CrossRef]
- Cruz, L.J.; Tacken, P.J.; Rueda, F.; Domingo, J.C.; Albericio, F.; Figdor, C.G. Targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol. 2012, 509, 143–163. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Xin, Y.; Jiang, C.; Yan, B.; Zhai, S. Interactions Between Nanoparticles and Dendritic Cells: From the Perspective of Cancer Immunotherapy. Front. Oncol. 2018, 8, 404. [Google Scholar] [CrossRef]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Wu, T.; Qin, X.; Qiao, Q.; Shang, L.; Song, Q.; Yang, C.; Zhang, Z. Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019, 19, 6635–6646. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeman, N.C. Nucleic acid junctions and lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Fu, T.J.; Seeman, N.C. DNA double-crossover molecules. Biochemistry 1993, 32, 3211–3220. [Google Scholar] [CrossRef]
- Sa-Ardyen, P.; Vologodskii, A.V.; Seeman, N.C. The flexibility of DNA double crossover molecules. Biophys. J. 2003, 84, 3829–3837. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.Q.; Sha, R.J.; Seeman, N.C. Pseudohexagonal 2D DNA crystals from double crossover cohesion. J. Am. Chem. Soc. 2004, 126, 10230–10231. [Google Scholar] [CrossRef]
- Wang, W.; Lin, T.; Zhang, S.Y.; Bai, T.X.; Mi, Y.L.; Wei, B.Y. Self-assembly of fully addressable DNA nanostructures from double crossover tiles. Nucleic Acids Res. 2016, 44, 7989–7996. [Google Scholar] [CrossRef] [Green Version]
- LaBean, T.H.; Yan, H.; Kopatsch, J.; Liu, F.R.; Winfree, E.; Reif, J.H.; Seeman, N.C. Construction, analysis, ligation, and self-assembly of DNA triple crossover complexes. J. Am. Chem. Soc. 2000, 122, 1848–1860. [Google Scholar] [CrossRef]
- Liu, D.; Park, S.H.; Reif, J.H.; LaBean, T.H. DNA nanotubes self-assembled from triple-crossover tiles as templates for conductive nanowires. Proc. Natl. Acad. Sci. USA 2004, 101, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Wei, B.; Mi, Y.L. A new triple crossover triangle (TXT) motif for DNA self-assembly. Biomacromolecules 2005, 6, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Yan, H.; Wang, T.; Seeman, N.C. Paranemic crossover DNA: A generalized Holliday structure with applications in nanotechnology. J. Am. Chem. Soc. 2004, 126, 1666–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.Y.; Wang, X.; Wang, T.; Sha, R.J.; Seeman, N.C. PX DNA triangle oligomerized using a novel three-domain motif. Nano Lett. 2008, 8, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winfree, E.; Liu, F.R.; Wenzler, L.A.; Seeman, N.C. Design and self-assembly of two-dimensional DNA crystals. Nature 1998, 394, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Park, S.H.; Finkelstein, G.; Reif, J.H.; LaBean, T.H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 2003, 301, 1882–1884. [Google Scholar] [CrossRef]
- Chen, J.; Seeman, N.C. The Synthesis from DNA of a Molecule with the Connectivity of a Cube. Nature 1991, 350, 631–633. [Google Scholar] [CrossRef]
- Shih, W.M.; Quispe, J.D.; Joyce, G.F. A 1.7-kilobase single-stranded DNA that folds into a nanoscale octahedron. Nature 2004, 427, 618–621. [Google Scholar] [CrossRef]
- Rothemund, P.W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.D.; Sun, W.Q.; Shen, Z.Y.; Seeman, N.C. A nanomechanical device based on the B-Z transition of DNA. Nature 1999, 397, 144–146. [Google Scholar] [CrossRef]
- Modi, S.; Swetha, M.G.; Goswami, D.; Gupta, G.D.; Mayor, S.; Krishnan, Y. A DNA nanomachine that maps spatial and temporal pH changes inside living cells. Nat. Nanotechnol. 2009, 4, 325–330. [Google Scholar] [CrossRef]
- Modi, S.; Nizak, C.; Surana, S.; Halder, S.; Krishnan, Y. Two DNA nanomachines map pH changes along intersecting endocytic pathways inside the same cell. Nat. Nanotechnol. 2013, 8, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.G.; Liang, X.G.; Mochizuki, T.; Asanuma, H. A Light-Driven DNA Nanomachine for the Efficient Photoswitching of RNA Digestion. Angew. Chem. Int. Ed. 2010, 49, 2167–2170. [Google Scholar] [CrossRef] [PubMed]
