Isoflavones Isolated from the Seeds of Millettia ferruginea Induced Apoptotic Cell Death in Human Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Ferrugone and DMI Isolated from M. ferruginea Had Potent Cytotoxic Activities against Human Ovarian Cancer Cells
2.2. Ferrugone and DMI Isolated from M. ferruginea Induced Apoptotic Cell Death in the Ovarian Cancer Cells
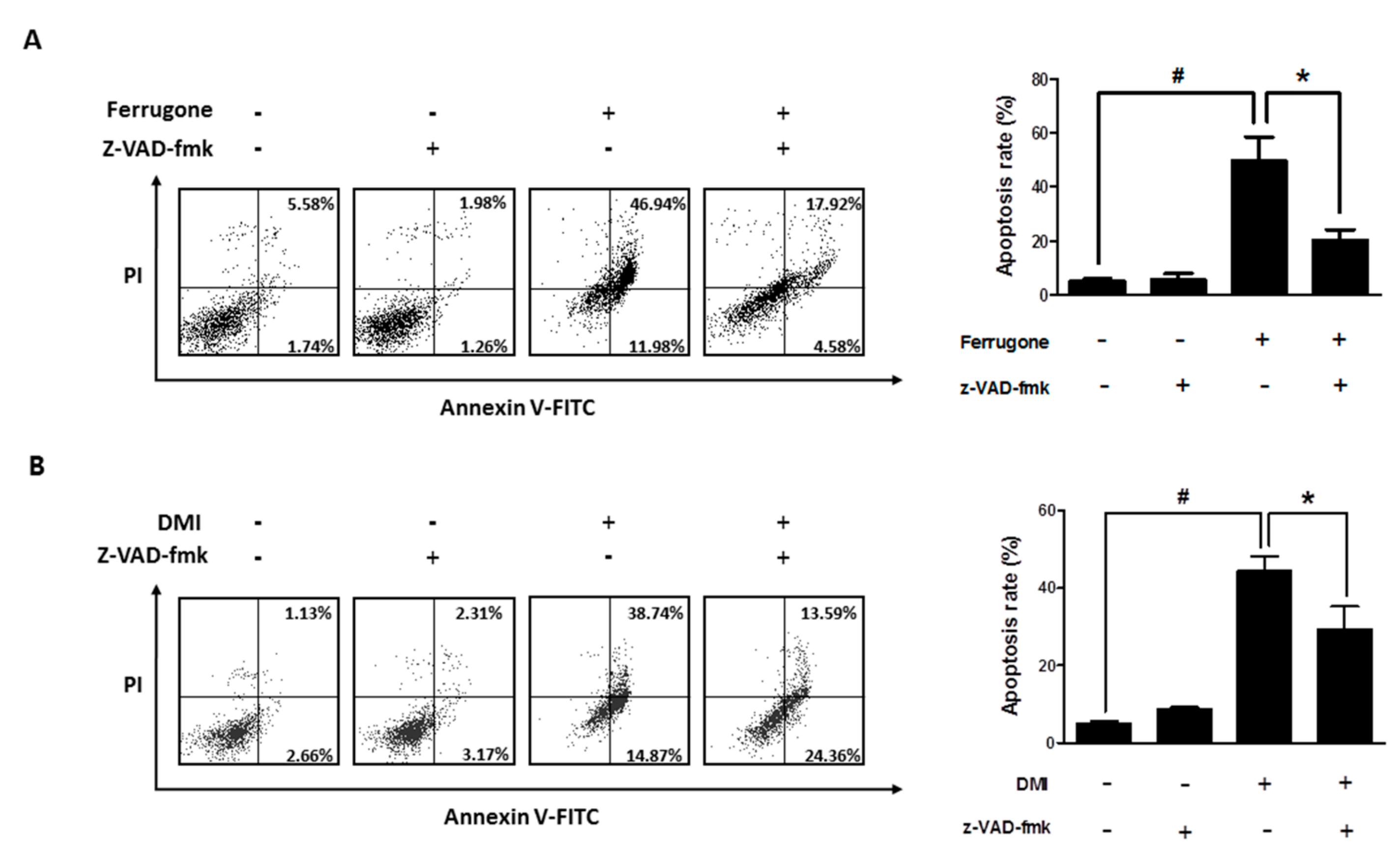
2.3. Ferrugone and DMI Induced Caspase-Dependent Apoptotic Cell Death in the Ovarian Cancer Cells
2.4. Apoptosis-Inducing Effect of DMI Is Associated with ROS Production
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Materials
4.3. Cell Culture
4.4. MTT Assay
4.5. Propidium Iodide (PI) Staining for Cell Cycle Analysis
4.6. Annexin V and PI Double Staining for Apoptosis Analysis
4.7. Western Blot Analysis
4.8. Measurement of Reactive Oxygen Species
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. Ca. Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar]
- Christian, J.; Thomas, H. Ovarian cancer chemotherapy. Cancer Treat. Rev. 2001, 27, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Banzouzi, J.T.; Prost, A.; Rajemiariaho, M.; Ongoka, P. Traditional uses of african millettia. Int. J. Bot. 2008, 4, 406–420. [Google Scholar] [CrossRef] [Green Version]
- Mollel, N.; Adema, F. Revision of millettia section truncaticalyces (leguminosae papilionoideae). Blumea 2006, 51, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Singhal, A.K.; Sharma, R.P.; Baruah, J.N.; Serengolam, V.; Govindant, S.V.; Herzt, W. Rotenoids from roots of millettia pachycarpa. Phytochemistry 1982, 21, 949–951. [Google Scholar] [CrossRef]
- Bueno Perez, L.; Pan, L.; Munoz Acuna, U.; Li, J.; Chai, H.B.; Gallucci, J.C.; Ninh, T.N.; Carcache de Blanco, E.J.; Soejarto, D.D.; Kinghorn, A.D. Caeruleanone A, a rotenoid with a new arrangement of the d-ring from the fruits of millettia caerulea. Org. Lett. 2014, 16, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.J.E.K.; Dankyi, E.; Kingsford-Adaboh, R.; Ishida, H. In search of new leads: A closer look at the therapeutic potential of the constituents of millettia thonningii, millettia pachycarpa and their structural analogues. Int. J. Pharm. Pharm. Sci. 2011, 3, 71–81. [Google Scholar]
- Zingue, S.; Magne, C.B.; Clyne, N.C.; Njamen, D. Elucidation of underlying mechanisms by which millettia macrophylla benth induces its estrogenic activity. Int. Sch. Res. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Deyou, T.; Gumula, I.; Pang, F.; Gruhonjic, A.; Mumo, M.; Holleran, J.; Duffy, S.; Fitzpatrick, P.A.; Heydenreich, M.; Landberg, G.; et al. Rotenoids, flavonoids, and chalcones from the root bark of millettia usaramensis. J. Nat. Prod. 2015, 78, 2932–2939. [Google Scholar] [CrossRef] [PubMed]