- Lund, K.; Manzo, A.J.; Dabby, N.; Michelotti, N.; Johnson-Buck, A.; Nangreave, J.; Taylor, S.; Pei, R.J.; Stojanovic, M.N.; Walter, N.G.; et al. Molecular robots guided by prescriptive landscapes. Nature 2010, 465, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, M.S.; Mao, C.D. An autonomous DNA nanomotor powered by a DNA enzyme. Angew. Chem. Int. Ed. 2004, 43, 3554–3557. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Yan, H.; Daniell, X.G.; Turberfield, A.J.; Reif, J.H. A unidirectional DNA walker that moves autonomously along a track. Angew. Chem. Int. Ed. 2004, 43, 4906–4911. [Google Scholar] [CrossRef] [PubMed]
- Bath, J.; Green, S.J.; Turberfield, A.J. A free-running DNA motor powered by a nicking enzyme. Angew. Chem. Int. Ed. 2005, 44, 4358–4361. [Google Scholar] [CrossRef]
- Yurke, B.; Turberfield, A.J.; Mills, A.P.; Simmel, F.C.; Neumann, J.L. A DNA-fuelled molecular machine made of DNA. Nature 2000, 406, 605–608. [Google Scholar] [CrossRef]
- Shin, J.S.; Pierce, N.A. A synthetic DNA walker for molecular transport. J. Am. Chem. Soc. 2004, 126, 10834–10835. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Mao, C.D. Molecular gears: A pair of DNA circles continuously rolls against each other. J. Am. Chem. Soc. 2004, 126, 11410–11411. [Google Scholar] [CrossRef] [Green Version]
- Douglas, S.M.; Bachelet, I.; Church, G.M. A Logic-Gated Nanorobot for Targeted Transport of Molecular Payloads. Science 2012, 335, 831–834. [Google Scholar] [CrossRef]
- Andersen, E.S.; Dong, M.; Nielsen, M.M.; Jahn, K.; Subramani, R.; Mamdouh, W.; Golas, M.M.; Sander, B.; Stark, H.; Oliveira, C.L.P.; et al. Self-assembly of a nanoscale DNA box with a controllable lid. Nature 2009, 459, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bujold, K.E.; Fakhoury, J.; Edwardson, T.G.W.; Carneiro, K.M.M.; Briard, J.N.; Godin, A.G.; Amrein, L.; Hamblin, G.D.; Panasci, L.C.; Wiseman, P.W.; et al. Sequence-responsive unzipping DNA cubes with tunable cellular uptake profiles. Chem. Sci. 2014, 5, 2449–2455. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Liang, L.; Li, J.; Wang, K.; Li, J.; Lv, M.; Chen, N.; Song, H.; Lee, J.; et al. Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nat. Commun. 2017, 8, 15646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, H.Y.; Li, X.F.; Zhang, H.Q.; Le, X.C. A microRNA-initiated DNAzyme motor operating in living cells. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, A.; Nakata, E.; Nakano, S.; Morii, T. Nucleic-Acid-Templated Enzyme Cascades. ChemBioChem 2017, 18, 696–716. [Google Scholar] [CrossRef]
- Gelinas, A.D.; Davies, D.R.; Janjic, N. Embracing proteins: Structural themes in aptamer-protein complexes. Curr. Opin. Struct. Biol. 2016, 36, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Sacca, B.; Niemeyer, C.M. Functionalization of DNA nanostructures with proteins. Chem. Soc. Rev. 2011, 40, 5910–5921. [Google Scholar] [CrossRef]
- Niemeyer, C.M.; Sano, T.; Smith, C.L.; Cantor, C.R. Oligonucleotide-Directed Self-Assembly of Proteins—Semisynthetic DNA Streptavidin Hybrid Molecules as Connectors for the Generation of Macroscopic Arrays and the Construction of Supramolecular Bioconjugates. Nucleic Acids Res. 1994, 22, 5530–5539. [Google Scholar] [CrossRef] [Green Version]
- Niemeyer, C.M.; Adler, M.; Gao, S.; Chi, L.F. Supramolecular nanocircles consisting of streptavidin and DNA. Angew. Chem. Int. Ed. 2000, 39, 3055–3059. [Google Scholar] [CrossRef]
- Li, H.Y.; Park, S.H.; Reif, J.H.; LaBean, T.H.; Yan, H. DNA-templated self-assembly of protein and nanoparticle linear arrays. J. Am. Chem. Soc. 2004, 126, 418–419. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Yin, P.; Liu, Y.; Reif, J.H.; LaBean, T.H.; Yan, H. Programmable DNA self-assemblies for nanoscale organization of ligands and proteins. Nano Lett. 2005, 5, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Koyfman, A.Y.; Braun, G.B.; Reich, N.O. Cell-Targeted Self-Assembled DNA Nanostructures. J. Am. Chem. Soc. 2009, 131, 14237–14239. [Google Scholar] [CrossRef] [PubMed]
- Malo, J.; Mitchell, J.C.; Venien-Bryan, C.; Harris, J.R.; Wille, H.; Sherratt, D.J.; Turberfield, A.J. Engineering a 2D protein-DNA crystal. Angew. Chem. Int. Ed. 2005, 44, 3057–3061. [Google Scholar] [CrossRef] [PubMed]
- Aptagen. Available online: https://www.aptagen.com/apta-index/ (accessed on 27 November 2019).
- Liu, Y.; Lin, C.X.; Li, H.Y.; Yan, H. Protein nanoarrays—Aptamer-directed self-assembly of protein arrays on a DNA nanostructure. Angew. Chem. Int. Ed. 2005, 44, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Katilius, E.; Liu, Y.; Zhang, J.P.; Yan, H. Self-assembled signaling aptamer DNA arrays for protein detection. Angew. Chem. Int. Ed. 2006, 45, 5296–5301. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, C.M. Bioorganic applications of semisynthetic DNA-protein conjugates. Chem. A Eur. J. 2001, 7, 3189–3195. [Google Scholar] [CrossRef]
- Niemeyer, C.M. The developments of semisynthetic DNA-protein conjugates. Trends Biotechnol. 2002, 20, 395–401. [Google Scholar] [CrossRef]
- Li, H.Y.; LaBean, T.H.; Leong, K.W. Nucleic acid-based nanoengineering: Novel structures for biomedical applications. Interface Focus 2011, 1, 702–724. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef]
- Yang, D.Y.; Campolongo, M.J.; Tran, T.N.N.; Ruiz, R.C.H.; Kahn, J.S.; Luo, D. Novel DNA materials and their applications. Wires Nanomed. Nanobiotechnol. 2010, 2, 648–669. [Google Scholar] [CrossRef]
- Seeman, N.; Sleiman, H. DNA nanotechnology. Nat. Rev. Mater. 2017, 3. [Google Scholar] [CrossRef]
- Bath, J.; Turberfield, A.J. DNA nanomachines. Nat. Nanotechnol 2007, 2, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Liedl, T.; Sobey, T.L.; Simmel, F.C. DNA-based nanodevices. Nano Today 2007, 2, 36–41. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Functional devices from DNA and proteins. Nano Today 2007, 2, 42–52. [Google Scholar] [CrossRef]
- Kwon, Y.W.; Lee, C.H.; Choi, D.H.; Jin, J.I. Materials science of DNA. J. Mater. Chem. 2009, 19, 1353–1380. [Google Scholar] [CrossRef]
- Carter, J.D.; LaBean, T.H. Organization of Inorganic Nanomaterials via Programmable DNA Self-Assembly and Peptide Molecular Recognition. ACS Nano 2011, 5, 2200–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torimoto, T.; Yamashita, M.; Kuwabata, S.; Sakata, T.; Mori, H.; Yoneyama, H. Fabrication of CdS nanoparticle chains along DNA double strands. J. Phys. Chem. B 1999, 103, 8799–8803. [Google Scholar] [CrossRef]
- Richter, J.; Seidel, R.; Kirsch, R.; Mertig, M.; Pompe, W.; Plaschke, J.; Schackert, H.K. Nanoscale palladium metallization of DNA. Adv. Mater. 2000, 12, 507–510. [Google Scholar] [CrossRef]
- Ford, W.E.; Harnack, O.; Yasuda, A.; Wessels, J.M. Platinated DNA as precursors to templated chains of metal nanoparticles. Adv. Mater. 2001, 13, 1793–1797. [Google Scholar] [CrossRef]
- Patolsky, F.; Weizmann, Y.; Lioubashevski, O.; Willner, I. Au-nanoparticle nanowires based on DNA and polylysine templates. Angew. Chem. Int. Ed. 2002, 41, 2323–2327. [Google Scholar] [CrossRef]
- Stoltenberg, R.M.; Woolley, A.T. DNA-templated nanowire fabrication. Biomed. Microdevices 2004, 6, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P.; Johnsson, K.P.; Peng, X.G.; Wilson, T.E.; Loweth, C.J.; Bruchez, M.P.; Schultz, P.G. Organization of ‘nanocrystal molecules’ using DNA. Nature 1996, 382, 609–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, J.D.; Pinto, Y.; Seeman, N.C.; Musier-Forsyth, K.; Taton, T.A.; Kiehl, R.A. DNA-templated self-assembly of metallic nanocomponent arrays on a surface. Nano Lett. 2004, 4, 2343–2347. [Google Scholar] [CrossRef]
- Pinto, Y.Y.; Le, J.D.; Seeman, N.C.; Musier-Forsyth, K.; Taton, T.A.; Kiehl, R.A. Sequence-encoded self-assembly of multiple-nanocomponent arrays by 2D DNA scaffolding. Nano Lett. 2005, 5, 2399–2402. [Google Scholar] [CrossRef]
- Sharma, J.; Chhabra, R.; Liu, Y.; Ke, Y.G.; Yan, H. DNA-templated self-assembly of two-dimensional and periodical gold nanoparticle arrays. Angew. Chem. Int. Ed. 2006, 45, 730–735. [Google Scholar] [CrossRef]
- Zhang, J.P.; Liu, Y.; Ke, Y.G.; Yan, H. Periodic square-like gold nanoparticle arrays templated by self-assembled 2D DNA nanogrids on a surface. Nano Lett. 2006, 6, 248–251. [Google Scholar] [CrossRef]
- Zheng, J.W.; Constantinou, P.E.; Micheel, C.; Alivisatos, A.P.; Kiehl, R.A.; Seeman, N.C. Two-dimensional nanoparticle arrays show the organizational power of robust DNA motifs. Nano Lett. 2006, 6, 1502–1504. [Google Scholar] [CrossRef] [Green Version]
- Nykypanchuk, D.; Maye, M.M.; van der Lelie, D.; Gang, O. DNA-guided crystallization of colloidal nanoparticles. Nature 2008, 451, 549–552. [Google Scholar] [CrossRef]
- Park, S.Y.; Lytton-Jean, A.K.R.; Lee, B.; Weigand, S.; Schatz, G.C.; Mirkin, C.A. DNA-programmable nanoparticle crystallization. Nature 2008, 451, 553–556. [Google Scholar] [CrossRef]
- Julin, S.; Nummelin, S.; Kostiainen, M.A.; Linko, V. DNA nanostructure-directed assembly of metal nanoparticle superlattices. J. Nanoparticle Res. 2018, 20, 119. [Google Scholar] [CrossRef] [Green Version]