- Deyou, T.; Jang, Y.P. A new prenylated isoflavone from the seeds of millettia ferruginea ssp ferruginea. S. Afr. J. Bot. 2018, 117, 155–157. [Google Scholar] [CrossRef]
- Choudhury, M.K.; Shiferaw, Y.; Sur, K.R.; Dey, D.; Debnath, S.; Ghosh, S.; Ray, R.; Hazra, B. Antimicrobial activity and DNA-fragmentation effect of isoflavonoids isolated from seeds of millettia ferruginea, and endemic legume tree in ethiopia. J. Coast. Life Med. 2016, 4, 556–563. [Google Scholar]
- Yang, J.H.; Hu, J.; Wan, L.; Chen, L.J. Barbigerone inhibits tumor angiogenesis, growth and metastasis in melanoma. Asian Pac. J. Cancer Prev. 2014, 15, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miadokova, E. Isoflavonoids - an overview of their biological activities and potential health benefits. Interdiscip. Toxicol. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- De Franciscis, P.; Colacurci, N.; Riemma, G.; Conte, A.; Pittana, E.; Guida, M.; Schiattarella, A. A nutraceutical approach to menopausal complaints. Medicina 2019, 55, 544. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.A.; Crider, M.; Hill, S.R. Hormone therapy and other treatments for symptoms of menopause. Am. Fam. Physician 2016, 94, 884–889. [Google Scholar]
- Wanda, G.J.; Starcke, S.; Zierau, O.; Njamen, D.; Richter, T.; Vollmer, G. Estrogenic activity of griffonianone c, an isoflavone from the root bark of millettia griffoniana: Regulation of the expression of estrogen responsive genes in uterus and liver of ovariectomized rats. Planta Med. 2007, 73, 512–518. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Kojima, N.; Tokuda, H.; Hirata, T.; Nishino, H.; Furukawa, H. Chemical constituents of millettia taiwaniana: Structure elucidation of five new isoflavonoids and their cancer chemopreventive activity. J. Nat. Prod. 2004, 67, 1125–1130. [Google Scholar] [CrossRef]
- Yan, W.; Yang, J.; Tang, H.; Xue, L.; Chen, K.; Wang, L.; Zhao, M.; Tang, M.; Peng, A.; Long, C.; et al. Flavonoids from the stems of millettia pachyloba drake mediate cytotoxic activity through apoptosis and autophagy in cancer cells. J. Adv. Res. 2019, 20, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Danial, N.N.; Korsmeyer, S.J. Cell death: Critical control points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef] [Green Version]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting apoptosis pathways in cancer therapy. Ca. Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef]