- Mastroianni, A.J.; Claridge, S.A.; Alivisatos, A.P. Pyramidal and Chiral Groupings of Gold Nanocrystals Assembled Using DNA Scaffolds. J. Am. Chem. Soc. 2009, 131, 8455–8459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, N.; Schlapak, R.; Kastner, M.; Armitage, D.; Chrzanowski, W.; Riener, J.; Hinterdorfer, P.; Ebner, A.; Howorka, S. A DNA Nanostructure for the Functional Assembly of Chemical Groups with Tunable Stoichiometry and Defined Nanoscale Geometry. Angew. Chem. Int. Ed. 2009, 48, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Chhabra, R.; Andersen, C.S.; Gothelf, K.V.; Yan, H.; Liu, Y. Toward reliable gold nanoparticle patterning on self-assembled DNA nanoscaffold. J. Am. Chem. Soc. 2008, 130, 7820–7821. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.Q.; Deng, Z.T.; Yan, H.; Cabrini, S.; Zuckermann, R.N.; Bokor, J. Gold Nanoparticle Self-Similar Chain Structure Organized by DNA Origami. J. Am. Chem. Soc. 2010, 132, 3248–3249. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Deng, Z.T.; Ding, B.Q.; Yan, H.; Liu, Y. DNA-Origami-Directed Self-Assembly of Discrete Silver-Nanoparticle Architectures. Angew. Chem. Int. Ed. 2010, 49, 2700–2704. [Google Scholar] [CrossRef]
- Helmi, S.; Ziegler, C.; Kauert, D.J.; Seidel, R. Shape-Controlled Synthesis of Gold Nanostructures Using DNA Origami Molds. Nano Lett. 2014, 14, 6693–6698. [Google Scholar] [CrossRef]
- Sun, W.; Boulais, E.; Hakobyan, Y.; Wang, W.L.; Guan, A.; Bathe, M.; Yin, P. Casting inorganic structures with DNA molds. Science 2014, 346, 1258361. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhang, X.P.; Shen, Z.Y.; Seeman, N.C. A robust DNA mechanical device controlled by hybridization topology. Nature 2002, 415, 62–65. [Google Scholar] [CrossRef]
- Mao, C.D.; LaBean, T.H.; Reif, J.H.; Seeman, N. Logical computation using algorithmic self-assembly of DNA triple-crossover molecules (vol 407, pg 493, 2000). Nature 2000, 408, 750. [Google Scholar] [CrossRef]
- Benenson, Y.; Paz-Elizur, T.; Adar, R.; Keinan, E.; Livneh, Z.; Shapiro, E. Programmable and autonomous computing machine made of biomolecules. Nature 2001, 414, 430–434. [Google Scholar] [CrossRef]
- Fujibayashi, K.; Hariadi, R.; Park, S.H.; Winfree, E.; Murata, S. Toward reliable algorithmic self-assembly of DNA tiles: A fixed-width cellular automaton pattern. Nano Lett. 2008, 8, 1791–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Xie, Y.J.; Zhou, S.H.; Zhou, C.J.; Zheng, X.D. Reversible Data Hiding Based on DNA Computing. Comput. Intell. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Lubrich, D.; Turberfield, A.J. DNA hairpins: Fuel for autonomous DNA devices. Biophys. J. 2006, 91, 2966–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataraman, S.; Dirks, R.M.; Rothemund, P.W.K.; Winfree, E.; Pierce, N.A. An autonomous polymerization motor powered by DNA hybridization. Nat. Nanotechnol. 2007, 2, 490–494. [Google Scholar] [CrossRef]
- Lubrich, D.; Lin, J.; Yan, J. A contractile DNA machine. Angew. Chem. Int. Ed. 2008, 47, 7026–7028. [Google Scholar] [CrossRef]
- Deng, Z.X.; Mao, C.D. Molecular lithography with DNA nanostructures. Angew. Chem. Int. Ed. 2004, 43, 4068–4070. [Google Scholar] [CrossRef]
- Edwardson, T.G.W.; Lau, K.L.; Bousmail, D.; Serpell, C.J.; Sleiman, H.F. Transfer of molecular recognition information from DNA nanostructures to gold nanoparticles. Nat. Chem. 2016, 8, 162–170. [Google Scholar] [CrossRef]
- Kuzyk, A.; Schreiber, R.; Fan, Z.Y.; Pardatscher, G.; Roller, E.M.; Hogele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sha, R.J.; Kristiansen, M.; Hernandez, C.; Hao, Y.D.; Mao, C.D.; Canary, J.W.; Seeman, N.C. An Organic Semiconductor Organized into 3D DNA Arrays by “Bottom-up” Rational Design. Angew. Chem. Int. Ed. 2017, 56, 6445–6448. [Google Scholar] [CrossRef]
- Tyagi, S.; Kramer, F.R. Molecular beacons: Probes that fluoresce upon hybridization. Nat. Biotechnol. 1996, 14, 303–308. [Google Scholar] [CrossRef]
- Drake, T.J.; Tan, W.H. Molecular beacon DNA probes and their bioanalytical applications. Appl. Spectrosc. 2004, 58, 269A–280A. [Google Scholar] [CrossRef] [PubMed]
- Mirkin, C.A. Programming the assembly of two- and three-dimensional architectures with DNA and nanoscale inorganic building blocks. Inorg. Chem. 2000, 39, 2258–2272. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; McLaughlin, C.K.; Aldaye, F.A.; Hamblin, G.D.; Rys, A.Z.; Rouiller, I.; Sleiman, H.F. Metal-nucleic acid cages. Nat. Chem. 2009, 1, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.M.; Stoeva, S.I.; Mirkin, C.A. Bio-bar-code-based DNA detection with PCR-like sensitivity. J. Am. Chem. Soc. 2004, 126, 5932–5933. [Google Scholar] [CrossRef]
- Stoeva, S.I.; Lee, J.S.; Thaxton, C.S.; Mirkin, C.A. Multiplexed DNA detection with biobarcoded nanoparticle probes. Angew. Chem. Int. Ed. 2006, 45, 3303–3306. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Lv, S.Z.; Lin, Z.Z.; Li, M.J.; Tang, D.P. Bio-bar-code-based photoelectrochemical immunoassay for sensitive detection of prostate-specific antigen using rolling circle amplification and enzymatic biocatalytic precipitation. Biosens. Bioelectron. 2018, 101, 159–166. [Google Scholar] [CrossRef]
- Delihas, N. Discovery and characterization of the first non-coding RNA that regulates gene expression, micF RNA: A historical perspective. World J. Biol. Chem. 2015, 6, 272–280. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Prigodich, A.E.; Patel, P.C.; Mirkin, C.A. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J. Am. Chem. Soc. 2009, 131, 2072–2073. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.C.; Hao, L.; Yeung, W.S.; Mirkin, C.A. Duplex end breathing determines serum stability and intracellular potency of siRNA-Au NPs. Mol. Pharm. 2011, 8, 1285–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, D.; Giljohann, D.A.; Chen, D.L.; Massich, M.D.; Wang, X.Q.; Iordanov, H.; Mirkin, C.A.; Paller, A.S. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 11975–11980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, S.A.; Day, E.S.; Ko, C.H.; Hurley, L.A.; Luciano, J.P.; Kouri, F.M.; Merkel, T.J.; Luthi, A.J.; Patel, P.C.; Cutler, J.I.; et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013, 5, 209ra152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Takemoto, H.; Yi, Y.; Zheng, M.; Maeda, Y.; Chaya, H.; Hayashi, K.; Mi, P.; Pittella, F.; Christie, R.J.; et al. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS Nano 2014, 8, 8979–8991. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, S.N.; Lee, A.; Mirkin, C.A. Probing the inherent stability of siRNA immobilized on nanoparticle constructs. Proc. Natl. Acad. Sci. USA 2014, 111, 9739–9744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396. [Google Scholar] [CrossRef] [PubMed]
- Koyfman, A.Y.; Braun, G.; Magonov, S.; Chworos, A.; Reich, N.O.; Jaeger, L. Controlled spacing of cationic gold nanoparticles by nanocrown RNA. J. Am. Chem. Soc. 2005, 127, 11886–11887. [Google Scholar] [CrossRef]
- Elbakry, A.; Zaky, A.; Liebl, R.; Rachel, R.; Goepferich, A.; Breunig, M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009, 9, 2059–2064. [Google Scholar] [CrossRef]
- Kim, S.T.; Chompoosor, A.; Yeh, Y.C.; Agasti, S.S.; Solfiell, D.J.; Rotello, V.M. Dendronized gold nanoparticles for siRNA delivery. Small 2012, 8, 3253–3256. [Google Scholar] [CrossRef] [Green Version]
- Shaat, H.; Mostafa, A.; Moustafa, M.; Gamal-Eldeen, A.; Emam, A.; El-Hussieny, E.; Elhefnawi, M. Modified gold nanoparticles for intracellular delivery of anti-liver cancer siRNA. Int. J. Pharm. 2016, 504, 125–133. [Google Scholar] [CrossRef]
- Conde, J.; Rosa, J.; de la Fuente, J.M.; Baptist, P.V. Gold-nanobeacons for simultaneous gene specific silencing and intracellular tracking of the silencing events. Biomaterials 2013, 34, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.F.; O’Driscoll, C.M.; Holmes, J.D.; Rahme, K. Bioconjugated gold nanoparticles enhance cellular uptake: A proof of concept study for siRNA delivery in prostate cancer cells. Int. J. Pharm. 2016, 509, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.X.; Wei, P.; Kong, L.D.; Guo, R.; Wang, S.G.; Shi, X.Y. Partially PEGylated dendrimer-entrapped gold nanoparticles: A promising nanoplatform for highly efficient DNA and siRNA delivery. J. Mater. Chem. B 2016, 4, 2933–2943. [Google Scholar] [CrossRef]
- Majer, O.; Liu, B.; Barton, G.M. Nucleic acid-sensing TLRs: Trafficking and regulation. Curr. Opin. Immunol. 2017, 44, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radovic-Moreno, A.F.; Chernyak, N.; Mader, C.C.; Nallagatla, S.; Kang, R.S.; Hao, L.L.; Walker, D.A.; Halo, T.L.; Merkel, T.J.; Rische, C.H.; et al. Immunomodulatory spherical nucleic acids. Proc. Natl. Acad. Sci. USA 2015, 112, 3892–3897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnaby, S.N.; Perelman, G.A.; Kohlstedt, K.L.; Chinen, A.B.; Schatz, G.C.; Mirkin, C.A. Design Considerations for RNA Spherical Nucleic Acids (SNAs). Bioconjug. Chem. 2016, 27, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. R 2003, 67, 657–685. [Google Scholar] [CrossRef] [Green Version]
- Hao, L.; Patel, P.C.; Alhasan, A.H.; Giljohann, D.A.; Mirkin, C.A. Nucleic acid-gold nanoparticle conjugates as mimics of microRNA. Small 2011, 7, 3158–3162. [Google Scholar] [CrossRef]
- Lee, J.S.; Green, J.J.; Love, K.T.; Sunshine, J.; Langer, R.; Anderson, D.G. Gold, poly(beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009, 9, 2402–2406. [Google Scholar] [CrossRef] [Green Version]