- Miller, W.H., Jr.; Schipper, H.M.; Lee, J.S.; Singer, J.; Waxman, S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002, 62, 3893–3903. [Google Scholar]
- Parkinson EI, H.P. Runaway ros as a selective anticancer strategy. Chem. Med. Chem. 2011, 6, 1957–1959. [Google Scholar] [CrossRef]
- Wu, C.C.; Bratton, S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadenbach, B.; Arnold, S.; Lee, I.; Huttemann, M. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim. Biophys. Acta 2004, 1655, 400–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Y.; Xiang, B.; Yang, J.; Sun, X.; Wang, Y.; Cang, H.; Yi, J. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with apaf-1. Cell Res. 2009, 19, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.H.; Yang, Y.I.; Lee, K.T.; Choi, J.H. Dieckol, isolated from the edible brown algae ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are not available from the authors. |





| Compound No | Name | IC50 a | |
|---|---|---|---|
| A2780 | SKOV3 | ||
| 1 | 6,7-dimethoxy-3′,4′-methylenedioxy-8- (3,3-dimethylallyl)isoflavone (DMI) | 0.45 ± 0.06 μM (0.18 ± 0.02 μg/mL) | > 100 μM (> 39.41 μg/mL) |
| 2 | Ferrugone | 0.51 ± 0.06 μM (0.21 ± 0.02 μg/mL) | 36.78 ± 5.26 μM (15.01 ± 2.15 μg/mL) |
| 3 | Prebarbigerone | 32.06 ± 8.31 μM (13.16 ± 3.41 μg/mL) | > 100 μM (> 41.05 μg/mL) |
| - | Cisplatin b | 14.14 ± 0.59 μM (4.24 ± 0.18 μg/mL) | 36.64 ± 0.96 μM (10.99 ± 0.29 μg/mL) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Y.; Kwak, J.H.; Lee, K.-T.; Deyou, T.; Jang, Y.P.; Choi, J.-H. Isoflavones Isolated from the Seeds of Millettia ferruginea Induced Apoptotic Cell Death in Human Ovarian Cancer Cells. Molecules 2020, 25, 207. https://doi.org/10.3390/molecules25010207
Wang Y-Y, Kwak JH, Lee K-T, Deyou T, Jang YP, Choi J-H. Isoflavones Isolated from the Seeds of Millettia ferruginea Induced Apoptotic Cell Death in Human Ovarian Cancer Cells. Molecules. 2020; 25(1):207. https://doi.org/10.3390/molecules25010207
Chicago/Turabian StyleWang, Yi-Yue, Jun Hyeok Kwak, Kyung-Tae Lee, Tsegaye Deyou, Young Pyo Jang, and Jung-Hye Choi. 2020. "Isoflavones Isolated from the Seeds of Millettia ferruginea Induced Apoptotic Cell Death in Human Ovarian Cancer Cells" Molecules 25, no. 1: 207. https://doi.org/10.3390/molecules25010207
APA StyleWang, Y.-Y., Kwak, J. H., Lee, K.-T., Deyou, T., Jang, Y. P., & Choi, J.-H. (2020). Isoflavones Isolated from the Seeds of Millettia ferruginea Induced Apoptotic Cell Death in Human Ovarian Cancer Cells. Molecules, 25(1), 207. https://doi.org/10.3390/molecules25010207