- Allhenn, D.; Boushehri, M.A.; Lamprecht, A. Drug delivery strategies for the treatment of malignant gliomas. Int. J. Pharm. 2012, 436, 299–310. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B. Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupold, S.E.; Hicke, B.J.; Lin, Y.; Coffey, D.S. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002, 62, 4029–4033. [Google Scholar] [PubMed]
- Javier, D.J.; Nitin, N.; Levy, M.; Ellington, A.; Richards-Kortum, R. Aptamer-targeted gold nanoparticles as molecular-specific contrast agents for reflectance imaging. Bioconjug. Chem. 2008, 19, 1309–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Jeong, Y.Y.; Jon, S. A Drug-Loaded Aptamer-Gold Nanoparticle Bioconjugate for Combined CT Imaging and Therapy of Prostate Cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Ferapontova, E.E.; Olsen, E.M.; Gothelf, K.V. An RNA aptamer-based electrochemical biosensor for detection of theophylline in serum. J. Am. Chem. Soc. 2008, 130, 4256–4258. [Google Scholar] [CrossRef] [PubMed]
- Garst, A.D.; Edwards, A.L.; Batey, R.T. Riboswitches: Structures and Mechanisms. CSH Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Zivarts, M.; Sudarsan, N.; Breaker, R.R. Immobilized RNA switches for the analysis of complex chemical and biological mixtures. Nat. Biotechnol. 2001, 19, 336–341. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, X.J.; Zhao, W.; Xu, M.C.; Xu, J.J.; Chen, H.Y. Regulation and imaging of gene expression via an RNA interference antagonistic biomimetic probe. Chem. Sci. 2017, 8, 4973–4977. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.Y.; Ling, K.; Tao, X.J.; Zhang, Q.Q. Theophylline detection in serum using a self-assembling RNA aptamer-based gold nanoparticle sensor. Biosens. Bioelectron. 2015, 70, 299–303. [Google Scholar] [CrossRef]
- Yao, G.B.; Pei, H.; Li, J.; Zhao, Y.; Zhu, D.; Zhang, Y.N.; Lin, Y.F.; Huang, Q.; Fan, C.H. Clicking DNA to gold nanoparticles: Poly-adenine-mediated formation of monovalent DNA-gold nanoparticle conjugates with nearly quantitative yield. NPG Asia Mater. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Ling, K.; Jiang, H.; Zhang, L.; Li, Y.; Yang, L.; Qiu, C.; Li, F.R. A self-assembling RNA aptamer-based nanoparticle sensor for fluorometric detection of Neomycin B in milk. Anal. Bioanal. Chem. 2016, 408, 3593–3600. [Google Scholar] [CrossRef] [PubMed]
- Moll, W.D.; Guo, P. Grouping of ferritin and gold nanoparticles conjugated to pRNA of the phage phi29 DNA-Packaging motor. J. Nanosci. Nanotechnol. 2007, 7, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.D.; Qiu, J.R.; Shi, X.Y. Multifunctional PEI-entrapped gold nanoparticles enable efficient delivery of therapeutic siRNA into glioblastoma cells. J. Control. Release 2017, 259, E83–E84. [Google Scholar] [CrossRef]
- Gugliotti, L.A.; Feldheim, D.L.; Eaton, B.E. RNA-mediated control of metal nanoparticle shape. J. Am. Chem. Soc. 2005, 127, 17814–17818. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, L.; Westhof, E.; Leontis, N.B. TectoRNA: Modular assembly units for the construction of RNA nano-objects. Nucleic Acids Res. 2001, 29, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Grabow, W.W.; Zakrevsky, P.; Afonin, K.A.; Chworos, A.; Shapiro, B.A.; Jaeger, L. Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett. 2011, 11, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczyk, D.; Gendaszewska-Darmach, E.; Pawlowska, R.; Chworos, A. Designing synthetic RNA for delivery by nanoparticles. J. Phys.Condens. Matter 2017, 29. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Chan, W.C.W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 2007, 7, 1542–1550. [Google Scholar] [CrossRef]
- Patel, P.C.; Giljohann, D.A.; Daniel, W.L.; Zheng, D.; Prigodich, A.E.; Mirkin, C.A. Scavenger Receptors Mediate Cellular Uptake of Polyvalent Oligonucleotide-Functionalized Gold Nanoparticles. Bioconjug. Chem. 2010, 21, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.D.; Zhang, J.Q.; Ekman, J.M.; Kenis, P.J.A.; Lu, Y. DNA-Mediated Control of Metal Nanoparticle Shape: One-Pot Synthesis and Cellular Uptake of Highly Stable and Functional Gold Nanoflowers. Nano Lett. 2010, 10, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.J.; Hao, L.L.; Narayan, S.P.; Auyeung, E.; Mirkin, C.A. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc. Natl. Acad. Sci. USA 2013, 110, 7625–7630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.; Zhang, X.B.; Lv, Y.; Gong, L.; Wang, R.; Zhu, X.; Yang, R.; Tan, W. Functional DNA-containing nanomaterials: Cellular applications in biosensing, imaging, and targeted therapy. Acc. Chem. Res. 2014, 47, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Kodiha, M.; Wang, Y.M.; Hutter, E.; Maysinger, D.; Stochaj, U. Off to the Organelles—Killing Cancer Cells with Targeted Gold Nanoparticles. Theranostics 2015, 5, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Nemati, H.; Ghahramani, M.H.; Faridi-Majidi, R.; Izadi, B.; Bahrami, G.; Madani, S.H.; Tavoosidana, G. Using siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation in psoriasis. J. Control. Release 2017, 268, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, A.G.; Xie, H.; Coleman, D.; Glomm, W.; Ryan, J.; Anderson, M.F.; Franzen, S.; Feldheim, D.L. Multifunctional gold nanoparticle-peptide complexes for nuclear targeting. J. Am. Chem. Soc. 2003, 125, 4700–4701. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Patel, P.C.; Millstone, J.E.; Rosi, N.L.; Mirkin, C.A. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007, 7, 3818–3821. [Google Scholar] [CrossRef]
- Dai, Q.; Walkey, C.; Chan, W.C.W. Polyethylene Glycol Backfilling Mitigates the Negative Impact of the Protein Corona on Nanoparticle Cell Targeting. Angew. Chem. Int. Ed. 2014, 53, 5093–5096. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular Transport of Silver and Gold Nanoparticles and Biological Responses: An Update. Int. J. Mol. Sci. 2018, 19, 1305. [Google Scholar] [CrossRef] [Green Version]
- Ohta, S.; Glancy, D.; Chan, W.C.W. DNA-controlled dynamic colloidal nanoparticle systems for mediating cellular interaction. Science 2016, 351, 841–845. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Vahidnezhad, H.; Youssefian, L.; Mosafer, J.; Baradaran, B.; Uitto, J. Applications of Spherical Nucleic Acid Nanoparticles as Delivery Systems. Trends Mol. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Chen, Z.; Ho, L.W.; Chan, P.S.; Li, Q.; Leung, S.C.; Zhang, B.; Lai, K.L.; Kwon, G.S.; Choi, C.H.J.; et al. Oligonucleotide-conjugated nanoparticles for targeted drug delivery via scavenger receptors class A: An in vitro assessment for proof-of-concept. Int. J. Pharm. 2017, 532, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, B.B.; He, M.; Li, X.T.; Chen, P.Y.; Hu, B. Study on uptake of gold nanoparticles by single cells using droplet microfluidic chip-inductively coupled plasma mass spectrometry. Talanta 2019, 200, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B. Intracellular uptake, transport, and processing of gold nanostructures. Mol. Membr. Biol. 2010, 27, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, D.B.; Dunne, M.; Stewart, J.; Allen, C.; Jaffray, D.A. Cellular uptake and transport of gold nanoparticles incorporated in a liposomal carrier. Nanomed. Nanotechnol. 2010, 6, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Song, W.C.; Kim, K.R.; Park, M.; Lee, K.E.; Ahn, D.R. Backbone-modified oligonucleotides for tuning the cellular uptake behaviour of spherical nucleic acids. Biomater. Sci. 2017, 5, 412–416. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Bourquin, J.; Septiadi, D.; Vanhecke, D.; Balog, S.; Steinmetz, L.; Spuch-Calvar, M.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Reduction of Nanoparticle Load in Cells by Mitosis but Not Exocytosis. ACS Nano 2019, 13, 7759–7770. [Google Scholar] [CrossRef]
- Levy, R.; Shaheen, U.; Cesbron, Y.; See, V. Gold nanoparticles delivery in mammalian live cells: A critical review. Nano Rev. 2010, 1. [Google Scholar] [CrossRef]
- Aoyama, Y.; Kanamori, T.; Nakai, T.; Sasaki, T.; Horiuchi, S.; Sando, S.; Niidome, T. Artificial viruses and their application to gene delivery. size-controlled gene coating with glycocluster nanoparticles. J. Am. Chem. Soc. 2003, 125, 3455–3457. [Google Scholar] [CrossRef]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Osaki, F.; Kanamori, T.; Sando, S.; Sera, T.; Aoyama, Y. A quantum dot conjugated sugar ball and its cellular uptake. On the size effects of endocytosis in the subviral region. J. Am. Chem. Soc. 2004, 126, 6520–6521. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Cho, M.; Jeong, J.; Choi, M.; Han, B.S.; Shin, H.S.; Hong, J.; Chung, B.H.; Jeong, J.; Cho, M.H. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharm. 2010, 245, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of Gold Nanoparticle Size on Their Properties as Contrast Agents for Computed Tomography. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, Z.G.; Wei, W.; Lv, P.P.; Yue, H.; Wang, L.Y.; Su, Z.G.; Ma, G.H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- de la Fuente, J.M.; Berry, C.C. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug. Chem 2005, 16, 1176–1180. [Google Scholar] [CrossRef]
- Gu, Y.J.; Cheng, J.P.; Lin, C.C.; Lam, Y.W.; Cheng, S.H.; Wong, W.T. Nuclear penetration of surface functionalized gold nanoparticles. Toxicol. Appl. Pharm. 2009, 237, 196–204. [Google Scholar] [CrossRef]
- Cesbron, Y.; Shaheen, U.; Free, P.; Levy, R. TAT and HA2 facilitate cellular uptake of gold nanoparticles but do not lead to cytosolic localisation. PLoS ONE 2015, 10, e0121683. [Google Scholar] [CrossRef] [Green Version]
- Feldherr, C.M.; Lanford, R.E.; Akin, D. Signal-mediated nuclear transport in simian virus 40-transformed cells is regulated by large tumor antigen. Proc. Natl. Acad. Sci. USA 1992, 89, 11002–11005. [Google Scholar] [CrossRef] [Green Version]
- Pujals, S.; Bastus, N.G.; Pereiro, E.; Lopez-Iglesias, C.; Punte, V.F.; Kogan, M.J.; Giralt, E. Shuttling Gold Nanoparticles into Tumoral Cells with an Amphipathic Proline-Rich Peptide. Chembiochem 2009, 10, 1025–1031. [Google Scholar] [CrossRef]
- Cho, W.S.; Cho, M.J.; Jeong, J.; Choi, M.; Cho, H.Y.; Han, B.S.; Kim, S.H.; Kim, H.O.; Lim, Y.T.; Chung, B.H.; et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol. Appl. Pharm. 2009, 236, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Nativo, P.; Prior, I.A.; Brust, M. Uptake and intracellular fate of surface-modified gold nanoparticles. ACS Nano 2008, 2, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Faria, H.; Soares, M.E.; Duarte, J.A.; Soares, L.; Pereira, E.; Costa-Pereira, C.; Teixeira, J.P.; de Lourdes Bastos, M.; Carmo, H. Influence of the surface coating on the cytotoxicity, genotoxicity and uptake of gold nanoparticles in human HepG2 cells. J. Appl. Toxicol. 2013, 33, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Harrison, N.; Richards-Kortum, R.; Sokolov, K. Plasmonic nanosensors for imaging intracellular biomarkers in live cells. Nano Lett. 2007, 7, 1338–1343. [Google Scholar] [CrossRef]
- Cruz, E.; Kayser, V. Synthesis and Enhanced Cellular Uptake In Vitro of Anti-HER2 Multifunctional Gold Nanoparticles. Cancers 2019, 11, 870. [Google Scholar] [CrossRef] [Green Version]
- Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar] [CrossRef]
- Yang, P.H.; Sun, X.S.; Chiu, J.F.; Sun, H.Z.; He, Q.Y. Transferrin-mediated gold nanoparticle cellular uptake. Bioconjug. Chem. 2005, 16, 494–496. [Google Scholar] [CrossRef]
- Soman, N.; Marsh, J.; Lanza, G.; Wickline, S. New mechanisms for non-porative ultrasound stimulation of cargo delivery to cell cytosol with targeted perfluorocarbon nanoparticles. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [Green Version]



| AuNP Size (nm) | Surface Group | Cell Line | Toxicity | Subcellular Localization | Reference |
|---|---|---|---|---|---|
| 1, 4 | Phospholipid | HeLa | Not reported | Lysosomal, perinuclear/nuclear | [198] |
| 2, 8 | Tat peptide | HTERT-BJ1 | Low cytotoxicity below 10 µM | Cytoplasmic around the mitochondria and nuclear | [209] |
| 3, 7 | PEG | HeLa | Non toxic | Nuclear | [210] |
| 5, 10, 15 | CALNN, TAT and/or HA2 viral peptides | HeLa | Not reported | Cytoplasmic vesicles, lysosomal, endosomal, membranes | [211] |
| 10 | Oligonucleotides | HaCaT, A549, BALB/c 3T3, C166 | Not reported | Cytoplasmic, endosomal | [185] |
| 11–32 | Nucleoplasmin | BALB/c 3T3 A31, MOP-8, SV-T2 | Not reported | Nuclear and cytoplasmic | [212] |
| 12 | Sweet arrow peptide (SAP) | HeLa | Not reported | Endosomal | [213] |
| 13 | PEG | In vivo studies | Induction of acute inflammation and apoptosis | Cytoplasmic vesicles and lysosomal | [214] |
| 16 | PEG, CALNN, NLS, CPPs | HeLa | Not reported | Cytoplasmic, nuclear, lysosomal | [215] |
| 20 | citrate (Cit) compared with 11-mercaptoundecanoic acid (11-MUA) | HepG2 | Non-toxic, DNA damage in Cit-AuNPS | Cytoplasmic, endosomal | [216] |
| 20 | BSA with NLS, receptor-mediated endocytosis peptides (RME) | HepG2 | 5% death | Nuclear | [189] |
| 20 | Biotinylated Tat-HA2, PEG-SH, anti-actin antibodies | BALB/c 3T3 | Not reported | Cytoskeleton (cytoplasmic) | [217] |
| 20–50 | Citrate, PEG, CPP, Trastuzumab | DLD-1, SKOV-3, MDA-MB-231, SKBR-3, MCF-7 | Cytotoxicity dependent on the surface group. | Intracellular | [218] |
| 30–90 | PEG | PC-3 | Non toxic | Cytoplasmic and nuclear | [219] |
| 14–100 | Transferrin | STO, HeLa, SNB19, NPC | Non toxic | Endosomal | [181,182,220] |
| 100 | DPPE | C-32 | Non toxic | Endosomal | [221] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk, A.; Pawlowska, R.; Jedrzejczyk, D.; Chworos, A. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules 2020, 25, 204. https://doi.org/10.3390/molecules25010204
Graczyk A, Pawlowska R, Jedrzejczyk D, Chworos A. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules. 2020; 25(1):204. https://doi.org/10.3390/molecules25010204
Chicago/Turabian StyleGraczyk, Anna, Roza Pawlowska, Dominika Jedrzejczyk, and Arkadiusz Chworos. 2020. "Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery" Molecules 25, no. 1: 204. https://doi.org/10.3390/molecules25010204
APA StyleGraczyk, A., Pawlowska, R., Jedrzejczyk, D., & Chworos, A. (2020). Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules, 25(1), 204. https://doi.org/10.3390/molecules25010204







